Volatile Composition of Smoked and Non-Smoked Iranian Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Extraction Method
2.3. Chromatographic Analyses
2.4. Statistical Analysis
3. Results
3.1. Identification of Volatile Compounds in Rice
3.2. Volatile Compositions of Non-Smoked and Smoked Iranian Rice
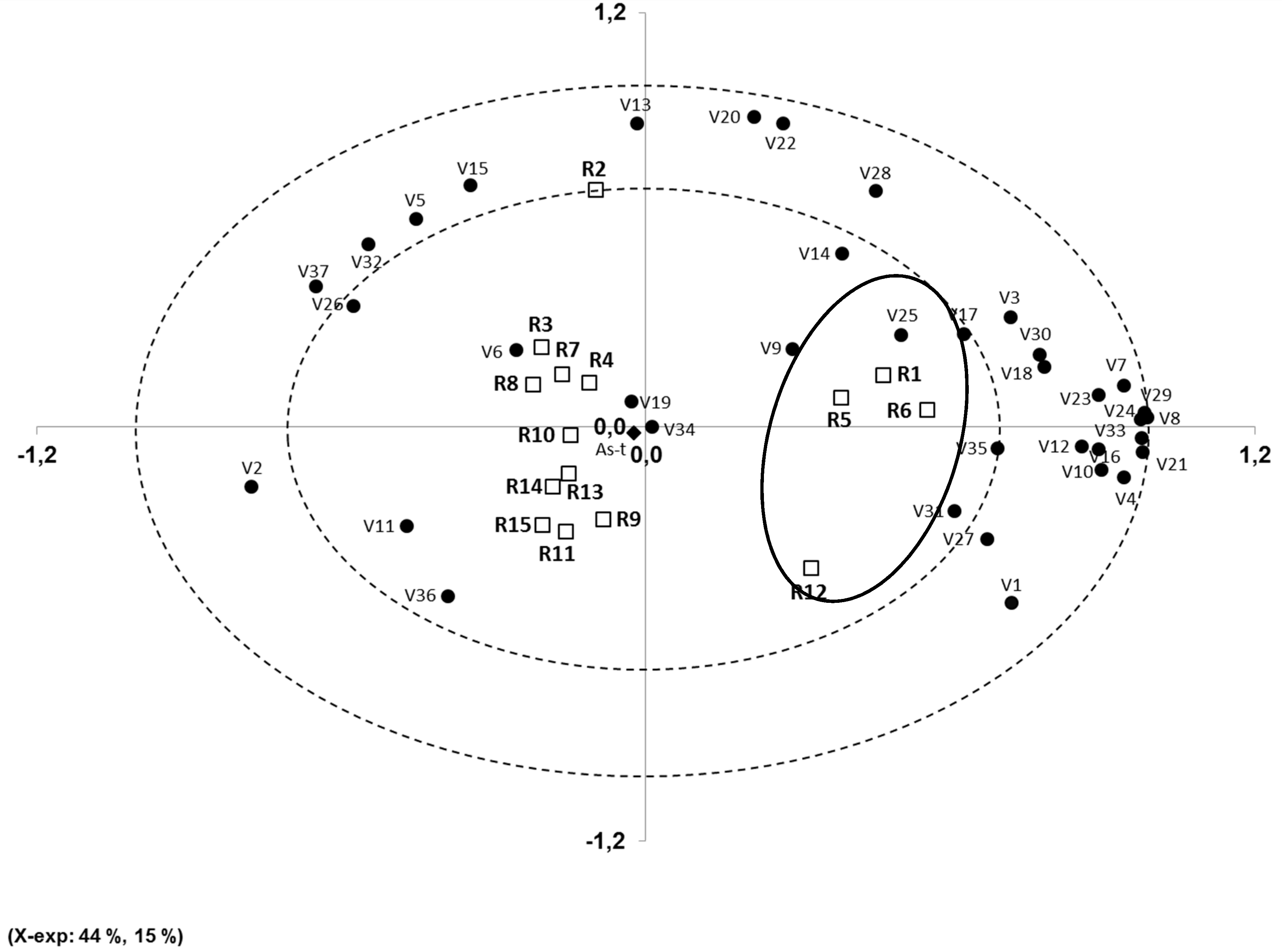
3.3. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Karizaki, V.M. Ethnic and traditional Iranian rice-based foods. J. Ethn. Foods 2016, 3, 124–134. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Munera-Picazo, S.; Burlo, F.; Hojjati, M.; Carbonell-Barrachina, A.A. Total and inorganic arsenic in Iranian rice. J. Food Sci. 2015, 80, T1129–T1135. [Google Scholar] [CrossRef] [PubMed]
- Tarang, A.; Gashti, A.B. The power of microsatellite markers and aflps in revealing the genetic diversity of hashemi aromatic rice from Iran. J. Integr. Agric. 2016, 15, 1186–1197. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization ot the United Nations). Available online: http://www.fao.org/docrep/003/w8595t/w8595t05.htm (accessed on 9 July 2016).
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z.; et al. Diversity of global rice markets and the science required for consumer-targeted rice breeding. PLoS ONE 2014, 9, e85106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahattanatawee, K.; Rouseff, R.L. Comparison of aroma active and sulfur volatiles in three fragrant rice cultivars using gc–olfactometry and GC-PFPD. Food Chem. 2014, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; McClung, A.M. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC-MS. Food Chem. 2011, 124, 501–513. [Google Scholar] [CrossRef]
- Griglione, A.; Liberto, E.; Cordero, C.; Bressanello, D.; Cagliero, C.; Rubiolo, P.; Bicchi, C.; Sgorbini, B. High-quality italian rice cultivars: Chemical indices of ageing and aroma quality. Food Chem. 2014, 172, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.I. Rice aroma and flavor: A literature review. Cereal Chem. 2008, 85, 447–456. [Google Scholar] [CrossRef]
- Malekzadeh, H.; Fatemi, M.H. Analysis of flavor volatiles of some iranian rice cultivars by optimized static headspace gas chromatography-mass spectrometry. J. Iran. Chem. Soc. 2015, 12, 2245–2251. [Google Scholar] [CrossRef]
- Ezquerro, O.; Pons, B.; Tena, M.T. Development of a headspace solid-phase microextraction-gas chromatography-mass spectrometry method for the identification of odour-causing volatile compounds in packaging materials. J. Chromatogr. A 2002, 963, 381–392. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fan, W.; Gao, Y.N.; Wu, S.F.; Wang, S.X. Study on Volatile Compounds in Rice by HS-SPME and GC-MS; Julius Kühn Institut, Bundesforschungsinstitut für Kulturpflanzen: Quedlinburg, Sweden, 2010; pp. 125–134. [Google Scholar]
- Golam, F.; NorZulaani, K.; Jennifer, A.H.; Subha, B.; Zulqarnain, M.; Osman, M.; Nazia, A.M.; Zulqarnian, M.; Mohammad, O. Evaluation of kernel elongation ratio and aroma association in global popular aromatic rice cultivars in tropical environment. Afr. J. Agric. Res. 2010, 5, 1515–1522. [Google Scholar]
- Malekzadeh, H.; Fatemi, M.H. Application of multivariate curve resolution approaches to improve analytical separation of iranian rice volatiles by GC-MS. Bull. Chem. Soc. Jpn. 2015, 88, 706–712. [Google Scholar] [CrossRef]
- Calin-Sanchez, A.; Martinez, J.J.; Vazquez-Araujo, L.; Burlo, F.; Melgarejo, P.; Carbonell-Barrachina, A.A. Volatile composition and sensory quality of Spanish pomegranates (punica granatum L.). J. Sci. Food Agric. 2011, 91, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Barrachina, A.A.; Memmi, H.; Noguera-Artiaga, L.; Gijon-Lopez, M.D.; Ciapa, R.; Perez-Lopez, D. Quality attributes of pistachio nuts as affected by rootstock and deficit irrigation. J. Sci. Food Agric. 2015, 95, 2866–2873. [Google Scholar] [CrossRef] [PubMed]
- Natinal Institute of Standards and Technology (NIST). Compounds database. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 7 November 2016).
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á. Volatile composition of essential oils from different aromatic herbs grown in mediterranean regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Carbonell-Barrachina, T.A. Irrigation dose and plant density affect the essential oil content and sensory quality of parsley (Petroselinum sativum). Sci. Hortic. 2016, 206, 1–6. [Google Scholar] [CrossRef]
- Hojjati, M.; Lipan, L.; Carbonell-Barrachina, Á.A. Effect of roasting on physicochemical properties of wild almonds (Amygdalus scoparia). J. Am. Oil Chem. Soc. 2016, 93, 1211–1220. [Google Scholar] [CrossRef]
- Calín-Sánchez, A.; Figiel, A.; Lech, K.; Szumny, A.; Martínez-Tomé, J.; Carbonell-Barrachina, A.A. Drying methods affect the aroma of origanum majorana l. Analyzed by GC-MS and descriptive sensory analysis. Ind. Crops Products 2015, 74, 218–227. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Flavors & Fragrances; Sigma-Aldrich: Madrid, Spain, 2014. [Google Scholar]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Kottearachchi, N.S.; Samarasekera, R. Volatile profiles of traditional aromatic rice varieties in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2014, 42, 87–93. [Google Scholar] [CrossRef]
- Tananuwong, K.; Lertsiri, S. Changes in volatile aroma compounds of organic fragrant rice during storage under different conditions. J. Sci. Food Agric. 2010, 90, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A. Characterisation of volatile compounds in a smoke flavouring from rice husk. Food Chem. 2014, 153, 81–86. [Google Scholar] [CrossRef] [PubMed]
| Sample Code | Cultivar | Comments | Province | City | Location |
|---|---|---|---|---|---|
| R1 | Sadri | Smoked | Guilan | Astaneh | North |
| R2 | Domsiah | - | Guilan | Astaneh | North |
| R3 | Shiroudi | - | Mazandaran | Amol | North |
| R4 | Hashemi | - | Guilan | Astaneh | North |
| R5 | Sadri | Smoked | Guilan | Rasht | North |
| R6 | Sadri | Smoked | Guilan | Talesh | North |
| R7 | Hashemi | - | Mazandaran | Mahmudabad | North |
| R8 | Tarem | - | Mazandaran | Mahmudabad | North |
| R9 | Hashemi | - | Mazandaran | Fereydun kenar | North |
| R10 | Tarem | - | Mazandaran | Amol | North |
| R11 | Champa | - | Khouzestan | Ramhormoz | West-South |
| R12 | Tarem | Smoked | Mazandaran | Fereydun kenar | North |
| R13 | Lenjun | - | Isfahan | Lenjan | Center |
| R14 | Tarem | - | Lorestan | Borujerd | West |
| R15 | Shamshiri | - | Ilam | Chardaval | West |
| Compound | Sample Code | RT † (min) | IT ‡ | Odor Descriptor ‡ | |
|---|---|---|---|---|---|
| Exp ‡ | Lit † | ||||
| 2-Methylfuran | V1 | 5.55 | 600 | 605 | Ethereal, acetone, chocolate |
| Hexanal | V2 | 7.39 | 804 | 801 | Fatty, green |
| 3-Furaldehyde | V3 | 7.69 | 820 | 831 | |
| Furfural | V4 | 8.07 | 839 | 830 | Almond, woody |
| 2-Heptanone | V5 | 9.18 | 895 | 891 | Banana, cinnamon, spicy, fruity |
| Heptanal | V6 | 9.50 | 908 | 903 | Oily, fruity, woody, fatty, nutty |
| 2-Methyl-2-cyclopenten-1-one | V7 | 9.80 | 918 | 915 | |
| 2-Acetylfuran | V8 | 9.87 | 920 | 921 | Almond, caramel, coffee |
| Anisole | V9 | 10.10 | 928 | 926 | Alcohol, butter, cheese, ethereal |
| 5-Methyl-furfural | V10 | 11.43 | 972 | 978 | Almond, caramel, spicy |
| Benzaldehyde | V11 | 11.71 | 982 | 978 | Almond, cherry, sweet |
| Phenol | V12 | 11.77 | 984 | 980 | Plastic |
| 6-Methyl-5-hepten-2-one | V13 | 11.95 | 990 | 994 | Oily, herbaceous, green |
| 2-Amylfuran | V14 | 12.23 | 999 | 1001 | Fruity, green, earth, bean |
| Octanal | V15 | 12.68 | 1011 | 1006 | Honey, fruity, fatty, citrus |
| Benzofuran | V16 | 12.86 | 1016 | 1015 | Burnt, coffee, woody |
| 2-Propionylfuran | V17 | 13.02 | 1020 | 1024 | |
| p-Methylanisole | V18 | 13.58 | 1034 | 1026 | Floral, earthy, walnut |
| Limonene | V19 | 13.89 | 1042 | 1039 | Herbaceous, minty |
| 3-Octen-2-one | V20 | 14.03 | 1046 | 1040 | Berry, nutty, earthy, vegetable |
| 2-Methyl-phenol | V21 | 14.75 | 1065 | 1075 | |
| 2-Octenal | V22 | 14.89 | 1068 | 1060 | Spicy, herbaceous, green |
| p-Cresol | V23 | 15.57 | 1086 | 1084 | Medicinal |
| Guaiacol | V24 | 16.28 | 1104 | 1102 | Woody, smoky |
| Methylbenzoate | V25 | 16.68 | 1113 | 1106 | |
| Nonanal | V26 | 16.78 | 1115 | 1105 | Fruity, citrus, grape, vegetable |
| 2-Ethylphenol | V27 | 19.12 | 1168 | 1169 | Oily, phenolic |
| 2-Nonenal | V28 | 19.35 | 1173 | 1171 | Waxy, fatty |
| 4-Ethylphenol | V29 | 19.80 | 1183 | 1178 | Alcohol, medicinal |
| 3-Ethylphenol | V30 | 19.92 | 1186 | 1175 | Musty, phenolic, burnt |
| 2-Methoxy-4-methylphenol | V31 | 20.96 | 1209 | 1198 | Almond |
| Decanal | V32 | 21.43 | 1219 | 1212 | Waxy, floral, citrus, sweet |
| Cinnamaldehyde | V33 | 22.37 | 1239 | 1234 | Cinnamon, clove, spicy |
| 2-Decenal | V34 | 24.18 | 1278 | 1274 | Floral, citrus, green, meaty |
| Tridecane | V35 | 24.78 | 1291 | 1300 | |
| p-Ethylguaiacol | V36 | 25.00 | 1296 | 1290 | Smoky, meat |
| Tetradecane | V37 | 25.26 | 1301 | 1290 | Mild waxy |
| Compound | ANOVA † | Non-Smoked Rice | Smoked Rice |
|---|---|---|---|
| Relative Abundance (%) | |||
| 2-Methylfuran | * | 0.29 ± 0.08 ‡ b ¥ | 0.41 ± 0.01 a |
| Hexanal | *** | 17.6 ± 2.5 a | 2.50 ± 0.36 b |
| 3-Furaldehyde | * | 0.11 ± 0.02 b | 0.26 ± 0.08 a |
| Furfural | *** | 0.14 ± 0.03 b | 26.7 ± 2.4 a |
| 2-Heptanone | ** | 0.49 ± 0.14 a | 0.06 ± 0.02 b |
| Heptanal | * | 0.46 ± 0.02 a | 0.30 ± 0.08 b |
| 2-Methyl-2-cyclopenten-1-one | * | 0.06 ± 0.01 b | 0.26 ± 0.07 a |
| 2-Acetylfuran | *** | 0.07 ± 0.01 b | 1.91 ± 0.24 a |
| Anisole | * | 0.17 ± 0.07 b | 0.29 ± 0.08 a |
| 5-Methyl-furfural | *** | 0.22 ± 0.13 b | 4.39 ± 0.50 a |
| Benzaldehyde | *** | 14.0 ± 2.9 a | 2.69 ± 0.10 b |
| Phenol | *** | 0.22 ± 0.06 b | 8.03 ± 0.14 a |
| 6-Methyl-5-hepten-2-one | NS | 0.45 ± 0.15 | 0.38 ± 0.09 |
| 2-Amylfuran | * | 0.33 ± 0.11 b | 1.03 ± 0.30 a |
| Octanal | * | 0.91 ± 0.12 a | 0.55 ± 0.10 b |
| Benzofuran | * | 0.06 ± 0.01 b | 0.66 ± 0.15 a |
| 2-Propionylfuran | NS | 0.06 ± 0.01 | 0.17 ± 0.09 |
| p-Methylanisole | * | 0.12 ± 0.04 b | 0.61 ± 0.16 a |
| Limonene | * | 0.60 ± 0.15 a | 0.34 ± 0.06 b |
| 3-Octen-2-one | NS | 0.18 ± 0.05 | 0.23 ± 0.07 |
| 2-Methyl-phenol | *** | 0.11 ± 0.04 b | 2.76 ± 0.09 a |
| 2-Octenal | * | 0.29 ± 0.11 b | 0.50 ± 0.08 a |
| p-Cresol | *** | 0.07 ± 0.02 b | 5.44 ± 0.83 a |
| Guaiacol | *** | 0.35 ± 0.17 b | 11.3 ± 1.1 a |
| Methylbenzoate | * | 0.12 ± 0.06 b | 0.41 ± 0.16 a |
| Nonanal | *** | 9.89 ± 1.30 a | 3.33 ± 0.45 b |
| 2-Ethylphenol | *** | 0.06 ± 0.02 b | 1.44 ± 0.34 a |
| 2-Nonenal | * | 0.15 ± 0.06 b | 0.43 ± 0.12 a |
| 4-Ethylphenol | *** | 0.05 ± 0.01 b | 3.48 ± 0.84 a |
| 3-Ethylphenol | ** | 0.08 ± 0.02 b | 0.84 ± 0.39 a |
| 2-Methoxy-4-methylphenol | *** | 2.06 ± 0.48 b | 6.99 ± 0.06 a |
| Decanal | *** | 2.33 ± 0.44 a | 0.45 ± 0.16 b |
| Cinnamaldehyde | * | 0.13 ± 0.03 b | 0.51 ± 0.06 a |
| 2-Decenal | NS | 0.84 ± 0.40 | 0.82 ± 0.36 |
| Tridecane | ** | 0.35 ± 0.09 b | 1.74 ± 0.47 a |
| p-Ethylguaiacol | *** | 13.7 ± 4.0 a | 3.74 ± 1.20 b |
| Tetradecane | *** | 32.9 ± 4.9 a | 4.12 ± 1.35 b |
| Compound | ANOVA † | Non-Smoked Rice | Smoked Rice |
|---|---|---|---|
| Aldehydes | *** | 46.5 ± 3.6 a ¥ | 12.1 ± 1.2 b |
| Ketones | NS | 1.19 ± 0.31 a | 0.92 ± 0.25 a |
| Phenol derivatives | *** | 17.0 ± 3.8 b | 44.9 ± 2.2 a |
| Furans | *** | 1.29 ± 0.24 b | 35.6 ± 2.5 a |
| Linear hydrocarbons | *** | 33.3 ± 4.9 a | 5.9 ± 1.6 b |
| Esters | *** | 0.12 ± 0.06 b | 0.41 ± 0.16 a |
| Terpenes | * | 0.60 ± 0.15 a | 0.34 ± 0.06 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipan, L.; Hojjati, M.; El-Zaeddi, H.; Sánchez-Rodríguez, L.; Carbonell-Barrachina, Á.A. Volatile Composition of Smoked and Non-Smoked Iranian Rice. Foods 2016, 5, 81. https://doi.org/10.3390/foods5040081
Lipan L, Hojjati M, El-Zaeddi H, Sánchez-Rodríguez L, Carbonell-Barrachina ÁA. Volatile Composition of Smoked and Non-Smoked Iranian Rice. Foods. 2016; 5(4):81. https://doi.org/10.3390/foods5040081
Chicago/Turabian StyleLipan, Leontina, Mohammad Hojjati, Hussein El-Zaeddi, Lucía Sánchez-Rodríguez, and Ángel Antonio Carbonell-Barrachina. 2016. "Volatile Composition of Smoked and Non-Smoked Iranian Rice" Foods 5, no. 4: 81. https://doi.org/10.3390/foods5040081







