Detection of Aryl Hydrocarbon Receptor Activation by Some Chemicals in Food Using a Reporter Gene Assay
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Evaluation of AhR Activity
3. Results and Discussion
3.1. PAHs
3.2. Pesticides
3.3. Amino Acids and Their Metabolites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsuda, R. Estimation of dietary intake of contaminants. Shokuhin Eisei Kenkyu 2013, 63, 9–19. [Google Scholar]
- Matsuda, R.; Watanabe, T. Shokuhin Karano Yugaibusshitsu Sessyuryousuitei to Sonoigi. Farumashia 2013, 49, 17–21. [Google Scholar]
- Tsutsumi, T.; Matsuda, R. Estimation of dietary intake of dioxins. Shokuhin Eisei Kenkyu 2013, 63, 7–19. [Google Scholar] [CrossRef]
- Jira, W.; Ziegenhals, K.; Speer, K. Gas chromatography-mass spectrometry (GC-MS) method for the determination of 16 European priority polycyclic aromatic hydrocarbons in smoked meat products and edible oils. Food Addit. Contam. 2008, 25, 704–712. [Google Scholar] [CrossRef]
- Daiokishinrui Ni Kakawaru Seibutsukenteihou Manual. In Environment Management Bureau; Ministry of Environment: Tokyo, Japan, 2010.
- Misaki, K.; Kawami, H.; Tanaka, T.; Handa, Y.; Nakamura, F.; Matsui, S.; Matsuda, T. Aryl hydrocarbon receptor ligand activity of polycyclic aromatic ketones and polycyclic aromatic quinones. Environ. Toxicol. Chem. 2007, 26, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Amakura, Y.; Nakamura, M.; Brown, D.J.; Clark, G.C.; Sasaki, K.; Toyoda, M.; Maitani, T. Validation of the CALUX bioassay for the screening of PCDD/Fs and dioxin-like PCBs in retail fish. Analyst 2003, 128, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Overmeire, I.V.; Clark, G.C.; Brown, D.J.; Chu, M.D.; Cooke, W.M.; Denison, M.S.; Baeyens, W.; Srebrnik, S.; Goeyens, L. Trace contamination with dioxin-like chemicals: Evaluation of bioassay-based TEQ determination for hazard assessment and regulatory responses. Environ. Sci. Policy 2001, 4, 345–357. [Google Scholar] [CrossRef]
- Hoogenboom, L.; Goeyens, L.; Carbonnelle, S.; van Loco, J.; Beernaert, H.; Baeyens, W.; Traag, W.; Bovee, T.; Jacobs, G.; Schoeters, G. The CALUX bioassay: Current status of its application to screening food and feed. Trends Anal. Chem. 2006, 25, 410–420. [Google Scholar] [CrossRef]
- Han, D.; Nagy, S.R.; Denison, M.S. Comparison of recombinant cell bioassays for the detection of Ah receptor agonists. BioFactors 2004, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 29, pp. 36–37. [Google Scholar]
- Hayakawa, K.; Tang, N.; Toriba, A.; Kameda, T. Hazardous atmospheric pollutants in East Asia: Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons. Bunseki 2008, 278–284. [Google Scholar]
- Zhao, B.; Bohonowych, J.E.S.; Timme-Laragy, A.; Jung, D.; Affatato, A.A.; Rice, R.H.; di Giulio, R.T.; Denison, M.S. Common commercial and consumer products contain activators of the aryl hydrocarbon (dioxin) receptor. PLoS ONE 2013, 8, e56860. [Google Scholar] [CrossRef] [PubMed]
- Machala, M.; Vondráček, J.; Bláha, L.; Ciganek, M.; Neča, J. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat. Res. 2001, 497, 49–62. [Google Scholar] [CrossRef]
- Behnisch, P.A.; Hosoe, K.; Sakai, S. Brominated dioxin-like compounds: In vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ. Int. 2003, 29, 861–877. [Google Scholar] [CrossRef]
- Gasiewicz, T.A.; Kende, A.S.; Rucci, G.; Whitney, B.; Willey, J.J. Analysis of structural requirements for Ah receptor antagonist activity: Ellipticines, flavones, and related compounds. Biochem. Pharmacol. 1996, 52, 1787–1803. [Google Scholar] [CrossRef]
- Ashida, H.; Nishiumi, S.; Fukuda, I. An update on the dietary ligands of the AhR. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Iida, M.; Yabushita, H.; Matsuda, T.; Kojima, H. In vitro screening for aryl hydrocarbon receptor agonistic activity in 200 pesticides using a highly sensitive reporter cell line, DR-EcoScreen cells, and in vivo mouse liver cytochrome P450-1A induction by propnil, diuron and linuron. Chemosphere 2008, 74, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Health-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef] [PubMed]
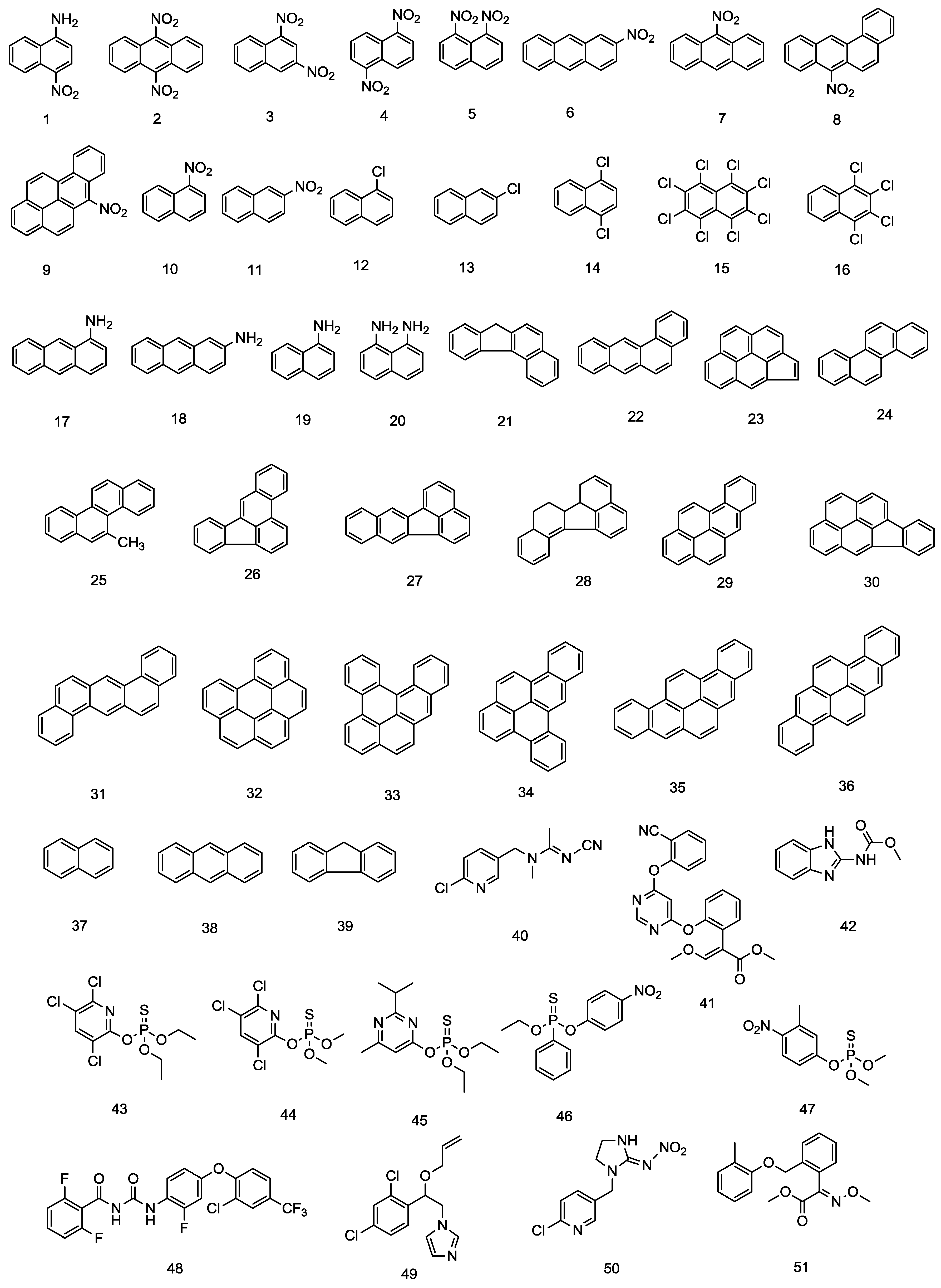


| No. | PAHs | No. | Pesticides Residues |
|---|---|---|---|
| 1 | 1-Amino-4-nitronaphthalene | 40 | Acetamiprid |
| 2 | 9,10-Dinitroanthracene | 41 | Azoxystorobin |
| 3 | 1,3-Dinitronaphthalene | 42 | Carbendazim |
| 4 | 1,5-Dinitronaphthalene | 43 | Chlorpyrifos |
| 5 | 1,8-Dinitronaphthalene | 44 | Chlorpyrifos methyl |
| 6 | 2-Nitroanthracene | 45 | Diazinon |
| 7 | 9-Nitroanthracene | 46 | EPN |
| 8 | 7-Nitrobenzo(a)anthracene | 47 | Fenitrothion |
| 9 | 6-Nitrobenzo(a)pyrene | 48 | Flufenoxuron |
| 10 | 1-Nitronaphthalene | 49 | Imazalil |
| 11 | 2-Nitroanthracene | 50 | Imidacloprid |
| 12 | 1-Chloronaphthalene | 51 | Kresoxim methyl |
| 13 | 2-Chloronaphthalene | 52 | Leucomalachite green |
| 14 | 1,4-Dichloronaphthalene | 53 | Malathion |
| 15 | Octachloronaphthalene | 54 | Methidathion |
| 16 | 1,2,3,4-Tetrachloronaphthalene | 55 | Parathion methyl |
| 17 | 1-Aminoanthracene | 56 | Phenthoate |
| 18 | 2-Aminoanthracene | 57 | Primiphos methyl |
| 19 | 1-Aminonaphthalene | 58 | Prothiofos |
| 20 | 1,8-Diaminonaphthalene | 59 | Pyraclostrobin |
| 21 | Benzo(c)fluorene | 60 | Thiabendazole |
| 22 | Benzo(a)anthracene | 61 | Tolclofos methyl |
| 23 | Cyclopenta(c,d)pyrene | 62 | Tribenuron methyl |
| 24 | Chrysene | No. | Amino acids and their metabolites |
| 25 | 5-Methylchrysene | 63 | 4-Aminobutanoic acid |
| 26 | Benzo(b)fluoranthene | 64 | Agmatine |
| 27 | Benzo(k)fluoranthene | 65 | l-Arginine |
| 28 | Benzo(j)fluoranthene | 66 | Cadaverine |
| 29 | Benzo(a)pyrene | 67 | l-Glutamic acid |
| 30 | Indeno(1,2,3-c,d)pyrene | 68 | Histamine |
| 31 | Dibenzo(a,h)anthrathene | 69 | Histidine |
| 32 | Benzo(g,h,i)perylene | 70 | l-Lysine |
| 33 | Dibenzo(a,l)pyrene | 71 | l-Ornithine |
| 34 | Dibenzo(a,e)pyrene | 72 | l-Tryptophan |
| 35 | Dibenzo(a,i)pyrene | 73 | l-Tyrosine |
| 36 | Dibenzo(a,h)pyrene | 74 | Putrescine |
| 37 | Naphthalene | 75 | Tryptamine |
| 38 | Fluorene | 76 | Tyramine |
| 39 | Anthracene |
| Compounds | EC RLU5000 a, nM (REP) b |
|---|---|
| TCDD | 0.01 (1) |
| 7-Nitrobenzo(a)anthracene (8) | 1.70 × 103 (5.9 × 10−6) |
| 6-Nitrobenzo(a)pyrene (9) | 1.76 × 102 (5.7 × 10−5) |
| 2-Nitroanthracene (11) | 3.81 × 104 (2.6 × 10−7) |
| 2-Chloronaphthalene (13) | 2.56 × 104 (3.9 × 10−7) |
| 1,4-Dichloronaphthalene (14) | 1.46 × 103 (6.8 × 10−6) |
| 1-Aminonaphthalene (19) | 5.00 × 103 (2.0 × 10−6) |
| Benzo(c)fluorene (21) | 2.31 × 103 (4.3 × 10−6) |
| Benzo(a)anthracene (22) | 8.87 × 102 (1.1 × 10−5) |
| Chrysene (24) | 2.41 × 102 (4.1 × 10−5) |
| 5-Methylchrysene (25) | 2.97 × 104 (3.4 × 10−7) |
| Benzo(b)fluoranthene (26) | 19.7 (5.1 × 10−4) |
| Benzo(k)fluoranthene (27) | 2.36 (4.2 × 10−3) |
| Benzo(j)fluoranthene (28) | 18.8 (5.3 × 10−4) |
| Benzo(a)pyrene (29) | 3.72 (2.7 × 10−3) |
| Indeno(1,2,3-c,d)pyrene (30) | 20.3 (4.9 × 10−4) |
| Dibenzo(a,e)pyrene (34) | 1.13 × 102 (8.8 × 10−5) |
| Dibenzo(a,i)pyrene (35) | 6.17 (1.6 × 10−3) |
| Dibenzo(a,h)pyrene (36) | 9.32 (1.1 × 10−3) |
| Naphthalene (37) | 3.77 × 104 (2.7 × 10−7) |
| Carbendazim (42) | 1.30 × 104 (7.7 × 10−7) |
| Thiabendazole (60) | 7.10 × 104 (1.4 × 10−7) |
| Tryptamine (75) | 2.51 × 104 (4.0 × 10−7) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amakura, Y.; Tsutsumi, T.; Yoshimura, M.; Nakamura, M.; Handa, H.; Matsuda, R.; Teshima, R.; Watanabe, T. Detection of Aryl Hydrocarbon Receptor Activation by Some Chemicals in Food Using a Reporter Gene Assay. Foods 2016, 5, 15. https://doi.org/10.3390/foods5010015
Amakura Y, Tsutsumi T, Yoshimura M, Nakamura M, Handa H, Matsuda R, Teshima R, Watanabe T. Detection of Aryl Hydrocarbon Receptor Activation by Some Chemicals in Food Using a Reporter Gene Assay. Foods. 2016; 5(1):15. https://doi.org/10.3390/foods5010015
Chicago/Turabian StyleAmakura, Yoshiaki, Tomoaki Tsutsumi, Morio Yoshimura, Masafumi Nakamura, Hiroshi Handa, Rieko Matsuda, Reiko Teshima, and Takahiro Watanabe. 2016. "Detection of Aryl Hydrocarbon Receptor Activation by Some Chemicals in Food Using a Reporter Gene Assay" Foods 5, no. 1: 15. https://doi.org/10.3390/foods5010015





