Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Preparation of the Aqueous Bitter Melon Extract
2.4. Experimental Design
| Coded Variable Levels | X1 Stock Solution Concentration % (w/w) | X2 Ratio of Extract to Stock Solution g/g (WW) |
|---|---|---|
| +1.682 | 37 | 1.71 |
| +1 | 35 | 1.50 |
| 0 | 30 | 1.00 |
| −1 | 25 | 0.50 |
| −1.682 | 23 | 0.30 |
2.5. Preparation of Spray Drying Infeed Solutions
2.6. Spray-Drying Conditions
2.7. Analytical Methods
2.7.1. Measurement of Bioactive Compounds
2.7.2. Measurement of Antioxidant Capacity
2.7.3. Determinants of the Infeed Solutions
2.7.4. Determinants of the Encapsulated Powders
Calculation of Process Yield
Retentions of Bioactive Concentration and Activity
Determination of the Physical Properties
Scanning Electron Microscope (SEM)
2.8. Statistical Analyses
3. Results and Discussion
3.1. The Maltodextrin to Gum Arabic Ratio
| Ratio of MD to GA g/g (WW) | EY (%) | TSC (%) | TPC (%) | TFC (%) | TAA (%) |
|---|---|---|---|---|---|
| 1:0 | 51.29 ± 4.52 a | 41.70 ± 0.95 a | 42.60 ± 0.12 a | 20.50 ± 0.40 a | 25.25 ± 1.35 a |
| 1:1 | 61.97 ± 4.24 a | 61.58 ± 1.33 bc | 57.85 ± 1.25 bc | 50.79 ± 1.12 b | 47.16 ± 2.78 b |
| 3:2 | 56.82 ± 3.06 a | 64.00 ± 1.39 b | 59.66 ± 0.73 b | 51.14 ± 1.28 b | 40.95 ± 1.75 c |
| 7:3 | 62.15 ± 4.32 a | 61.57 ± 1.27 c | 59.04 ± 1.39 b | 43.84 ± 0.77 c | 35.84 ± 2.41 c |
| 4:1 | 57.63 ± 4.63 a | 60.29 ± 0.44 c | 55.41 ± 0.30 c | 36.92 ± 0.78 d | 28.40 ± 0.71 a |
3.2. Fitting the Response Surface Methodology Model
| Pattern * | X1 Concentration % (w/w) | X2 Ratio g/g (WW) | EY (%) | TSC (%) | TPC (%) | TFC (%) | TAA (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | |||
| 00 | 30 | 1.00 | 63.47 | 63.38 | 60.10 | 63.52 | 54.98 | 56.04 | 48.68 | 49.49 | 45.79 | 44.11 |
| 00 | 30 | 1.00 | 65.89 | 63.38 | 62.67 | 63.52 | 57.28 | 56.04 | 51.16 | 49.49 | 43.54 | 44.11 |
| 0A | 30 | 1.71 | 52.15 | 54.17 | 74.23 | 79.13 | 87.81 | 87.21 | 62.95 | 61.68 | 90.02 | 89.15 |
| +− | 35 | 0.50 | 70.78 | 70.61 | 71.49 | 72.18 | 95.50 | 95.38 | 81.08 | 80.97 | 87.70 | 86.64 |
| 0a | 30 | 0.30 | 72.81 | 72.59 | 63.07 | 65.65 | 91.61 | 90.21 | 63.37 | 63.98 | 87.48 | 87.19 |
| 00 | 30 | 1.00 | 62.54 | 63.38 | 61.98 | 63.52 | 55.28 | 56.04 | 51.34 | 49.49 | 43.66 | 44.11 |
| ++ | 35 | 1.50 | 68.21 | 66.35 | 79.97 | 81.81 | 90.18 | 93.24 | 78.35 | 79.33 | 83.31 | 88.04 |
| A0 | 37 | 1.00 | 70.50 | 70.62 | 79.92 | 79.06 | 97.84 | 94.17 | 92.91 | 89.02 | 85.27 | 81.92 |
| −+ | 25 | 1.50 | 43.15 | 47.25 | 77.47 | 73.41 | 80.19 | 75.04 | 53.82 | 50.63 | 80.37 | 74.54 |
| a0 | 23 | 1.00 | 64.46 | 56.14 | 59.06 | 67.13 | 63.95 | 68.32 | 44.28 | 48.27 | 58.35 | 62.75 |
| −− | 25 | 0.50 | 63.56 | 69.31 | 63.20 | 63.78 | 79.46 | 77.18 | 57.07 | 52.27 | 74.01 | 73.14 |
| Independent Variables | Regression Coefficient Values | ||||
|---|---|---|---|---|---|
| EY (Y1) | TSC (Y2) | TPC(Y3) | TFC (Y4) | TAA (Y5) | |
| Intercept | 63.97 | 61.58 | 56.51 | 50.39 | 44.33 |
| Linear | |||||
| X1 | 5.10 * | 5.04 * | 9.24 ** | 14.66 *** | 6.84 * |
| X2 | −6.53 * | 4.82 * | −1.07 | −0.82 | 0.70 |
| Quadratic | |||||
| X1 X1 | 0.87 | 4.94 | 12.51 ** | 9.53 * | 14.01 ** |
| X2 X2 | −1.63 | 4.53 * | 16.67 ** | 6.81 * | 22.48 *** |
| Interaction | |||||
| X1X2 | 4.46 | −1.45 | −1.51 | 0.13 | −2.69 |
| R2 | 0.86 | 0.87 | 0.98 | 0.97 | 0.98 |
| p-value of lack of fit | 0.083 | 0.062 | 0.071 | 0.100 | 0.075 |
3.3. Effects of the Concentration and Ratio on Encapsulation Yield and Encapsulation Efficiency
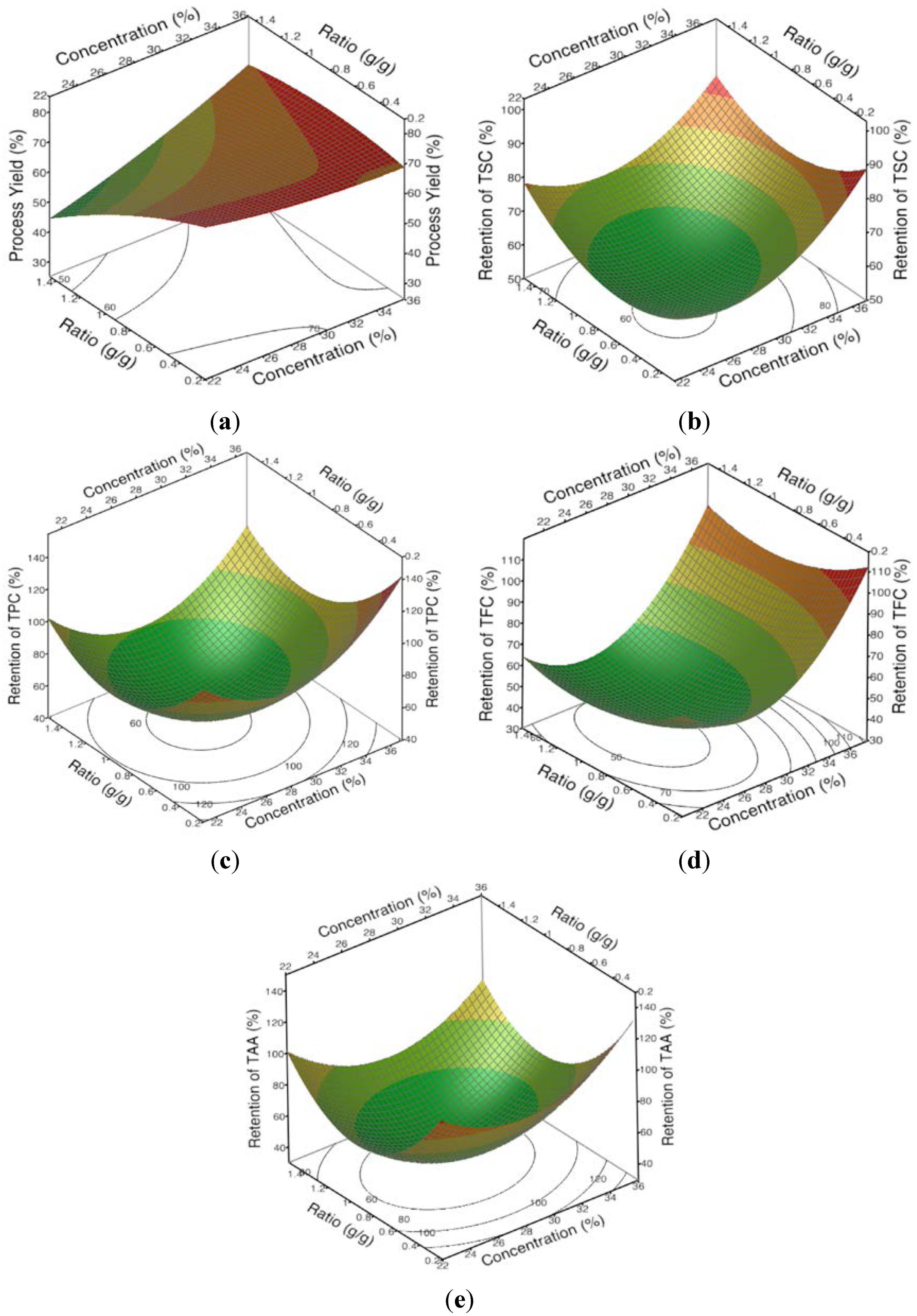
3.4. The Optimal Formulation and Validation of the Models

| EY (Y1) | TSC (Y2) | TPC (Y3) | TFC (Y4) | TAA (Y5) | |
|---|---|---|---|---|---|
| Predicted values | 66.4 ± 4.4 | 80.9 ± 3.9 | 93.9 ± 3.5 | 80.6 ± 3.3 | 88.3 ± 3.9 |
| Experimental values | 62.4 ± 0.4 | 82.7 ± 1.6 | 92.0 ± 3.6 | 79.6 ± 3.5 | 83.6 ± 3.9 |
3.5. Properties of the Optimised Encapsulated Powder
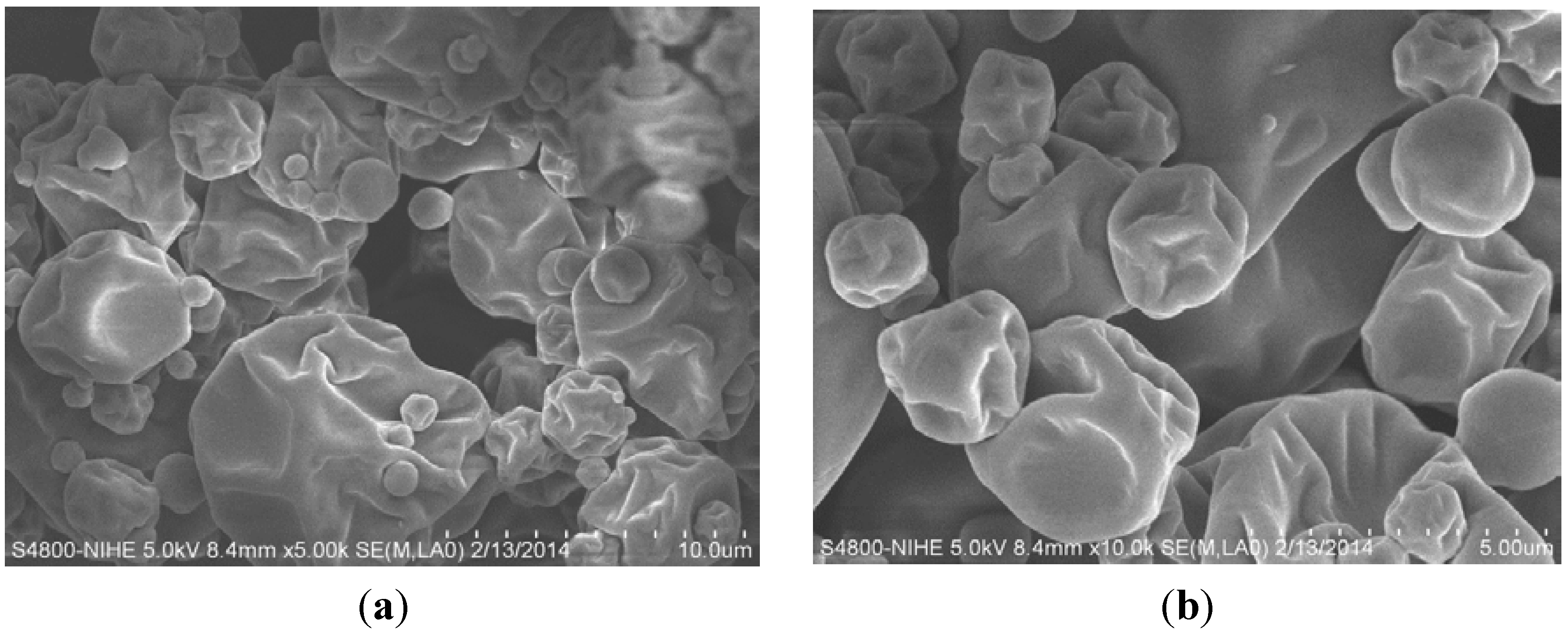
| Physical Properties | ||
|---|---|---|
| Moisture content (%) | 2.82 ± 0.24 | |
| Water activity | 0.33 ± 0.01 | |
| Colour | Lightness | 92.41 ± 0.51 |
| Chroma | 7.82 ± 0.19 | |
| Hue | 94.79 ± 0.38 | |
| Bulk density (g/mL) | 0.50 ± 0.04 | |
| Water solubility index (%) | 93.20 ± 0.36 | |
| Water absorption index (%) | 1.63 ± 0.03 | |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joseph, B.; Jini, D. Antidiabetic effects of momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Paul, A.; Raychaudhuri, S.S. Medicinal uses and molecular identification of two momordica charantia varieties—A review. Electron. J. Biol. 2010, 6, 43–51. [Google Scholar]
- Zänker, K.S.; Mang, B.; Wolters, M.; Hahn, A. Personalized diabetes and cancer medicine: A rationale for anti-diabetic nutrition (bitter melon) in a supportive setting. Curr. Cancer Ther. Rev. 2012, 8, 66–77. [Google Scholar]
- Celia, G.; Cummings, E.; David, A.P.; Jaipaul, S. Beneficial effect and mechanism of action of momordica charantia in the treatment of diabetes mellitus: A mini review. Int. J. Diabetes Metab. 2003, 11, 46–55. [Google Scholar]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Extraction of flavonoids from bitter melon. Food Nutr. Sci. 2014, 5, 458–465. [Google Scholar] [CrossRef]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. An optimised aqueous extract of phenolic compounds from bitter melon with high antioxidant capacity. Antioxidants 2014, 3, 814–829. [Google Scholar] [CrossRef]
- Habicht, S.D.; Rudloff, S.; Borsch, C.; Mueller, A.S.; Pallauf, J.; Yang, R.Y.; Krawinkel, M.B.; Kind, V. Quantification of antidiabetic extracts and compounds in bitter gourd varieties. Food Chem. 2011, 126, 172–176. [Google Scholar] [CrossRef]
- Tan, S.P.; Vuong, Q.V.; Stathopoulos, C.E.; Parks, S.E.; Roach, P.D. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J. Food Sci. 2014, 79, E1372–E1381. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pegg, R. Encapsulation, stabilization, and controlled release of food ingredients and bioactives. In Handbook of Food Preservation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 509–568. [Google Scholar]
- Schutyser, M.A.I.; Perdana, J.; Boom, R.M. Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci. Technol. 2012, 27, 73–82. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D. Effects of spray drying conditions on the physicochemical and antioxidant properties of the gac (Momordica cochinchinensis) fruit aril powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2012, 115, 443–451. [Google Scholar] [CrossRef]
- Cilek, B.; Luca, A.; Hasirci, V.; Sahin, S.; Sumnu, G. Microencapsulation of phenolic compounds extracted from sour cherry pomace: Effect of formulation, ultrasonication time and core to coating ratio. Eur. Food Res. Technol. 2012, 235, 587–596. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Medica 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive folin-ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia linn. Var. Abbreviata ser.) in Taiwan. LWT Food Sci. Technol. 2008, 41, 323–330. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Conway, H.F.; Pfeifer, V.F.; Griffin, J.R. Gelatinization of corn grits by roll and extrusion cooking. Cereal Sci. Today 1969, 14, 4–12. [Google Scholar]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci. Technol. 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Santana, A.A.; Kurozawa, L.E.; de Oliveira, R.A.; Park, K.J. Influence of process conditions on the physicochemical properties of Pequi powder produced by spray drying. Dry. Technol. 2013, 31, 825–836. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. Microencapsulation of gac oil by spray drying: Optimization of wall material concentration and oil load using response surface methodology. Dry. Technol. 2014, 32, 385–397. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray-dried encapsulation of lycopene. Dry. Technol. 2012, 30, 641–652. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; Chapter 6. [Google Scholar]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Mestry, A.P.; Mujumdar, A.S.; Thorat, B.N. Optimization of spray drying of an innovative functional food: Fermented mixed juice of carrot and watermelon. Dry. Technol. 2011, 29, 1121–1131. [Google Scholar] [CrossRef]
- Nijdam, J.J.; Langrish, T.A.G. The effect of surface composition on the functional properties of milk powders. J. Food Eng. 2006, 77, 919–925. [Google Scholar] [CrossRef]
- Osorio, C.; Acevedo, B.; Hillebrand, S.; Carriazo, J.; Winterhalter, P.; Morales, A.L.A. Microencapsulation by spray-drying of anthocyanin pigments from corozo (Bactris guineensis) fruit. J. Agric. Food Chem. 2010, 58, 6977–6985. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P.J. Food Processing Technology—Principles and Practice, 3rd ed.; Woodhead Publishing: New York, NY, USA, 2009; pp. 11–95. [Google Scholar]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Caliskan, G.; Nur Dirim, S. The effects of the different drying conditions and the amounts of maltodextrin addition during spray drying of sumac extract. Food Bioprod. Process. 2013, 91, 539–548. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Morassi, A.G.; Vanzo, A.A.; Park, K.J.; Hubinger, M.D. Influence of spray drying conditions on physicochemical properties of chicken meat powder. Dry. Technol. 2009, 27, 1248–1257. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: I. Drying kinetics and product recovery. Dry. Technol. 2008, 26, 714–725. [Google Scholar] [CrossRef]
- Bakar, J.; Ee, S.C.; Muhammad, K.; Hashim, D.; Adzahan, N. Spray-drying optimization for red pitaya peel (Hylocereus polyrhizus). Food Bioprocess Technol. 2013, 6, 1332–1342. [Google Scholar] [CrossRef]
- Moreira, G.É.G.; Maia Costa, M.G.; Souza, A.C.R.D.; Brito, E.S.D.; Medeiros, M.D.F.D.D.; Azeredo, H.M.C.D. Physical properties of spray dried Acerola pomace extract as affected by temperature and drying aids. LWT Food Sci. Technol. 2009, 42, 641–645. [Google Scholar] [CrossRef]
- Hancock, B.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400-419. https://doi.org/10.3390/foods4030400
Tan SP, Kha TC, Parks S, Stathopoulos C, Roach PD. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods. 2015; 4(3):400-419. https://doi.org/10.3390/foods4030400
Chicago/Turabian StyleTan, Sing Pei, Tuyen Chan Kha, Sophie Parks, Costas Stathopoulos, and Paul D. Roach. 2015. "Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying" Foods 4, no. 3: 400-419. https://doi.org/10.3390/foods4030400
APA StyleTan, S. P., Kha, T. C., Parks, S., Stathopoulos, C., & Roach, P. D. (2015). Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods, 4(3), 400-419. https://doi.org/10.3390/foods4030400






