1. Introduction
Active food packaging may include oxygen scavengers, moisture absorbers, ultraviolet barriers, or compounds that deliver flavoring, antioxidant, or antimicrobial agents [
1]. In the context of increasing the demand of multiple hurdle technology to achieve high food safety standards, the development of antimicrobial and antioxidant packaging systems is of great interest. Packaging materials are usually made of synthetic polymers, such as plastic films and multicomponent packages, and they can be used as carriers of active compounds; however the use of edible materials has safety advantages and is more likely to be accepted by consumers. Active edible films can be prepared from plant or animal based proteins, starches, cellulose derivatives, chitin/chitosan, gums, lipids, or mixtures [
2]. Chitosan has the ability to form edible and biodegradable films that can carry and release compounds with antimicrobial or antioxidant abilities [
3,
4,
5,
6].
The use of natural products, such as essential oils (EOs), as food preserving agents is being promoted given the current trend towards green consumerism. Several essential oils (EO) have shown antioxidant properties as well as antimicrobial effects against mold, yeasts, bacteria, and viruses, mainly due to their bioactive components such as flavonoids, terpenes, carotenes, etc. [
7,
8]. However, EO impairs strong flavor, odor, and even some colors, thus their use is limited to such foods that allow sensory modifications. Given that their direct use in food is limited, other options like EO encapsulation or systems with no contact food-EO may be preferred. Building an active package with no contact between the EO and the food has many advantages like: no taste transfer, reduced organoleptic changes and even distribution of the active compounds in the headspace [
1]. There is a clear consumer preference for food with no additives, and so, if the chemical is not added to the food but to the package, it does not need to be declared in the label [
8]. Actually, since spoilage occurs mainly on the food surface there is no need to add antimicrobial agents in the bulk of the food, but just to the headspace. EOs may be added to synthetic polymers or sachets as well, however by using edible films there are no safety issues of concern (disintegration and release into the food as well as accidental ingestion of sachets or absorbent pads) [
1]. Selecting mild odor EOs may help in reducing the inconvenience of strong flavors.
The antimicrobial activity of EOs is very difficult to compare due to the high variability of the chemical composition of EOs within the same species, affected by many factors (ecological, geographical, and physiological, among others) [
9]. EO composition needs to be determined in each study in order to properly define the preservation properties of each EO and conditions of use.
Chitosan edible films containing mild odor thyme EO may be an innovative preservation technique to extend the commercial shelf life of ready to eat meat products, and even replace the use of artificial chemical preservation agents. Although a large number of studies on the antimicrobial effectiveness of EOs are available, very few studies are available on food products. The aim of this study was to evaluate an active packaging system including a layer of chitosan film containing the EO of mild flavored thyme (0%, 0.5%, 1% and 2% EO) for sliced ready to eat (RTE) cooked pork and evaluate the pH, color, evolution of microbial populations, and sensory changes on the RTE meat during refrigerated storage. The main purpose was to evaluate if a layer of chitosan film with thyme EO could be successfully incorporated in a packaging system for RTE meat products to provide an active effect to the packaging and promote an increase in the shelf-life of the cooked meat.
2. Materials and Methods
Essential oil: Thyme (Thymus vulgaris L.) commercial essential oil (EO) was purchased from Herbes del Molí (Benimarfull, Alicante, Spain). The company reported that the EO was extracted from the whole plant of organic grown autochthonous thyme by hydro distillation. The company is certified by the Comité de Agricultura Ecológica de la Comunidad Valenciana.
GC-MS and GC-FID Analytical Conditions for oil analysis: The volatile compounds were isolated, identified, and quantified as described in a previous work [
10,
11], with the only difference being the column used: a Rxi-1301Sil MS (Restek Corporation, 110 Benner Cir, Bellefonte, PA 16823, USA; 30 m, 0.25 mm ID, 1 µm film thickness). Most of the compounds were identified by simultaneously using two different analytical methods [
5]: (a) KI, Kováts Index in reference to n-alkanes (C
8–C
32); and (b) mass spectra (authentic chemicals and Wiley spectral library collection). Identification was considered to be tentative when it was based on mass spectral data only. Semi-quantification of the compounds was run in a Shimadzu GC-2100 equipped with an FID detector and the same column and conditions as the GC-MS. Quantitative data were obtained electronically from FID area data without using correction factors. All the tests were performed in triplicate.
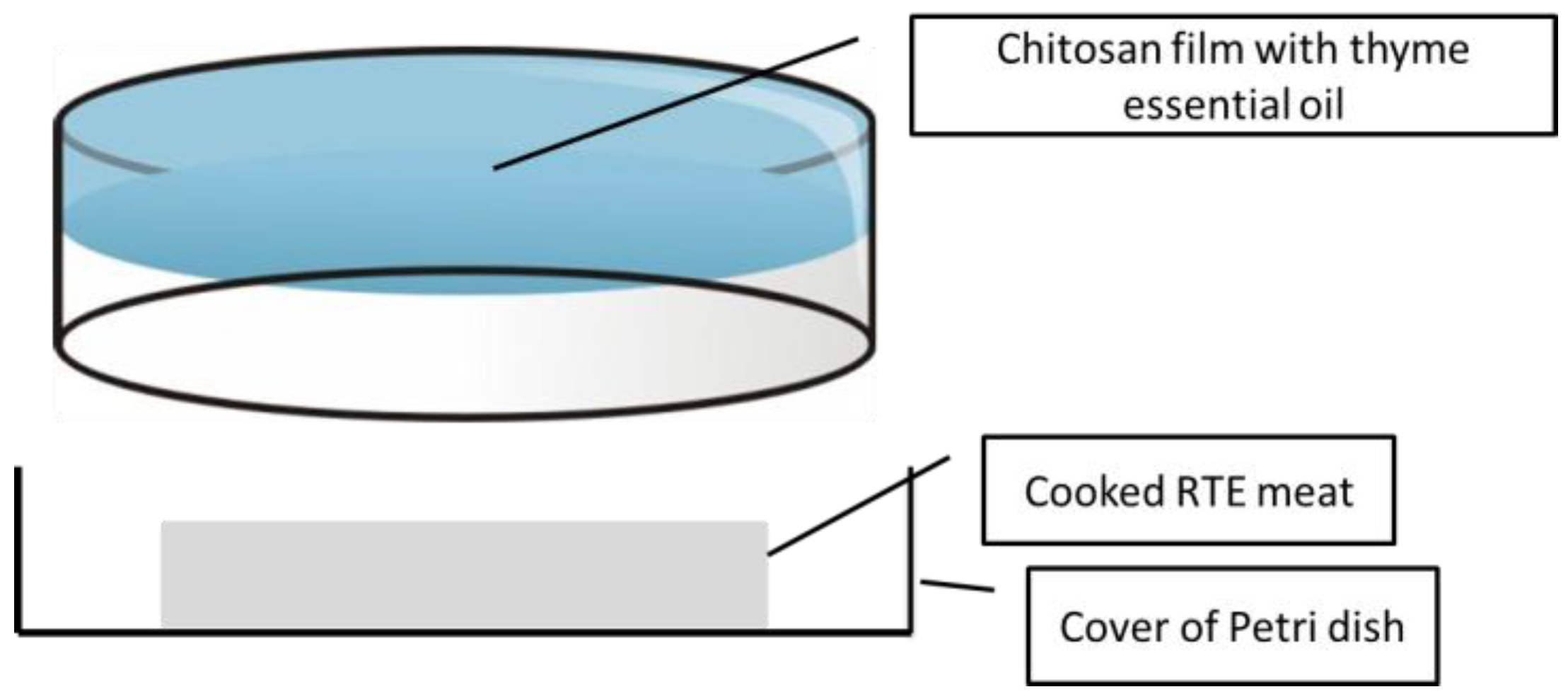
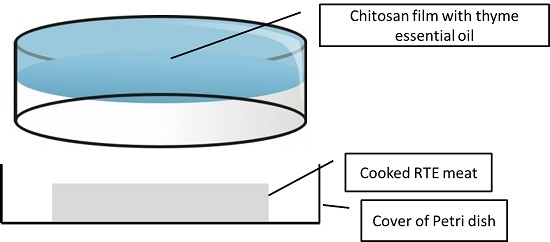
Film preparation: High molecular weight chitosan of analytical grade was obtained from Sigma Aldrich (800,000 cps; >75% deacetylation degree). The chitosan solution was prepared as follows: 1% chitosan, 1% lactic acid, 0.1% tween, and 0.25% glycerol with different concentrations of EO (0%, 0.5%, 1%, and 2%). To prepare the active packaging, 20 g of each solution were poured into the inner surface of the cover of a Petri dish (90 mm diameter) and were allowed to dry for 72 h at 37 °C, and then the plates were stored at 53% relative humidity until they were used.
Meat material: Commercially cooked ham (400 g pieces, 5 cm diameter, 15 cm length) was used to obtain slices under hygienic conditions. Overall composition, as indicated in the label, was: 18.5% proteins, 1.5% carbohydrates, 1.5% fat, and <0.2% fiber.
Package design: The basic design was a Petri dish in which the cover had a chitosan film, and one slice of RTE cooked pork was introduced in the opposite cover (no film), and each individual plate was packed in a plastic bag under 50% vacuum (to avoid the collapse of the Petri dish). The package was sealed to avoid uncontrolled release of EO compounds outside of it (
Figure 1 and
Figure 2). The volatile materials of the EO could be easily released to the headspace of the package without leakage. Packaged samples were kept under refrigeration (3 ± 1 °C) for four weeks, and were sampled weekly for determinations.
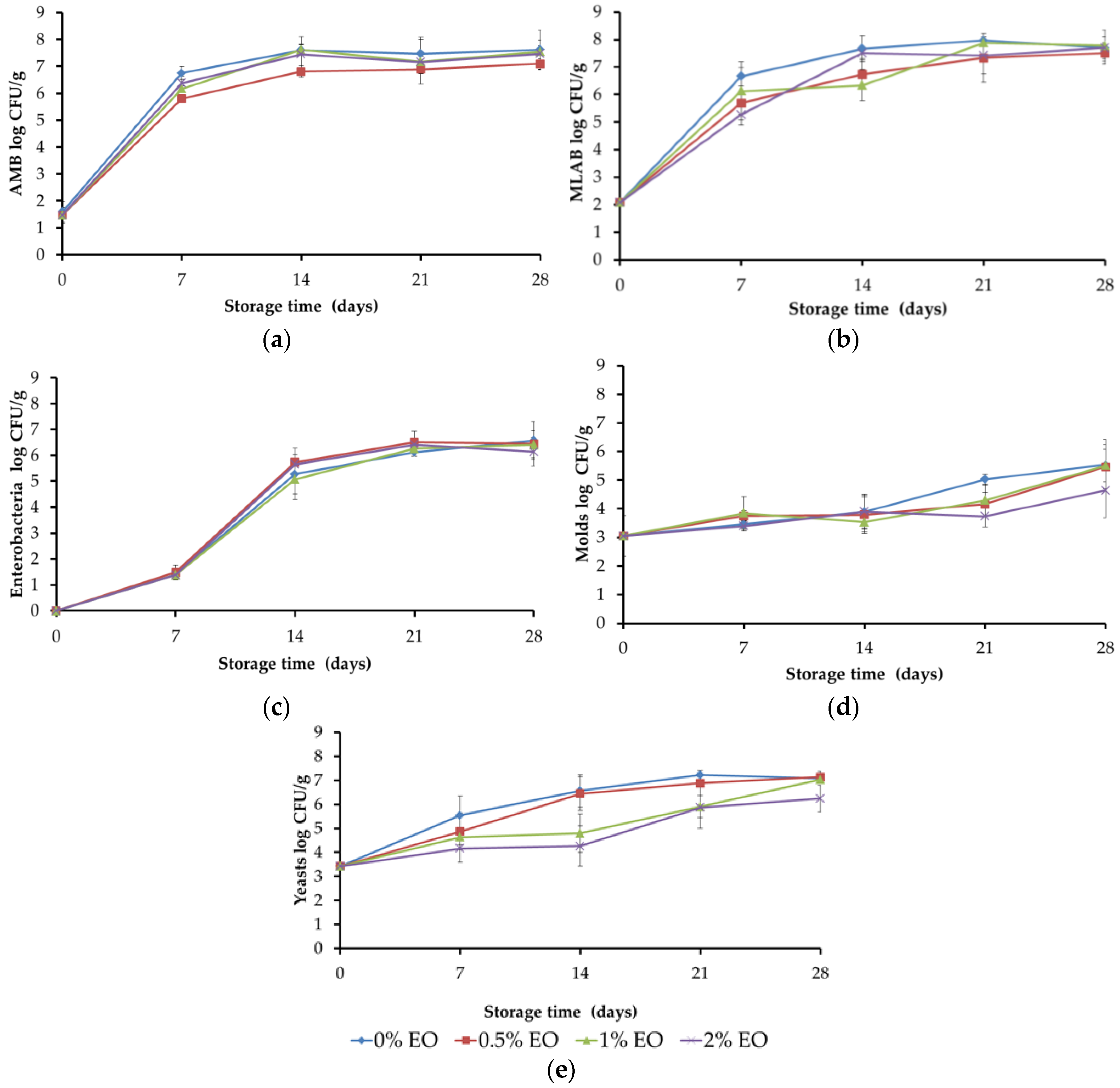
Microbial analysis and sampling of meat: Samples were taken weekly for the determination of counts of total aerobic mesophilic bacteria in Plate Count Agar (PCA), incubated at 37 °C for 48 h [
5,
12]. Mesophilic lactic acid bacteria (MLAB) were determined in MRS agar (Man Rogosa Sharpe) incubated under anaerobiosis at 37 °C for 72 h [
5,
13]. Enterobacteria were determined in VRBG Agar (Violet Red Bile Glucose), with a double layer to provide microaerophilic conditions, incubated at 37 °C for 24 h. Molds and yeasts were determined in Rose Bengal Agar with Cloramphenicol incubated at 28 °C for 3 days for the yeast count and 5 days for the mold count. Results were expressed as logarithms of colony forming units per gram of cooked meat.
pH determination of meat: The pH was measured in a GLP21 pH-meter (Crison Instruments, Barcelona, Spain) with a punction electrode, and 3 measurements were taken per sample.
Color determination of meat: CIELAB (Commission internationale de l'éclairage, L*, a* and b*) color space was used to provide L *, a *, and b * values. A spectrocolorimeter Minolta CM-2022 (Minolta Camera Co. Osaka, Japan) with illuminant D65 and 10° observer was used. A CR-A51 glass (Minolta Camera Co. Osaka, Japan) was put between the sample and the equipment following the American Meat Science Association recommendations (AMSA, 2012). Ten replicates per sample were taken: measurements were taken on both sides of the slices. Hue, Chroma, and ΔE were calculated.
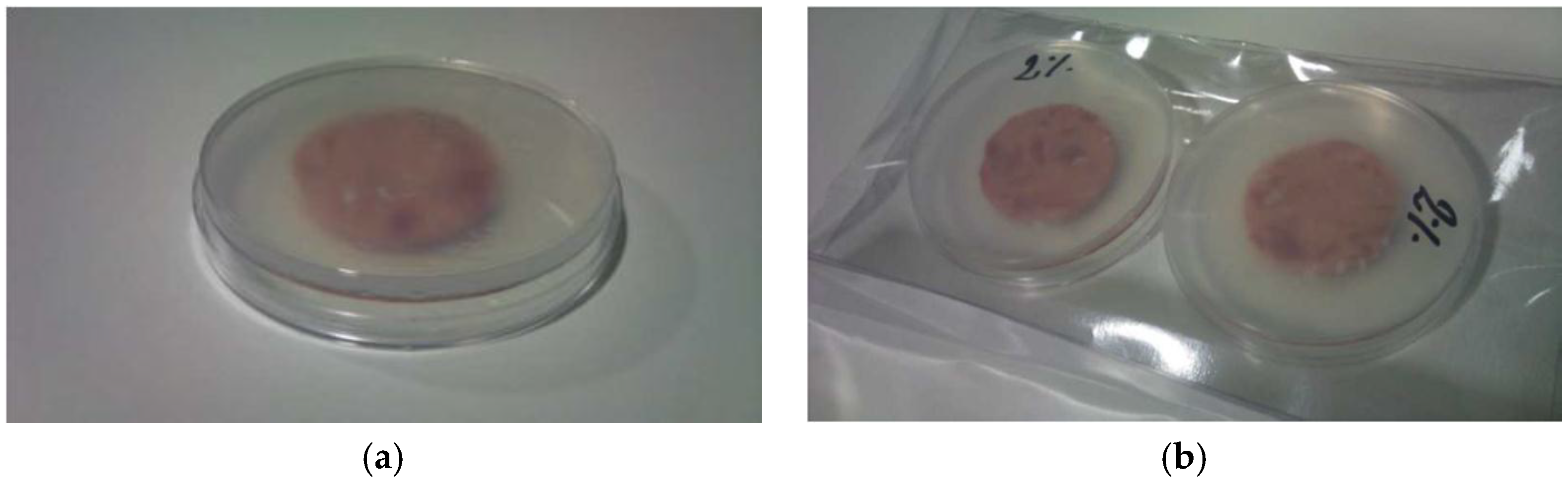
Sensory evaluation of meat: Ten trained judges evaluated the samples for odor, color, and exudates. On each sampling day, 5 closed packages (
Figure 2b) from each treatment were provided to the panel for sensory analysis. They were opened by 5 judges and immediately each judge was given one Petri dish containing one slice (
Figure 2a). The 10 trained judges evaluated: ham odor, thyme odor, off-odors, color, and presence of exudates. Seven point scales were used for grading the attributes and were described: (a) for all evaluated odors, odor perception was defined as follows 1 = imperceptible, 2 = slight odor perception, 3 = low intensity odor perception, 4 = perceptible odor, 5 = moderate odor intensity, 6 = high odor intensity, 7 = extremely intense odor; (b) for color 1 = extremely light, 2 = moderately light, 3 = slightly light, 4 = regular color, 5 = slightly dark, 6 = moderately dark, 7 = extremely dark; (c) for exudates, 1 = imperceptible presence of exudates, 2 = slight presence of exudates, 3 = low amount of exudates, 4 = evident presence of exudates, 5 = moderate presence of exudates, 6 = high amounts of exudates, 7 = extremely high presence of exudates.
Statistical analysis: The whole experiment was run in duplicate, and triplicate analyses were run for all determinations (10 for color). The IBM SPSS statistics package was used for analysis (SPSS Statistical Software, Inc., Chicago, IL, USA). Microbial counts, color, and pH data were analyzed by an ANOVA with two factors. Thyme EO concentration (4 levels: 0%, 0.5%, 1% and 2%), storage time (5 levels: 0, 7, 14, 21, and 28 days), and their interactions were considered. Tukey’s test was used for mean comparison (p < 0.05). Regarding sensory data, a Kruskal-Wallis H test was carried out on the medians.
4. Conclusions
We designed a model package with the inclusion of a layer of chitosan incorporated with thyme EO with the advantage of possible automation. Sachets and pads may be also incorporated but they need to be manually added to the packages, whereas applying a coating to a plastic layer or side of the package may provide technical benefits. Edible films with EOs are safe for consumers and there is no need to use warnings labels to not eat the package.
Regarding the use of the active packaging with thyme EO (1,8-cineole chemotype) in RTE cooked pork, higher antimicrobial activity of the EO was demonstrated against yeast, whereas the other studied microbial populations were not affected by the presence of the EO. The presence of the chitosan-EO layer avoided water condensation, whereas the packages containing only chitosan had evident water droplets. The most remarkable sensory effects were observed for odor, with ham odor intensity decreasing with increased EO concentration, as a result of a masking process produced by thyme odor. However, thyme odor was perceived as desirable for this RTE. Other issues need to be addressed in future works such as EO migration kinetics, duration of the activity of the films, and, when brought to the industrial scale, the packaging processing effect on film retention and properties. In the present work, our aim was to reduce the impact of EO on meat sensory properties by using thyme from a chemotype with mild odor, which happened to have low content of antimicrobial compounds (thymol and carvacrol). Even under such conditions, yeast populations were controlled, color preservation was enhanced, and exudates in the package were reduced, which gave a better appearance to the packaged meat. In future studies, combining EOs with organic acids or salts, or even with isolated active compounds, should also be explored.