Compositional Signatures of Conventional, Free Range, and Organic Pork Meat Using Fingerprint Techniques
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples
2.2. Chemical and Reagents
2.3. Fat Extraction
2.4. Fatty Acid Methyl Ester (FAME) Analyses
2.5. ESI-MS/MS
2.6. PTR-MS
2.7. Statistical Analysis
2.8. Variable Selection
3. Results and Discussion
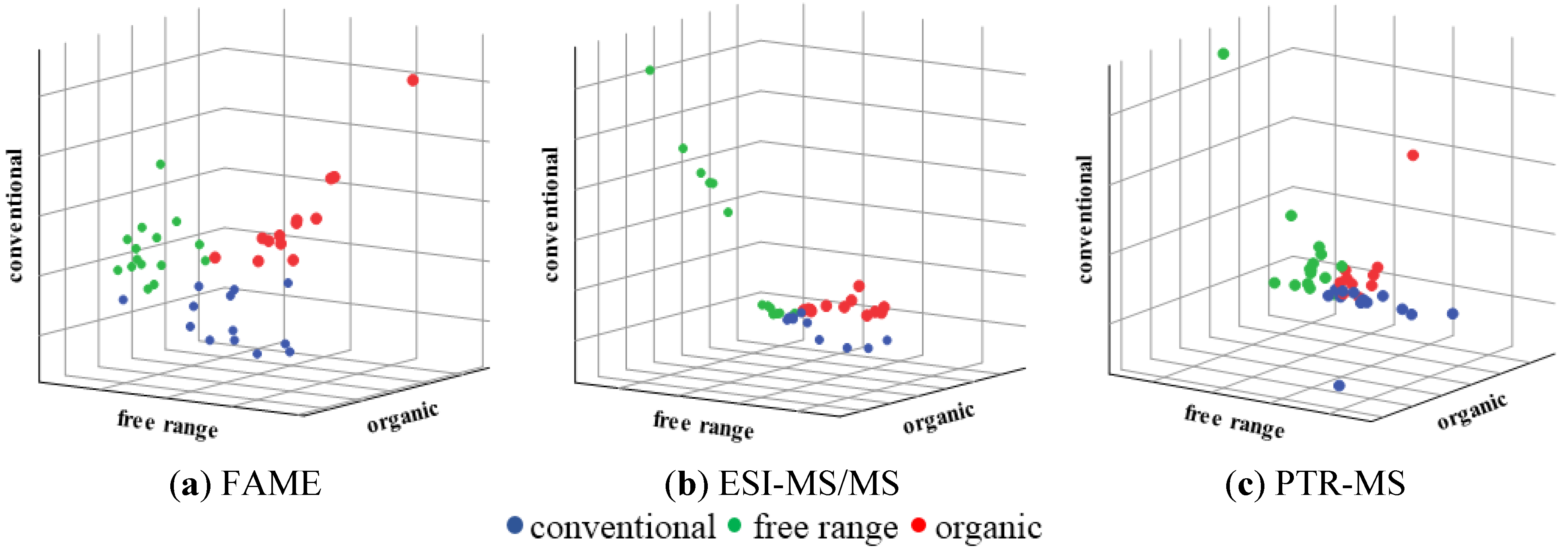
3.1. Three Animal Welfare Categories
| Fatty Acid Methyl Ester (FAME) | Electrospray ionization-tandem mass spectrometry (ESI-MS/MS) | Proton-transfer-reaction mass spectrometry (PTR-MS) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| samples classified as | samples classified as | samples classified as | ||||||||||||||
| All data | correct | correct | correct | |||||||||||||
| Conventional | Free range | Organic | Not classified | classification (%) | Conventional | Free range | Organic | Not classified | classification (%) | Conventional | Free range | Organic | Not classified | Classification (%) | ||
| Conventional | 13 | 0 | 0 | 0 | 100 | 12 | 0 | 0 | 0 | 100 | 13 | 0 | 0 | 0 | 100 | |
| Free range | 0 | 15 | 0 | 0 | 100 | 1 | 13 | 0 | 0 | 93 | 1 | 14 | 0 | 0 | 93 | |
| Organic | 0 | 0 | 13 | 0 | 100 | 0 | 0 | 12 | 0 | 100 | 0 | 0 | 10 | 0 | 100 | |
| Average of training set | 100 | 95.3 | 95.3 | |||||||||||||
| Model performance evaluation | ||||||||||||||||
| Training set | correct | correct | correct | |||||||||||||
| Conventional | Free range | Organic | Not classified | classification (%) | Conventional | Free range | Organic | Not classified | classification (%) | Conventional | Free range | Organic | Not classified | classification (%) | ||
| Conventional | 10 | 0 | 0 | 0 | 100 | 9 | 0 | 0 | 0 | 100 | 10 | 0 | 0 | 0 | 100 | |
| Free range | 0 | 12 | 0 | 0 | 100 | 0 | 11 | 0 | 0 | 100 | 1 | 11 | 0 | 0 | 92 | |
| Organic | 0 | 0 | 10 | 0 | 100 | 0 | 0 | 9 | 0 | 100 | 0 | 0 | 8 | 0 | 100 | |
| Validation set | ||||||||||||||||
| Conventional | 3 | 0 | 0 | 0 | 100 | 3 | 0 | 0 | 0 | 100 | 3 | 0 | 0 | 0 | 100 | |
| Free range | 0 | 3 | 0 | 0 | 100 | 1 | 2 | 0 | 0 | 67 | 0 | 3 | 0 | 0 | 100 | |
| Organic | 0 | 0 | 2 | 1 | 67 | 1 | 0 | 2 | 0 | 67 | 0 | 0 | 2 | 0 | 100 | |
| Average of validation set | 89 | 78 | 100 | |||||||||||||
| Fatty acid | Conventional | Free Range | Organic |
|---|---|---|---|
| Myristoleic acid | 0.02 a ± 0.01 | 0.02 a ± 0.00 | 0.01 b ± 0.00 |
| Pentadecylic acid | 0.06 a,b ± 0.02 | 0.06 a ± 0.01 | 0.07 b ± 0.02 |
| Palmitic acid | 25.32 a ± 1.10 | 25.37 a ± 0.91 | 22.98 b ± 1.86 |
| Palmitoleic acid | 2.09 a ± 0.40 | 2.10 a ± 0.29 | 1.59 b ± 0.21 |
| Palmitoleic acid | 0.01 a ± 0.00 | 0.01 a ± 0.01 | 0.01 a ± 0.00 |
| Margaric acid | 0.32 a ± 0.07 | 0.30 a ± 0.09 | 0.40 b ± 0.11 |
| Heptadecanoic acid | 0.23 a ± 0.05 | 0.23 a ± 0.08 | 0.27a ± 0.08 |
| Stearic acid | 13.86 a,b ± 2.38 | 14.23 a ± 1.17 | 12.78 b ± 1.44 |
| Trans elaidic acid | 0.23 a ± 0.05 | 0.22 a,b ± 0.06 | 0.18 b ± 0.05 |
| Oleic acid | 36.79 a ± 3.04 | 38.93 b ± 2.05 | 35.97 a ± 1.56 |
| Vaccenic acid | 2.55 a ± 0.28 | 2.63 a ± 0.32 | 2.28 b ± 0.17 |
| Unknown 20.4 min | 0.04 a,b ± 0.01 | 0.04 a ± 0.01 | 0.03 b ± 0.01 |
| Linoleic acid | 14.97 a ± 3.64 | 12.58 b ± 2.17 | 18.98 c ± 2.98 |
| Unknown 21.2 | 0.03 a ± 0.01 | 0.04 a ± 0.01 | 0.04 a ± 0.01 |
| Gamma-linolenic acid | 0.01 a,b ± 0.00 | 0.01 a ± 0.00 | 0.01 b ± 0.00 |
| Alpha-linolenic acid | 1.44 a ± 0.40 | 1.15 b ± 0.25 | 2.02 c ± 0.34 |
| Eicosenoic acid | 0.71 a ± 0.11 | 0.83 b ± 0.11 | 0.73 a ± 0.08 |
| Eicosadienoic acid | 0.52 a ± 0.08 | 0.51 b ± 0.07 | 0.70 c ± 0.10 |
| Unknown 23.3 min | 0.03 a,b ± 0.01 | 0.03 a ± 0.01 | 0.02 b ± 0.01 |
| Unknown 23.5 min | 0.03 a ± 0.01 | 0.03 a ± 0.01 | 0.03 a ± 0.01 |
| Dihomo-gamma-linolenic acid | 0.09 a ± 0.02 | 0.09 a ± 0.01 | 0.11 a ± 0.02 |
| Eicosatrienoic acid + arachidonic acid | 0.41 a ± 0.08 | 0.37 a ± 0.06 | 0.5 b ± 0.08 |
| Adrenic acid | 0.10 a ± 0.02 | 0.10 a ± 0.02 | 0.11 a ± 0.03 |
| Docosapentaenoic acid (osbond acid) | 0.02 a ± 0.01 | 0.01 b ± 0.00 | 0.02 a ± 0.00 |
| Docosapentaenoic acid (clupanodonic acid) | 0.12 a ± 0.03 | 0.12 a ± 0.05 | 0.15 b ± 0.03 |
| ∑ Saturated | 39.56 a ± 2.63 | 39.95 a ± 1.48 | 36.24 b ± 2.35 |
| ∑ Monounsaturated | 42.63 a ± 3.10 | 44.97 b ± 2.10 | 41.03 a ± 1.58 |
| ∑ Polyunsaturated | 17.68 a ± 3.67 | 14.95 b ± 2.19 | 22.60 c ± 2.98 |
| ∑ Unknown | 0.13 a ± 0.02 | 0.14 a ± 0.02 | 0.13 a ± 0.01 |
| ∑ Omega-3 | 1.97 a ± 0.41 | 1.64 b ± 0.26 | 2.67 c ± 0.35 |

| Fatty Acid Methyl Ester (FAME) | Electrospray ionization-tandem mass spectrometry (ESI-MS/MS) | Proton-transfer-reaction mass spectrometry (PTR-MS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| samples classified as | samples classified as | samples classified as | ||||||||||
| All data | correct | correct | correct | |||||||||
| Non-organic | Organic | Not classified | Classification (%) | Non-organic | Organic | Not classified | Classification (%) | Non-organic | Organic | Not classified | Classification (%) | |
| Non-organica | 28 | 0 | 0 | 100 | 26 | 0 | 0 | 100 | 28 | 0 | 0 | 100 |
| Organic | 0 | 13 | 0 | 100 | 0 | 12 | 0 | 100 | 0 | 10 | 0 | 100 |
| Average of training set | 100 | 100 | 100 | |||||||||
| Model performance evaluation | correct | correct | correct | |||||||||
| Training set | Non-organic | Organic | Not classified | classification (%) | Non-organic | Organic | Not classified | classification (%) | Non-organic | Organic | Not classified | classification (%) |
| Non-organica | 22 | 0 | 0 | 100 | 20 | 0 | 0 | 100 | 22 | 0 | 0 | 100 |
| Organic | 0 | 10 | 0 | 100 | 0 | 9 | 0 | 100 | 0 | 8 | 0 | 100 |
| Validation set | ||||||||||||
| Non-organica | 6 | 0 | 0 | 100 | 6 | 0 | 0 | 100 | 6 | 0 | 0 | 100 |
| Organic | 0 | 3 | 0 | 100 | 1 | 2 | 0 | 67 | 0 | 1 | 1 | 50 |
| Average of validation set | 100 | 83 | 75 | |||||||||
3.2. Organic Versus Conventional Meat
| Fatty acid | Non-Organic | Organic |
|---|---|---|
| Myristoleic acid | 0.02 a ± 0.01 | 0.01 b ± 0.00 |
| Pentadecylic acid | 0.06 a ± 0.02 | 0.07 b ± 0.02 |
| Palmitic acid | 25.34 a ± 0.98 | 22.98 b ± 1.86 |
| Palmitoleic acid | 2.09 a ± 0.34 | 1.59 b ± 0.21 |
| Palmitoleic acid | 0.01 a ± 0.00 | 0.01 a ± 0.01 |
| Margaric acid | 0.31 a ± 0.08 | 0.40 b ± 0.11 |
| Heptadecanoic acid | 0.23 a ± 0.07 | 0.27 a ± 0.08 |
| Stearic acid | 14.06 a ± 1.81 | 12.78 b ± 1.44 |
| Trans elaidic acid | 0.23 a ± 0.06 | 0.18 b ± 0.05 |
| Oleic acid | 37.94 a ± 2.73 | 35.97 b ± 1.56 |
| Vaccenic acid | 2.59 a ± 0.30 | 2.28 b ± 0.17 |
| Unknown 20.4 | 0.04 a ± 0.01 | 0.03 a ± 0.01 |
| Linoleic acid | 13.69 a ± 3.13 | 18.98 b ± 2.98 |
| Unknown 21.2 | 0.03 a ± 0.01 | 0.04 a ± 0.01 |
| Gamma-linolenic acid | 0.01 a ± 0.00 | 0.01 b ± 0.00 |
| Alpha-linolenic acid | 1.29 a ± 0.35 | 2.02 b ± 0.34 |
| Eicosenoic acid | 0.77 a ± 0.12 | 0.73 a ± 0.08 |
| Eicosadienoic acid | 0.51 a ± 0.07 | 0.7 b ± 0.10 |
| Unknown 23.3 | 0.03 a ± 0.01 | 0.02 b ± 0.01 |
| Unknown 23.5 | 0.03 a ± 0.01 | 0.03 a ± 0.01 |
| Dihomo-gamma-linolenic acid | 0.09 a ± 0.02 | 0.11 b ± 0.02 |
| Eicosatrienoic acid + arachidonic acid | 0.39 a ± 0.07 | 0.5 b ± 0.08 |
| Adrenic acid | 0.1 a ± 0.02 | 0.11 a ± 0.03 |
| Docosapentaenoic acid (osbond acid) | 0.01 a ± 0.01 | 0.02 b ± 0.00 |
| Docosapentaenoic acid (clupanodonic acid) | 0.12 a ± 0.04 | 0.15 b ± 0.03 |
| ∑ Saturated | 39.77a ± 2.06 | 36.24 a ± 2.35 |
| ∑ Monounsaturated | 43.88 a ± 2.77 | 41.03 b ± 1.58 |
| ∑ Polyunsaturated | 16.22 a ± 3.15 | 22.60 b ± 3.00 |
| ∑ Unknown | 0.14 a ± 0.02 | 0.13 a ± 0.01 |
| ∑ Omega-3 | 1.79 a ± 0.36 | 2.67 b ± 0.35 |
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Ballin, N.Z. Authentication of meat and meat products. Meat Sci. 2010, 86, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Nakyinsige, K.; Man, Y.B.C.; Sazili, A.Q. Halal authenticity issues in meat and meat products. Meat Sci. 2012, 91, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Siderer, Y.; Maquet, A.; Anklam, E. Need for research to support consumer confidence in the growing organic food market. Trends Food Sci.Technol. 2005, 16, 332–343. [Google Scholar] [CrossRef]
- Harper, G.; Henson, S. Consumer Concerns about Animal Welfare and the Impact on Food Choice - EU FAIR CT98–3678; The University of Reading; Centre for Food Economics Research (CeFER); Department of Agricultural and Food Economics: Reading, UK, 2001. [Google Scholar]
- Kriflik, L. Consumer citizenship: Acting to minimise environmental health risks related to the food system. Appetite 2006, 46, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Wandel, M.; Bugge, A. Environmental concern in consumer evaluation of food quality. Food Qual. Prefer. 1997, 8, 19–26. [Google Scholar] [CrossRef]
- Shafie, F.A.; Rennie, D. Consumer perceptions towards organic food. Procedia Soc. Behav. Sci. 2012, 49, 360–367. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Geng, Y.; Qi, Y. Green food consumption intention, behaviors and influencing factors among chinese consumers. Food Qual. Prefer. 2013, 28, 279–286. [Google Scholar] [CrossRef]
- Falguera, V.; Aliguer, N.; Falguera, M. An integrated approach to current trends in food consumption: Moving toward functional and organic products? Food Control 2012, 26, 274–281. [Google Scholar] [CrossRef]
- Alonso, R.; Rodríguez-Estévez, V.; Domínguez-Vidal, A.; Ayora-Cañada, M.J.; Arce, L.; Valcárcel, M. Ion mobility spectrometry of volatile compounds from iberian pig fat for fast feeding regime authentication. Talanta 2008, 76, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kneafsey, M.; Dowler, E.; Lambie-Mumford, H.; Inman, A.; Collier, R. Consumers and food security: Uncertain or empowered? J. Rural Stu. 2013, 29, 101–112. [Google Scholar] [CrossRef]
- European Union Strategy for the Protection and Welfare of Animals, 2012–2015. Available online: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//NONSGML+REPORT+A7-2012-0216+0+DOC+PDF+V0//EN (acessed on 21 August 2015).
- Janssen, M.; Hamm, U. Product labelling in the market for organic food: Consumer preferences and willingness-to-pay for different organic certification logos. Food Qual. Prefer. 2012, 25, 9–22. [Google Scholar] [CrossRef]
- Harvey, D.; Hubbard, C. Reconsidering the political economy of farm animal welfare: An anatomy of market failure. Food Policy 2013, 38, 105–114. [Google Scholar] [CrossRef]
- Dennis, M.J. Recent developments in food authentication. Analyst 1998, 123, 151R–156R. [Google Scholar] [CrossRef]
- Monahan, F.J.; Moloney, A.P.; Osorio, M.T.; Röhrle, F.T.; Schmidt, O.; Brennan, L. Authentication of grass-fed beef using bovine muscle, hair or urine. Trends Food Sci. Technol. 2012, 28, 69–76. [Google Scholar] [CrossRef]
- Primrose, S.; W.oolfe, M.; Rollinson, S. Food forensics: Methods for determining the authenticity of foodstuffs. Trends Food Sci. Technol. 2010, 21, 582–590. [Google Scholar] [CrossRef]
- Lebret, B. Effects of feeding and rearing systems on growth, carcass composition and meat quality in pigs. Animal 2008, 2, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Ngapo, T.M.; Gariépy, C. Factors affecting the eating quality of pork. Crit. Rev. Food Sci. Nutr. 2008, 48, 599–633. [Google Scholar] [CrossRef] [PubMed]
- Prache, S.; Cornu, A.; Berdagué, J.L.; Priolo, A. Traceability of animal feeding diet in the meat and milk of small ruminants. Small Rumin. Res. 2005, 59, 157–168. [Google Scholar] [CrossRef]
- Morel, P.; McIntosh, J.; Janz, J. Alteration of the fatty acid profile of pork by dietary manipulation. Asian Austral. J. Anim. Sci. 2006, 19, 431–437. [Google Scholar] [CrossRef]
- Wood, J.; Richardson, R.; Nute, G.; Fisher, A.; Campo, M.; Kasapidou, E.; Sheard, P.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Osorio, M.T.; Downey, G.; Moloney, A.P.; Röhrle, F.T.; Luciano, G.; Schmidt, O.; Monahan, F.J. Beef authentication using dietary markers: Chemometric selection and modelling of significant beef biomarkers using concatenated data from multiple analytical methods. Food Chem. 2013, 141, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
- Razminowicz, R.H.; Kreuzer, M.; Scheeder, M.R.L. Quality of retail beef from two grass-based production systems in comparison with conventional beef. Meat Sci. 2006, 73, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Spangenberg, J.; Willems, H.; Kreuzer, M.; Leiber, F. Stable carbon isotope composition of perirenal adipose tissue fatty acids from engadine sheep grazing either mountain or lowland pasture. J. Anim. Sci. 2012, 90, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.P.; Alves, S.P.; Martins, S., IV; Costa, A.S.; Fontes, C.M.; Lemos, J.P.; Bessa, R.J.; Prates, J.A. Effect of the feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Kim, D.H.; Seong, P.N.; Cho, S.H.; Kim, J.H.; Lee, J.M.; Jo, C.; Lim, D.G. Fatty acid composition and meat quality traits of organically reared korean native black pigs. Livest. Sci. 2009, 120, 96–102. [Google Scholar] [CrossRef]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef]
- Angood, K.M.; Wood, J.D.; Nute, G.R.; Whittington, F.M.; Hughes, S.I.; Sheard, P.R. A comparison of organic and conventionally-produced lamb purchased from three major UK supermarkets: Price, eating quality and fatty acid composition. Meat Sci. 2008, 78, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Husak, R.L.; Sebranek, J.G.; Bregendahl, K. A survey of commercially available broilers marketed as organic, free-range, and conventional broilers for cooked meat yields, meat composition, and relative value. Poult. Sci. 2008, 87, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, P.; Casale, M.; Casolino, M.C.; Baldo, M.A.; Nizzi Grifi, F.; Forina, M. Comparison between classical and innovative class-modelling techniques for the characterisation of a PDO olive oil. Anal. Bioanal. Chem. 2011, 399, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kliks, M.M.; Qu, W.; Jun, S.; Shi, G.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef] [PubMed]
- Chambery, A.; del Monaco, G.; di Maro, A.; Parente, A. Peptide fingerprint of high quality campania white wines by MALDI-TOF mass spectrometry. Food Chem. 2009, 113, 1283–1289. [Google Scholar] [CrossRef]
- Capuano, E.; Ruth, S.M.V. QA: Fraud control for foods and other biomaterials by product fingerprinting. In Latest Research into Quality Control; Akyar, I., Ed.; InTech.: Rijeka, Croatia, 2012; pp. 111–143. [Google Scholar]
- Catharino, R.R.; Haddad, R.; Cabrini, L.G.; Cunha, I.B.S.; Sawaya, A.C.H.F.; Eberlin, M.N. Characterization of vegetable oils by electrospray ionization mass spectrometry fingerprinting: Classification, quality, adulteration, and aging. Anal. Chem. 2005, 77, 7429–7433. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, J.M.; Ortea, I.; Carrera, M. Proteomics and its applications for food authentication and food-technology research. TrAC Trend. Anal. Chem. 2013, 52, 135–141. [Google Scholar] [CrossRef]
- Montowska, M.; Alexander, M.R.; Tucker, G.A.; Barrett, D.A. Rapid detection of peptide markers for authentication purposes in raw and cooked meat using ambient liquid extraction surface analysis mass spectrometry. Anal. Chem. 2014, 86, 10257–10265. [Google Scholar] [CrossRef] [PubMed]
- Von Bargen, C.; Brockmeyer, J.; Humpf, H.U. Meat authentication: A new HPLC-MS/MS based method for the fast and sensitive detection of horse and pork in highly processed food. J. Agric. Food Chem. 2014, 62, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.; Ratel, J. Correction of the data generated by mass spectrometry analyses of biological tissues: Application to food authentication. J. Chromatogr. A 2007, 1154, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.S.; Scampicchio, M.; Benedetti, S. Chapter 8—Electronic noses and tongues. In Chemical Analysis of Food: Techniques and Applications; Picó, Y., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 219–247. [Google Scholar]
- Nurjuliana, M.; Che Man, Y.B.; Mat Hashim, D.; Mohamed, A.K.S. Rapid identification of pork for halal authentication using the electronic nose and gas chromatography mass spectrometer with headspace analyzer. Meat Sci. 2011, 88, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Biasioli, F.; Gasperi, F.; Yeretzian, C.; Märk, T.D. PTR-MS monitoring of VOCS and BVOCS in food science and technology. TrAC Trend. Anal. Chem. 2011, 30, 968–977. [Google Scholar] [CrossRef]
- Araghipour, N.; Colineau, J.; Koot, A.; Akkermans, W.; Rojas, J.M.M.; Beauchamp, J.; Wisthaler, A.; Märk, T.D.; Downey, G.; Guillou, C.; et al. Geographical origin classification of olive oils by PTR-MS. Food Chem. 2008, 108, 374–383. [Google Scholar] [CrossRef]
- Ruiz-Samblás, C.; Tres, A.; Koot, A.; van Ruth, S.M.; González-Casado, A.; Cuadros-Rodríguez, L. Proton transfer reaction-mass spectrometry volatile organic compound fingerprinting for monovarietal extra virgin olive oil identification. Food Chem. 2012, 134, 589–596. [Google Scholar] [CrossRef]
- Dimitri, G.; van Ruth, S.M.; Sacchetti, G.; Piva, A.; Alewijn, M.; Arfelli, G. PTR-MS monitoring of volatiles fingerprint evolution during grape must cooking. LWT-Food Sci. Technol. 2013, 51, 356–360. [Google Scholar] [CrossRef]
- Maçatelli, M.; Akkermans, W.; Koot, A.; Buchgraber, M.; Paterson, A.; van Ruth, S. Verification of the geographical origin of european butters using PTR-MS. J. Food Comp. Anal. 2009, 22, 169–175. [Google Scholar] [CrossRef]
- Odake, S.; Shimamura, T.; Akuzawa, R.; Shimono, A.; van Ruth, S.M.; Wender, L.P.B.; Mikael Agerlin, P. Volatile compounds of wagyu (japanese black cattle) beef analysed by PTR-MS. In Developments in Food Science, 43. Flavour Science; Bredie, W., Petersen, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 43, pp. 29–32. [Google Scholar]
- Özdestan, Ö.; van Ruth, S.M.; Alewijn, M.; Koot, A.; Romano, A.; Cappellin, L.; Biasioli, F. Differentiation of specialty coffees by proton transfer reaction-mass spectrometry. Food Res. Int. 2013, 53, 433–439. [Google Scholar] [CrossRef]
- Van Ruth, S.M.; Floris, V.; Fayoux, S. Characterisation of the volatile profiles of infant formulas by proton transfer reaction-mass spectrometry and gas chromatography-mass spectrometry. Food Chem. 2006, 98, 343–350. [Google Scholar] [CrossRef]
- Galle, S.A.; Koot, A.; Soukoulis, C.; Cappellin, L.; Biasioli, F.; Alewijn, M.; van Ruth, S.M. Typicality and geographical origin markers of protected origin cheese from the netherlands revealed by PTR-MS. J. Agric. Food Chem. 2011, 59, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Luykx, D.M.A.M.; van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Holm, E.S.; Adamsen, A.P.; Feilberg, A.; Schafer, A.; Lokke, M.M.; Petersen, M.A. Quality changes during storage of cooked and sliced meat products measured with PTR-MS and HS-GC-MS. Meat Sci. 2013, 95, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mayr, D.; Margesin, R.; Klingsbichel, E.; Hartungen, E.; Jenewein, D.; Schinner, F.; Märk, T.D. Rapid detection of meat spoilage by measuring volatile organic compounds by using proton transfer reaction mass spectrometry. Appl. Environ. Microbiol. 2003, 69, 4697–4705. [Google Scholar] [CrossRef] [PubMed]
- Tres, A.; van Ruth, S.M. Verification of organic feed identity by fatty acid fingerprinting. J. Agric. Food Chem. 2011, 59, 8816–8821. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.B.; Alewijn, M.; Boerrigter-Eenling, R.; Van Ruth, S.M. Compositional Signatures of Conventional, Free Range, and Organic Pork Meat Using Fingerprint Techniques. Foods 2015, 4, 359-375. https://doi.org/10.3390/foods4030359
Oliveira GB, Alewijn M, Boerrigter-Eenling R, Van Ruth SM. Compositional Signatures of Conventional, Free Range, and Organic Pork Meat Using Fingerprint Techniques. Foods. 2015; 4(3):359-375. https://doi.org/10.3390/foods4030359
Chicago/Turabian StyleOliveira, Gislene B., Martin Alewijn, Rita Boerrigter-Eenling, and Saskia M. Van Ruth. 2015. "Compositional Signatures of Conventional, Free Range, and Organic Pork Meat Using Fingerprint Techniques" Foods 4, no. 3: 359-375. https://doi.org/10.3390/foods4030359





