Profiling of Nutritional and Health-Related Compounds in Oat Varieties
Abstract
:1. Introduction
2. Experimental Section
2.1. Field Trial
2.2. Kilning and Milling of Oat Seeds
2.3. Starch, Total Protein, and Moisture Content
2.4. Avenins
2.5. β-Glucans
2.6. Fatty Acids
2.7. Vitamin E
2.8. Antioxidant Capacity
2.9. Data Analysis
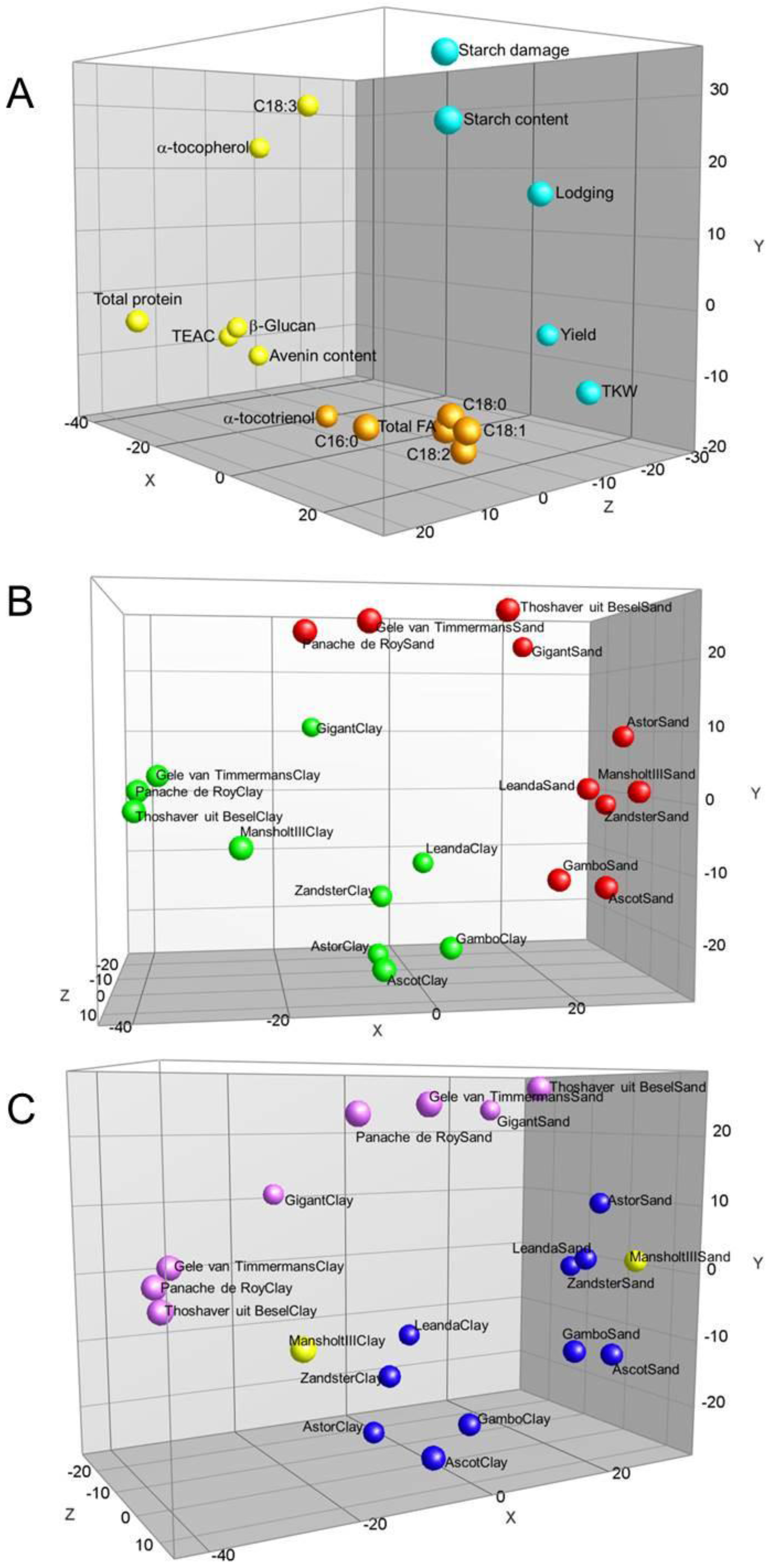
3. Results and Discussion
| Sand | Clay | |||||
|---|---|---|---|---|---|---|
| Range | Average | Stdev | Range | Average | Stdev | |
| Total protein (%) | 10.9–16.6 | 13.2 | 1.8 | 11.6–15.5 | 13.7 | 1.2 |
| Avenin content (%) | 0.4–0.6 | 0.5 | 0.1 | 0.6–0.7 | 0.7 | 0.1 |
| Starch content (%) | 52.4–66.5 | 58.0 | 5.2 | 45.6–52.2 | 48.1 | 2.2 |
| Starch damage (%) | 1.8–4.0 | 2.7 | 0.7 | 1.4–2.2 | 1.7 | 0.3 |
| β-Glucan (%) | 3.3–4.9 | 4.0 | 0.5 | 3.8–5.6 | 4.6 | 0.5 |
| C16:0 (mg/100 g) | 193.5–292.9 | 245.1 | 31.8 | 178.7–262.3 | 237.0 | 42.8 |
| C18:0 (mg/100 g) | 11.5–33.3 | 20.1 | 7.6 | 10.0–29.9 | 16.2 | 5.7 |
| C18:1 (mg/100 g) | 385.0–718.1 | 515.4 | 113.7 | 310.5–723.3 | 438.5 | 125.9 |
| C18:2 (mg/100 g) | 532.3–748.9 | 626.9 | 69.5 | 500.3–849.0 | 608.0 | 104.0 |
| C18:3 (mg/100 g) | 12.3–16.1 | 15.1 | 1.2 | 13.3–17.1 | 15.2 | 1.2 |
| Total FA (%) | 1.2–1.8 | 1.4 | 0.2 | 1.0–2.0 | 1.3 | 0.3 |
| TEAC (µmol/100 g) | 190.6–356.5 | 305.0 | 46.5 | 373.0–478.6 | 415.3 | 40.0 |
| α-Tocotrienol (mg/100 g) | 2.8–4.4 | 3.4 | 0.5 | 3.0–4.4 | 3.7 | 0.4 |
| α-Tocopherol (mg/100 g) | 0.7–1.6 | 1.3 | 0.3 | 0.8–1.8 | 1.4 | 0.3 |
| Yield (kg/ha) | 4698–7401 | 6018 | 1194 | 4880–7570 | 6139 | 895 |
| TKW (g/1000 kernels) | 23.0–35.0 | 29.7 | 4.2 | 26.0–33.0 | 29.3 | 2.5 |
| Lodging | 6–9 | 8 | 1 | 4–9 | 7 | 2 |

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strychar, R. Chapter 1: World oat production, trade, and usage. In Oats: Chemistry and Technology; AACC International, Inc.: St. Paul, MN, USA, 2011; pp. 1–10. [Google Scholar]
- Food and Drug Administration (FDA). Food Labeling; Gluten-Free Labeling of Foods. Available online: https://www.federalregister.gov/articles/2013/08/05/2013-18813/food-labeling-gluten-free-labeling-of-foods (accessed on 21 December 2015).
- European Commission. Commission Implementing Regulation (eu) no 828/2014. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32014R0828 (accessed on 21 December 2015).
- Londono, D.M.; van’t Westende, W.P.C.; Goryunova, S.; Salentijn, E.M.J.; van den Broeck, H.C.; van der Meer, I.M.; Visser, R.G.F.; Gilissen, L.J.W.J.; Smulders, M.J.M. Avenin diversity analysis of the genus Avena (oat). Relevance for people with celiac disease. J. Cereal Sci. 2013, 58, 170–177. [Google Scholar] [CrossRef]
- Peterson, D.M. Chapter 8: Storage proteins. In Oats: Chemistry and technology; AACC International, Inc.: St. Paul, MN, USA, 2011; pp. 123–142. [Google Scholar]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Zhang, H. Oat beta-glucan: Its role in health promotion and prevention of diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Othman, R.A.; Moghadasian, M.H.; Jones, P.J.H. Cholesterol-lowering effects of oat β-glucan. Nutr. Rev. 2011, 69, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.; Gibbs, A.; Brand-Miller, J.; Duncan, A.; Hart, V.; Lamarche, B.; Tosh, S.; Duss, R. Bioactive oat beta-glucan reduces ldl cholesterol in caucasians and non-caucasians. Nutr. J. 2011, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Charlton, K.E.; Tapsell, L.C.; Batterham, M.J.; O'Shea, J.; Thorne, R.; Beck, E.; Tosh, S.M. Effect of 6 weeks’ consumption of β-glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br. J. Nutr. 2012, 107, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- FDA. Code of Federal Regulations Title 21 (21cfr101.81): Soluble Fiber from Certain Foods and Risk of Coronary Heart Disease. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.81 (accessed on 21 December 2015).
- NAD; EFSA Panel on Dietetic Products. Scientific opinion on the substantiation of a health claim related to oat beta glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to article 14 of regulation (ec) no 1924/2006. EFSA J. Nutr. Allerg. 2010, 8, 1885. [Google Scholar]
- NAD; EFSA Panel on Dietetic Products. Scientific opinion on the substantiation of health claims related to oat and barley grain fibre and increase in faecal bulk (id 819, 822) pursuant to article 13(1) of regulation (ec) no 1924/2006. EFSA J. Nutr. Allerg. 2011, 9, 2249. [Google Scholar]
- Rose, D.J. Impact of whole grains on the gut microbiota: The next frontier for oats? Br. J. Nutr. 2014, 112, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Webster, F.H.; Wood, P.J. Oats: Chemistry and technology; American Association of Cereal Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.W. Chapter 6: Nutrient composition and nutritional quality of oats and comparisons with other cereals. In Oats: Chemistry and technology; AACC International, Inc.: St. Paul, MN, USA, 2011; pp. 95–107. [Google Scholar]
- Zhou, M.; Robards, K.; Glennie-Holmes, M.; Helliwell, S. Oat lipids. J. Am. Oil. Chem. Soc. 1999, 76, 159–169. [Google Scholar] [CrossRef]
- Wicklund, T.; Magnus, E.M. Effect of extrusion cooking on extractable lipids and fatty acid composition in sifted oat flour. Cereal Chem. 1997, 74, 326–329. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations Title 21 (21cfr101.75): Health Claims: Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.75 (accessed on 21 December 2015).
- Ekstrand, B.; Gangby, I.; Akesson, G. Lipase activity in oats - distribution, ph dependence, and heat inactivation. Cereal Chem. 1992, 69, 379–381. [Google Scholar]
- Frank, J.; Chin, X.W.D.; Schrader, C.; Eckert, G.P.; Rimbach, G. Do tocotrienols have potential as neuroprotective dietary factors? Ageing Res. Rev. 2012, 11, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, L.H.; Theander, O.; Lingnert, H. Avenanthramides - a group of phenolic antioxidants in oats. Cereal Chem. 1993, 70, 637–641. [Google Scholar]
- Collins, F.W. Oat phenolics: Avenanthramides, novel substituted n-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 1989, 37, 60–66. [Google Scholar] [CrossRef]
- Chu, Y.-F.; Wise, M.L.; Gulvady, A.A.; Chang, T.; Kendra, D.F.; Jan-Willem van Klinken, B.; Shi, Y.; O’Shea, M. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013, 139, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Meydani, M. Potential health benefits of avenanthramides of oats. Nutr Rev 2009, 67, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Gates, F.K.; Sontag-Strohm, T.; Stoddard, F.L.; Dobraszczyk, B.J.; Salovaara, H. Interaction of heat–moisture conditions and physical properties in oat processing: Ii. Flake quality. J. Cereal Sci. 2008, 48, 288–293. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 9th ed.; The Association: St. Paul, MN, USA, 1995. [Google Scholar]
- Sebecic, B.; Balenovic, J. Rapid ecologically acceptable method for wheat protein content determination—Comparison of methods. Deut. Lebensm-Rundsch. 2001, 97, 221–225. [Google Scholar]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of ingredients and baking process for improved wholemeal oat bread quality. LWT Food Sci. Technol. 2007, 40, 860–870. [Google Scholar] [CrossRef]
- Van den Broeck, H.C.; Gilissen, L.J.W.J.; Smulders, M.J.M.; van der Meer, I.M.; Hamer, R.J. Dough quality of bread wheat lacking α-gliadins with celiac disease epitopes and addition of celiac-safe avenins to improve dough quality. J. Cereal Sci. 2011, 53, 206–216. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bouwmeester, H.J.; Verstappen, F.W.A.; Posthumus, M.A.; Dicke, M. Spider mite-induced (3s)-(e)-nerolidol synthase activity in cucumber andlima bean. The first dedicated step in acyclic c11-homoterpene biosynthesis. Plant Physiol. 1999, 121, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Londono, D.M.; Gilissen, L.J.W.J.; Visser, R.G.F.; Smulders, M.J.M.; Hamer, R.J. Understanding the role of oat β-glucan in oat-based dough systems. J. Cereal Sci. 2015, 62, 1–7. [Google Scholar] [CrossRef]
- Oomah, B.D. Baking and related properties of wheat-oat composite flours. Cereal Chem. 1983, 60, 220–225. [Google Scholar]
- Mariotti, M.; Lucisano, M.; Pagani, M.A. Development of a baking procedure for the production of oat-supplemented wheat bread. Int. J. Food Sci. Technol. 2006, 41, 151–157. [Google Scholar] [CrossRef]
- Lásztity, R. Oat grain - a wonderful reservoir of natural nutrients and biologically active substances. Food Rev. Int. 1998, 14, 99–119. [Google Scholar] [CrossRef]
- Banaś, A.; Debski, H.; Banaś, W.; Heneen, W.K.; Dahlqvist, A.; Bafor, M.; Gummeson, P.; Marttila, S.; Ekman, Å.; Carlsson, A.S.; et al. Lipids in grain tissues of oat (Avena sativa): Differences in content, time of deposition, and fatty acid composition. J. Exp. Bot. 2007, 58, 2463–2470. [Google Scholar] [PubMed]
- Peterson, D.M. Oat tocols: Concentration and stability in oat products and distribution within the kernel. Cereal Chem. 1995, 72, 21–24. [Google Scholar]
- Peterson, D.M.; Smith, D. Changes in nitrogen and carbohydrate fractions in developing oat groats. Crop Sci. 1976, 16, 67–71. [Google Scholar] [CrossRef]
- Pennock, J.F.; Hemming, F.W.; Kerr, J.D. A reassessment of tocopherol chemistry. Biochem. Biophys. Res. Commun. 1964, 17, 542–548. [Google Scholar] [CrossRef]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar] [PubMed]
- Robinson, D.S.; Wu, Z.; Domoney, C.; Casey, R. Lipoxygenases and the quality of foods. Food Chem. 1995, 54, 33–43. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Dimberg, L.H.; Kamal-Eldin, A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa l.). J. Agric. Food Chem. 2002, 50, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci. 2001, 41, 1676–1681. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Molteberg, E.L.; Solheim, R.; Frølich, W. Variation in oat groats due to variety, storage and heat treatment. I: Phenolic compounds. J. Cereal Sci. 1996, 24, 263–272. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Sunnerheim, K.; Sundberg, B.; Walsh, K. Stability of oat avenanthramides. Cereal Chem. J. 2001, 78, 278–281. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Broeck, H.C.; Londono, D.M.; Timmer, R.; Smulders, M.J.M.; Gilissen, L.J.W.J.; Van der Meer, I.M. Profiling of Nutritional and Health-Related Compounds in Oat Varieties. Foods 2016, 5, 2. https://doi.org/10.3390/foods5010002
Van den Broeck HC, Londono DM, Timmer R, Smulders MJM, Gilissen LJWJ, Van der Meer IM. Profiling of Nutritional and Health-Related Compounds in Oat Varieties. Foods. 2016; 5(1):2. https://doi.org/10.3390/foods5010002
Chicago/Turabian StyleVan den Broeck, Hetty C., Diana M. Londono, Ruud Timmer, Marinus J. M. Smulders, Ludovicus J. W. J. Gilissen, and Ingrid M. Van der Meer. 2016. "Profiling of Nutritional and Health-Related Compounds in Oat Varieties" Foods 5, no. 1: 2. https://doi.org/10.3390/foods5010002






