1H NMR and Multivariate Analysis for Geographic Characterization of Commercial Extra Virgin Olive Oil: A Possible Correlation with Climate Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Nuclear Magnetic Resonance Spectroscopy
2.3. Multivariate Data Processing
2.4. Chemicals
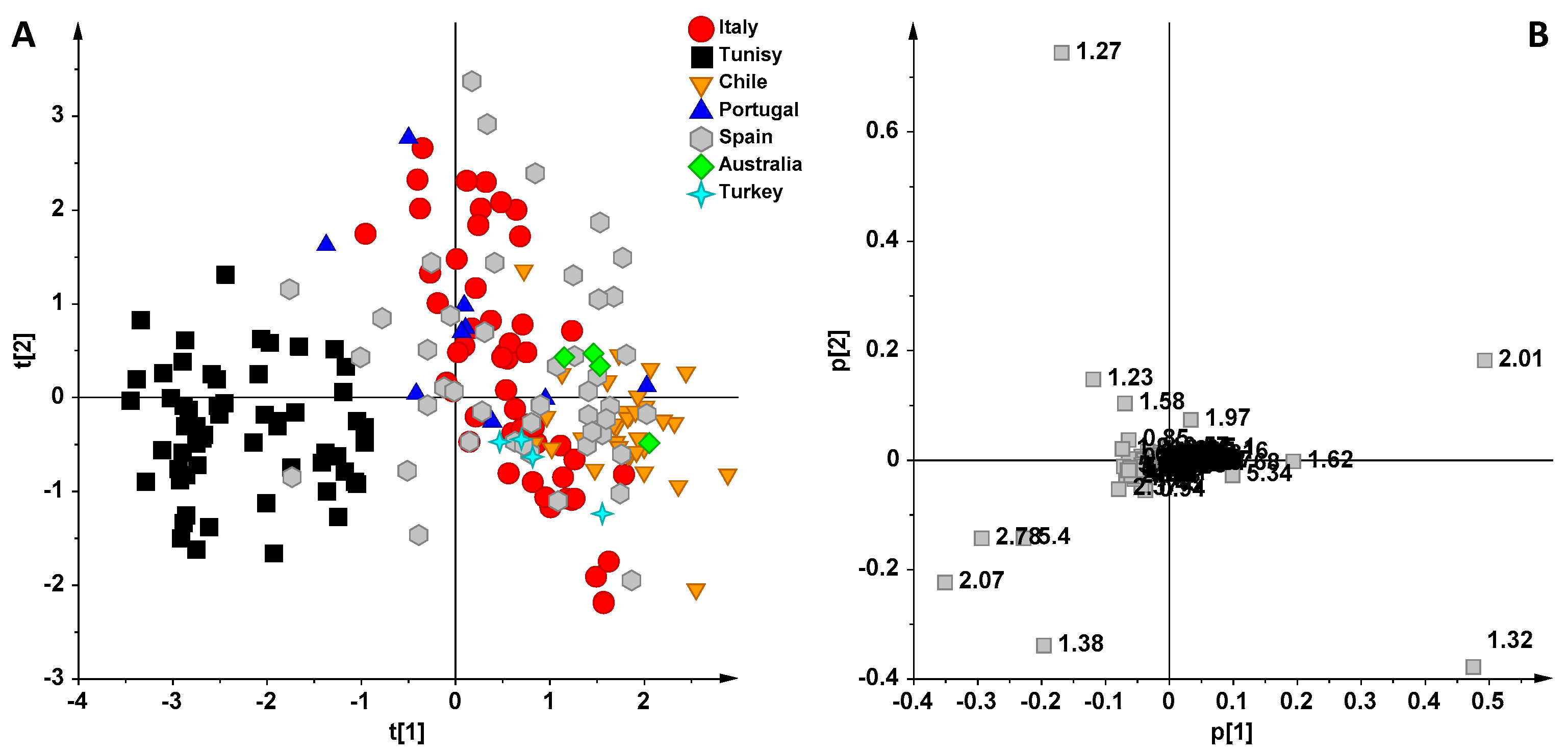
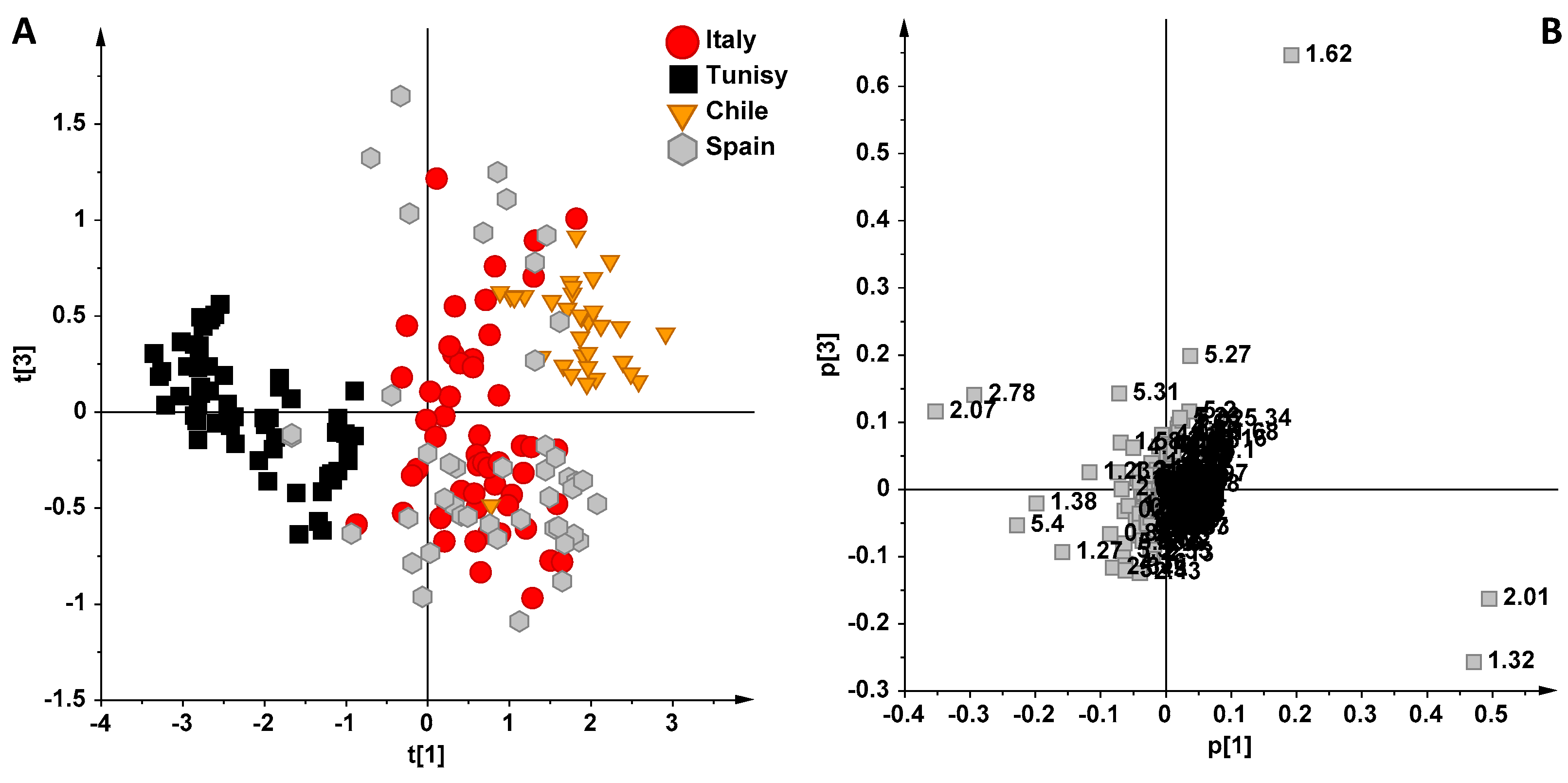
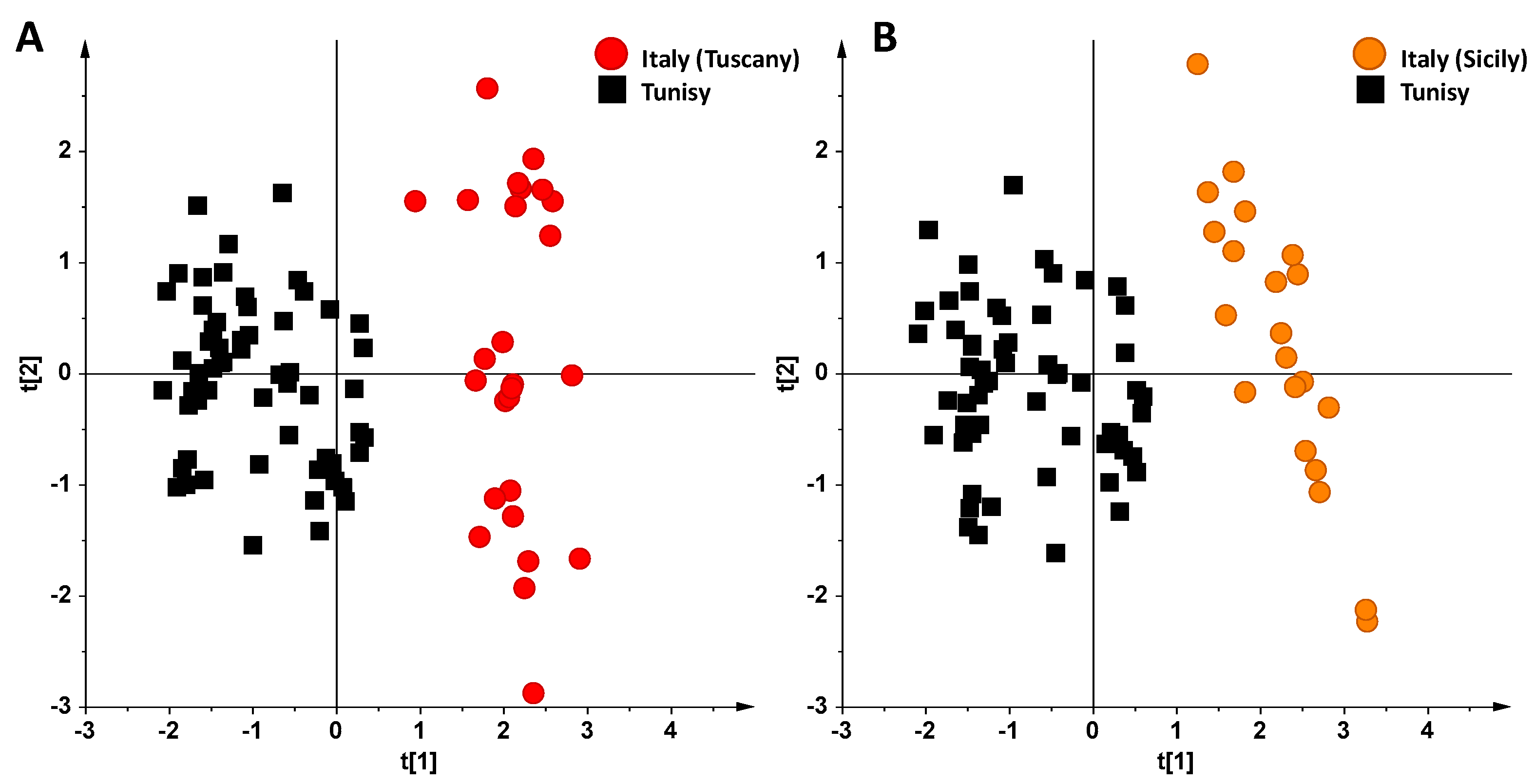
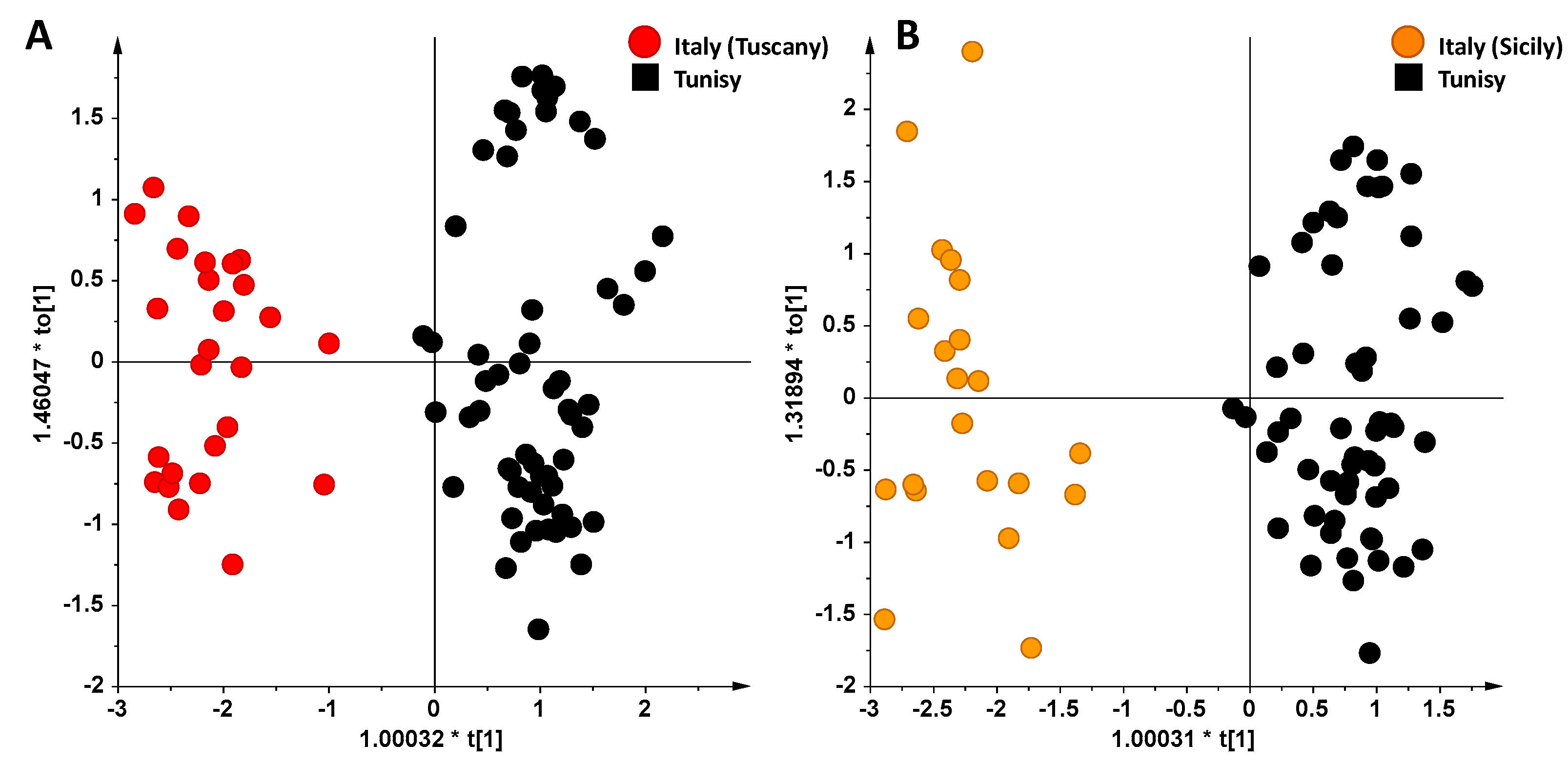
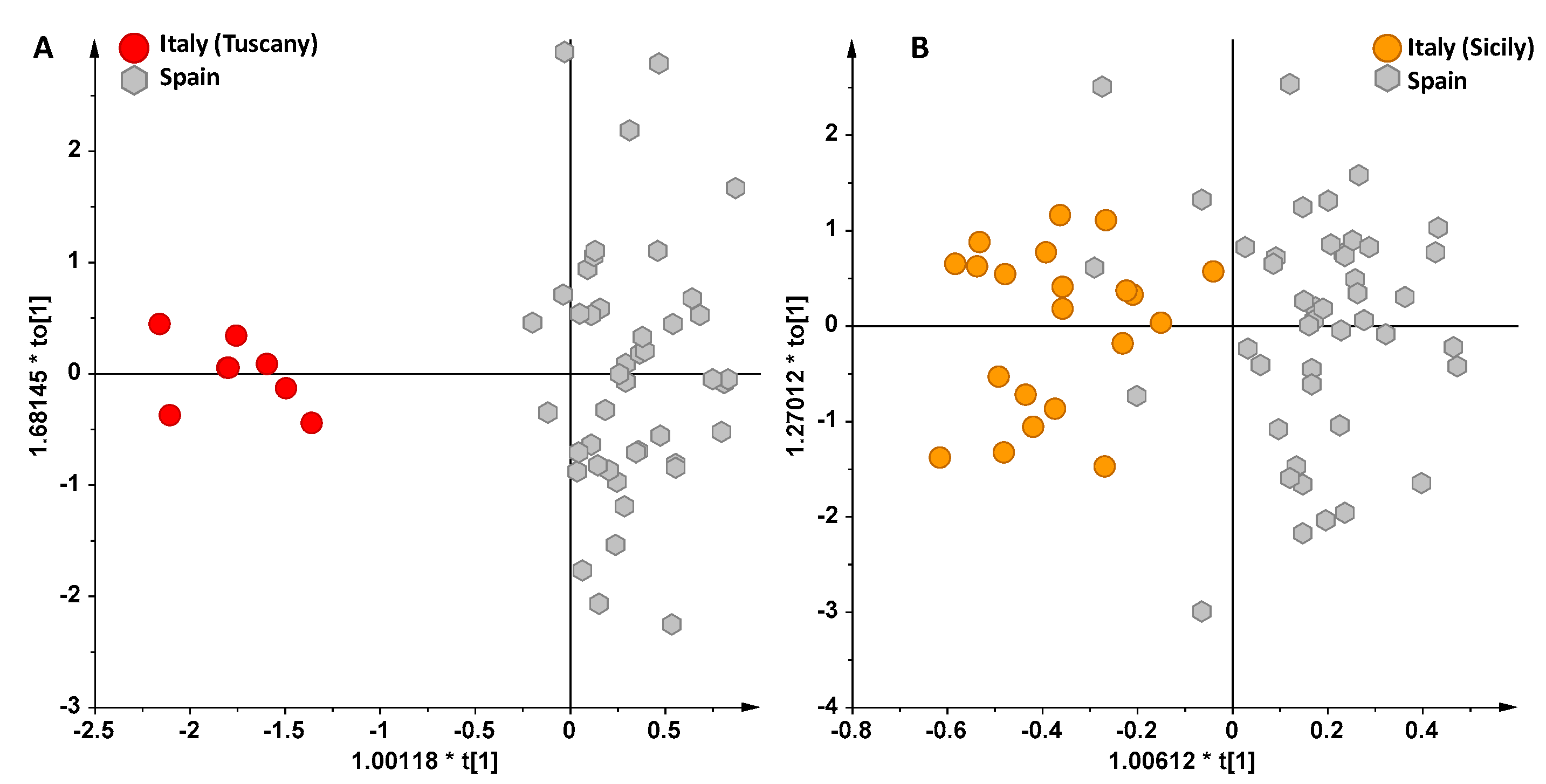
3. Results and Discussion
NMR Spectroscopy and MVA
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Regulation Regulation 2081/92/EEC. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:31992R2081 (accessed on 6 November 2017).
- Bianchi, G.; Angerosa, F.; Camera, L.; Reniero, F.; Anglani, C. Stable carbon-isotope ratios (carbon-13/carbon-12) of olive oil components. J. Agric. Food Chem. 1993, 41, 1936–1940. [Google Scholar] [CrossRef]
- Angerosa, F.; Breas, O.; Contento, S.; Guillou, C.; Reniero, F.; Sada, E. Application of stable isotope ratio analysis to the characterization of the geographical origin of olive oils. J. Agric. Food Chem. 1999, 47, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, G.; Shaw, A.D.; Kell, D.B. Use of 13C nuclear magnetic resonance distortionless enhancement by polarization transfer pulse sequence and multivariate analysis to discriminate olive oil cultivars. J. Am. Oil Chem. Soc. 1999, 76, 1223–1231. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Moreno-Rojas, J.M.; Holland, M.V.; Reniero, F.; Guillou, C.; Heberger, K. Virgin olive oil authentication by multivariate analyses of 1H NMR fingerprints and δ13C and δ2H data. J. Agric. Food Chem. 2010, 58, 5586–5596. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Riddellova, K.; Klimankova, E.; Cerna, M.; Pudil, F.; Hajslova, J. Traceability of olive oil based on volatiles pattern and multivariate analysis. Food Chem. 2010, 121, 282–289. [Google Scholar] [CrossRef]
- Bertran, E.; Blanco, M.; Coello, J.; Iturriaga, H.; Maspoch, S.; Montoliu, I. Near infrared spectrometry and pattern recognition as screening methods for the authentication of virgin olive oils of very close geographical origins. J. Near Infrared Spectrosc. 2000, 8, 45–52. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) No 29/2012. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0029 (accessed on 6 November 2017).
- Del Coco, L.; Mondelli, D.; Mezzapesa, G.N.; Miano, T.; De Pascali, S.A.; Girelli, C.R.; Fanizzi, F.P. Protected Designation of Origin extra virgin olive oils assessment by nuclear magnetic resonance and multivariate statistical analysis:“Terra di Bari”, an Apulian (Southeast Italy) case study. J. Am. Oil Chem. Soc. 2016, 93, 373–381. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Capitani, D. Applications of NMR metabolomics to the study of foodstuffs: Truffle, kiwifruit, lettuce, and sea bass. Electrophoresis 2012, 33, 2290–2313. [Google Scholar] [CrossRef] [PubMed]
- Vlahov, G.; Del Re, P.; Simone, N. Determination of geographical origin of olive oils using 13C nuclear magnetic resonance spectroscopy. I—Classification of olive oils of the puglia region with denomination of protected origin. J. Agric. Food Chem. 2003, 51, 5612–5615. [Google Scholar] [CrossRef] [PubMed]
- Camin, F.; Pavone, A.; Bontempo, L.; Wehrens, R.; Paolini, M.; Faberi, A.; Marianella, R.M.; Capitani, D.; Vista, S.; Mannina, L. The use of IRMS, 1H NMR and chemical analysis to characterise Italian and imported Tunisian olive oils. Food Chem. 2016, 196, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Girelli, C.R.; De Pascali, S.A.; Del Coco, L.; Fanizzi, F.P. Metabolic profile comparison of fruit juice from certified sweet cherry trees (Prunus avium L.) of ferrovia and giorgia cultivars: A preliminary study. Food Res. Int. 2016, 90, 281–287. [Google Scholar] [CrossRef]
- De Pascali, S.A.; Coletta, A.; Del Coco, L.; Basile, T.; Gambacorta, G.; Fanizzi, F.P. Viticultural practice and winemaking effects on metabolic profile of negroamaro. Food Chem. 2014, 161, 112–119. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Mannina, L.; Capitani, D.; Bidet, O.; Rossi, E.; Bucarelli, F.M.; Quaglia, G.B.; Segre, A. NMR and statistical study of olive oils from lazio: A geographical, ecological and agronomic characterization. Food Chem. 2007, 105, 1256–1267. [Google Scholar] [CrossRef]
- Del Coco, L.; De Pascali, S.A.; Fanizzi, F.P. 1H NMR spectroscopy and multivariate analysis of monovarietal EVOOs as a tool for modulating coratina-based blends. Foods 2014, 3, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillen, M.D. Direct study of minor extra-virgin olive oil components without any sample modification. 1H NMR multisupression experiment: A powerful tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schutz, B.; Schafer, H.; Kontominas, M.G.; Sacco, A. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 2012, 130, 177–183. [Google Scholar] [CrossRef]
- Del Coco, L.; De Pascali, S.A.; Fanizzi, F.P. 1H NMR metabolic profiling of Apulian EVOOs: Fine pedoclimatic influences in salento cultivars. In Royal Society of Chemistry; The Royal Society of Chemistry: London, UK, 2015; pp. 154–160. [Google Scholar]
- Mannina, L.; Sobolev, A.P. High resolution NMR characterization of olive oils in terms of quality, authenticity and geographical origin. Magn. Reson. Chem. 2011, 49, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Rezzi, S.; Axelson, D.E.; Heberger, K.; Reniero, F.; Mariani, C.; Guillou, C. Classification of olive oils using high throughput flow 1H NMR fingerprinting with principal component analysis, linear discriminant analysis and probabilistic neural networks. Anal. Chim. Acta 2005, 552, 13–24. [Google Scholar] [CrossRef]
- Girelli, C.R.; Del Coco, L.; Papadia, P.; De Pascali, S.A.; Fanizzi, F.P. Harvest year effects on Apulian EVOOs evaluated by 1H NMR based metabolomics. PeerJ 2016, 4, e2740. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E. The Handbook of Metabonomics and Metabolomics; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: London, UK, 2005. [Google Scholar]
- Girelli, C.R.; Del Coco, L.; Fanizzi, F.P. 1H NMR spectroscopy and multivariate analysis as possible tool to assess cultivars, from specific geographical areas, in evoos. Eur. J. Lipid Sci. Technol. 2015, 118, 1380–1388. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; van Velzen, E.J.; Hoefsloot, H.C.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J. O2-PLS for qualitative and quantitative analysis in multivariate calibration. J. Chemom. 2002, 16, 283–293. [Google Scholar] [CrossRef]
- Merchak, N.; El Bacha, E.; Khouzam, R.B.; Rizk, T.; Akoka, S.; Bejjani, J. Geoclimatic, morphological, and temporal effects on lebanese olive oils composition and classification: A 1H NMR metabolomic study. Food Chem. 2017, 217, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2016/580. Available online: http://www.mvo.nl/media/reg_2016_580_tunisian_olive_oil.pdf (accessed on 20 July 2017).
- Girelli, C.R.; Del Coco, L.; Fanizzi, F.P. Tunisian extra virgin olive oil traceability in the EEC market: Tunisian/Italian (Coratina) EVOOs blend as a case study. Sustainability 2017, 9, 1471. [Google Scholar]
- Del Coco, L.; Schena, F.P.; Fanizzi, F.P. 1H nuclear magnetic resonance study of olive oils commercially available as Italian products in the United States of America. Nutrients 2012, 4, 343–355. [Google Scholar] [CrossRef] [PubMed]
| Country (Region) | Cultivars | Number of Samples | Average Monthly Cumulative Rainfall (mm) | Average Temperature (°C) |
|---|---|---|---|---|
| Tuscany (Italy) | frantoio, moraiolo, correggiolo, leccino, pendolino | 29 | 76.0 | 14.6 |
| Apulia (Italy) | coratina, ogliarola, nociara, leccino, peranzana | 9 | 48.8 | 15.7 |
| Sicily (Italy) | moresca, verrese, nocellara del belice, cerasuola, biancolilla, nocellara etnea | 23 | 45.6 | 17.3 |
| Australia | barnea, frantoio, correggiola | 4 | 45.4 | 21.7 |
| Chile | arbequina, leccino, bosana koroneiki, picua | 32 | 48.7 | 8.4 |
| Portugal | madural, cordovil, negrucha | 14 | 84.4 | 15.6 |
| Spain | picual, hojiblanca, arbequina, blanqueta, picudo | 62 | 50.3 | 14.0 |
| Turkey | sari ulak, ayvalik, beylik | 4 | 53.8 | 12.1 |
| Tunisia | chemlali, chetoui, chemchali | 58 | 22.2 | 20.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rongai, D.; Sabatini, N.; Del Coco, L.; Perri, E.; Del Re, P.; Simone, N.; Marchegiani, D.; Fanizzi, F.P. 1H NMR and Multivariate Analysis for Geographic Characterization of Commercial Extra Virgin Olive Oil: A Possible Correlation with Climate Data. Foods 2017, 6, 96. https://doi.org/10.3390/foods6110096
Rongai D, Sabatini N, Del Coco L, Perri E, Del Re P, Simone N, Marchegiani D, Fanizzi FP. 1H NMR and Multivariate Analysis for Geographic Characterization of Commercial Extra Virgin Olive Oil: A Possible Correlation with Climate Data. Foods. 2017; 6(11):96. https://doi.org/10.3390/foods6110096
Chicago/Turabian StyleRongai, Domenico, Nadia Sabatini, Laura Del Coco, Enzo Perri, Paolo Del Re, Nicola Simone, Donato Marchegiani, and Francesco Paolo Fanizzi. 2017. "1H NMR and Multivariate Analysis for Geographic Characterization of Commercial Extra Virgin Olive Oil: A Possible Correlation with Climate Data" Foods 6, no. 11: 96. https://doi.org/10.3390/foods6110096






