Combined Effect of Pressure-Assisted Thermal Processing and Antioxidants on the Retention of Conjugated Linoleic Acid in Milk
Abstract
:1. Introduction
2. Experimental Section
2.1 Obtaining CLA-Enriched Milk and Sample Preparation
2.2. Pressure-Assisted Thermal Processing
2.3. Analytical Determinations
2.3.1. CLA Determination
2.3.2. Dissolved Oxygen
2.4. Data Analysis
2.4.1. Retention of CLA
2.4.2. Quenching Ability
3. Results and Discussion
3.1. Pressure, Temperature and Time History
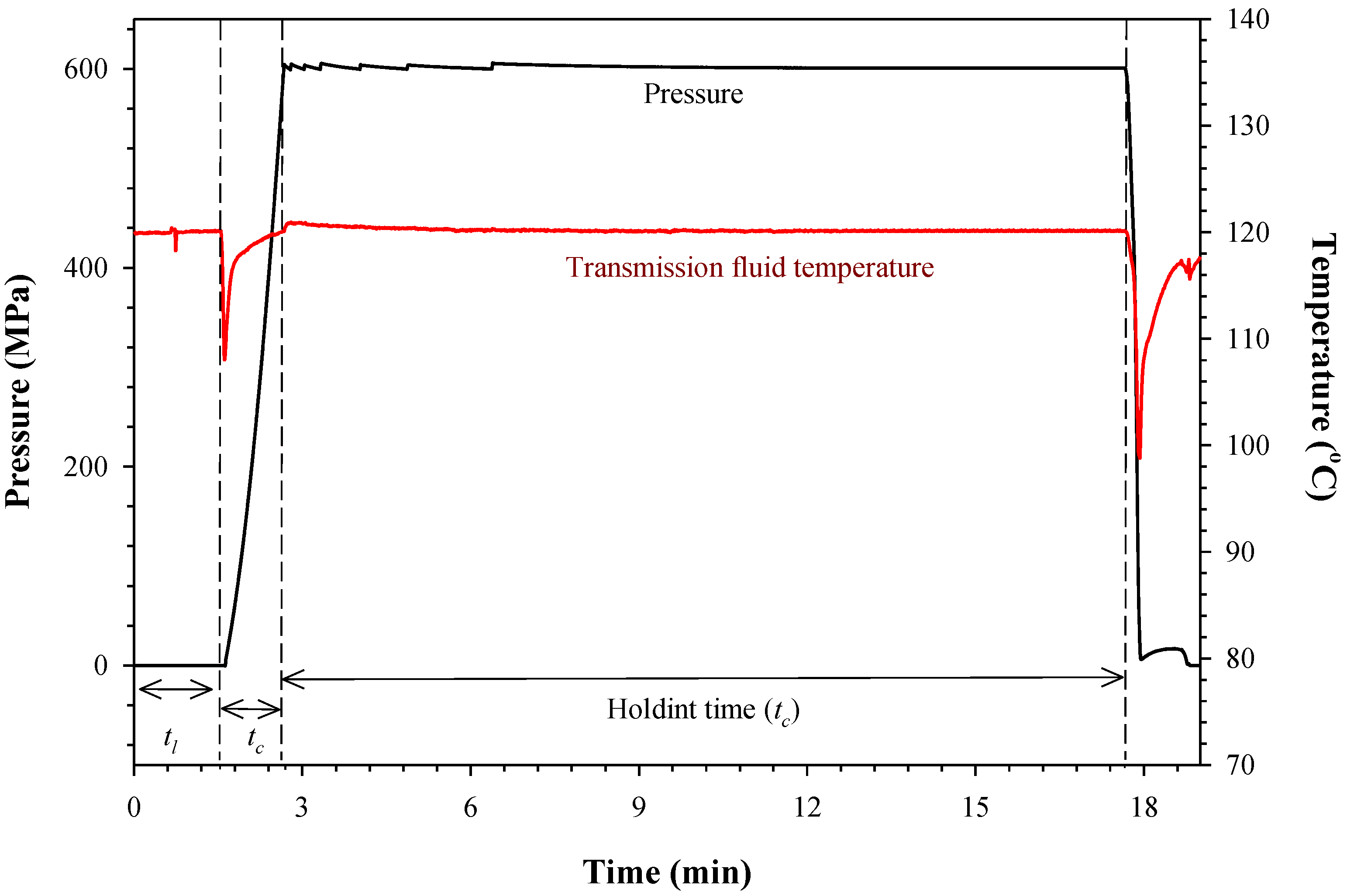
3.2. CLA-Enriched Milk

| Antioxidant | 600 MPa | 0.1 MPa | ||
|---|---|---|---|---|
| k1, min−1 | 95% CI | k1, min−1 | 95% CI | |
| Milk | 0.059 | 0.006 | 0.091 | 0.010 |
| Ascorbic acid | 0.081 | 0.009 | 0.366 | 0.050 |
| Cysteine | 0.030 | 0.005 | 0.194 | 0.051 |
| Caffeic acid | 0.041 | 0.015 | 0.078 | 0.020 |
| Catechin | 0.051 | 0.009 | 0.139 | 0.015 |
| Gallic acid | 0.092 | 0.018 | 0.150 | 0.036 |
| p-Coumaric acid | 0.042 | 0.011 | 0.118 | 0.011 |
| Tannic acid | 0.069 | 0.019 | 0.084 | 0.017 |
3.3. Ascorbic Acid
3.4. Cysteine
| Holding time (min) | Normalized retention of conjugated linoleic acid (CLA) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cysteine | Caffeic acid | Catechin | |||||||
| 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | ||||
| 0 | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | |||
| 1 | 0.73 ± 0.03b | 0.81 ± 0.03b | 0.91 ± 0.03a | 0.74 ± 0.03b | 0.95 ± 0.03b | 0.87 ± 0.03b | |||
| 5 | 0.63 ± 0.02c | 0.72 ± 0.02c | 0.79 ± 0.02b | 0.71 ± 0.02c | 0.77 ± 0.02c | 0.81 ± 0.02c | |||
| 10 | 0.59 ± 0.03d | 0.73 ± 0.03cd | 0.70 ± 0.02c | 0.71 ± 0.03c | 0.69 ± 0.02d | 0.79 ± 0.03c | |||
| 15 | 0.56 ± 0.02e | 0.70 ± 0.03d | 0.69 ± 0.02c | 0.66 ± 0.03d | 0.39 ± 0.02e | 0.75 ± 0.03d | |||
| Holding time (min) | Normalized dissolved oxygen (DO2) | ||||||||
| Cysteine | Caffeic acid | Catechin | |||||||
| 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | ||||
| 0 | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | |||
| 1 | 0.13 ± 0.02b | 0.79 ± 0.03b | 0.88 ± 0.03b | 0.78 ± 0.03b | 0.78 ± 0.02b | 0.71 ± 0.03b | |||
| 5 | 0.11 ± 0.03c | 0.64 ± 0.01c | 0.54 ± 0.01c | 0.65 ± 0.01c | 0.65 ± 0.03c | 0.30 ± 0.04c | |||
| 10 | 0.11 ± 0.01c | 0.61 ± 0.03d | 0.29 ± 0.03d | 0.56 ± 0.02d | 0.56 ± 0.01d | 0.14 ± 0.03d | |||
| 15 | 0.05 ± 0.02d | 0.42 ± 0.04e | 0.25 ± 0.02e | 0.18 ± 0.03e | 0.18 ± 0.02e | 0.09 ± 0.03e | |||
3.5. Phenolic Antioxidants
| Holding time (min) | Normalized retention of conjugated linoleic acid (CLA) | |||||
|---|---|---|---|---|---|---|
| Gallic acid | p-Coumaric acid | Tannic acid | ||||
| 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | |
| 0 | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a |
| 1 | 0.69 ± 0.03b | 0.98 ± 0.02b | 0.91 ± 0.03b | 0.87 ± 0.03b | 0.91 ± 0.03b | 0.78 ± 0.03b |
| 5 | 0.61 ± 0.02c | 0.94 ± 0.02c | 0.76 ± 0.04c | 0.75 ± 0.02c | 0.78 ± 0.02c | 0.76 ± 0.02c |
| 10 | 0.51 ± 0.02d | 0.89 ± 0.03d | 0.53 ± 0.02d | 0.72 ± 0.03d | 0.75 ± 0.02d | 0.72 ± 0.03d |
| 15 | 0.36 ± 0.02e | 0.85 ± 0.03e | 0.43 ± 0.03e | 0.63 ± 0.03e | 0.64 ± 0.02e | 0.62 ± 0.03e |
| Holding time (min) | Normalized dissolved oxygen (DO2) | |||||
| Gallic acid | ρ-Coumaric acid | Tannic acid | ||||
| 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | 0.1 MPa | 600 MPa | |
| 0 | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a |
| 1 | 0.18 ± 0.03b | 0.68 ± 0.02b | 0.84 ± 0.02b | 0.47 ± 0.03b | 0.63 ± 0.03b | 0.27 ± 0.03b |
| 5 | 0.17 ± 0.02b | 0.41 ± 0.04c | 0.27 ± 0.04c | 0.18 ± 0.01c | 0.33 ± 0.02c | 0.21 ± 0.01c |
| 10 | 0.13 ± 0.02c | 0.12 ± 0.03d | 0.23 ± 0.03d | 0.11 ± 0.02d | 0.30 ± 0.03d | 0.19 ± 0.02d |
| 15 | 0.11 ± 0.02c | 0.11 ± 0.03d | 0.21 ± 0.02e | 0.10 ± 0.02d | 0.11 ± 0.02e | 0.13 ± 0.02e |
3.6. Different Concentrations of Catechin and Gallic Acid

3.7. Possible Reaction Mechanisms of Phenolic Antioxidants
| Holding time (min) | Antioxidant added (500 mg kg−1) | ||
|---|---|---|---|
| Gallic acid | Catechin | Caffeic acid | |
| 0 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| 1 | 0.94 ± 0.02 | 0.72 ± 0.03 | 0.94 ± 0.02 |
| 5 | 0.81 ± 0.01 | 0.53 ± 0.04 | 0.81 ± 0.01 |
| 10 | 0.61 ± 0.02 | 0.44 ± 0.05 | 0.61 ± 0.04 |
| 15 | 0.48 ± 0.07 | 0.40 ± 0.02 | 0.48 ± 0.05 |
| k1 (min−1) | 0.029 ± 0.006 | 0.078 ± 0.005 | 0.014 ± 0.005 |
| k2 (min−1) | 0.081 ± 0.006 | 0.051 ± 0.019 | 0.061 ± 0.025 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure-based technologies in the food industry. Ann. Rev. Food Sci. Tech. 2015, 6, 1–28. [Google Scholar] [CrossRef]
- Gupta, R.; Kopec, R.E.; Schwartz, S.J.; Balasubramaniam, V.M. Combined pressure-temperature on carotenoid retention and bioaccessibility in tomato juice. J. Agric. Food Chem. 2011, 59, 7808–7817. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Chemical reactions in food systems at high hydrostatic pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]
- Oey, I.; Verlinde, P.; Hendrickx, M.E.; van Loey, A. Temperature and pressure stability of l-ascorbic acid and/or 6s 5-methyltetrahydrofolic acid: A kinetic study. Eur. Food Res. Tech. 2006, 223, 71–77. [Google Scholar] [CrossRef]
- Verbeyst, L.; Bogaerts, R.; van der Plancken, I.; Hendrickx, M.E.; van Loey, A. Modelling of vitamin C degradation during thermal and high-pressure treatments of red fruits. Food Bioprocess. Tech. 2013, 6, 1015–1023. [Google Scholar] [CrossRef]
- Butz, P.; Serfert, Y.; Garcia, A.F.; Dieterich, S.; Lindauer, R.; Bognar, A.; Tauscher, B. Influence of high-pressure treatment at 25 degrees C and 80 degrees C on folates in orange juice and model media. J. Food Sci. 2004, 69, S117–S121. [Google Scholar]
- Matser, A.A.; Krebbers, B.; van den Berg, R.W.; Bartels, P.V. Advantages of high pressure sterilisation on quality of food products. Trends Food Sci. Technol. 2004, 15, 79–85. [Google Scholar] [CrossRef]
- Verbeyst, L.; Oey, I.; van der Plancken, I.; Hendrickx, M.; van Loey, A. Kinetic study on the thermal and pressure degradation of anthocyanins in strawberries. Food Chem. 2010, 123, 269–274. [Google Scholar] [CrossRef]
- Gupta, R.; Balasubramaniam, V.M.; Schwartz, S.J.; Francis, D.M. Storage stability of lycopene in tomato juice subjected to combined pressure-heat treatments. J. Agric. Food Chem. 2010, 58, 8305–8313. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Modeling the retention kinetic of conjugated linoleic acid during high-pressure sterilization of milk. Food Res. Int. 2014, 62, 169–176. [Google Scholar] [CrossRef]
- Sevenich, R.; Kleinstueck, E.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. High-pressure thermal sterilization: Food safety and food quality of baby food puree. J. Food Sci. 2014, 79, M230–M237. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.E.; Pariza, M. The role of conjugated linoleic acid (CLA) in health. Int. Dairy J. 1998, 8, 459–462. [Google Scholar] [CrossRef]
- Campbell, W.; Drake, M.A.; Larick, D.K. The impact of fortification with conjugated linoleic acid (CLA) on the quality of fluid milk. J. Dairy Sci. 2003, 86, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Herzallah, S.M.; Humeid, M.A.; Al-Ismai, K.M. Effect of heating and processing methods of milk and dairy products on conjugated linoleic acid and trans fatty acid isomer content. J. Dairy Sci. 2005, 88, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Retention of bioactive lipids in heated milk: Experimental and modelling. Food Bioprod. Process. 2015, 94, 290–296. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Leal-Dávila, M.; Curtis, J.M.; Saldaña, M.D.A. Oxidative stability of ultra high temperature milk enriched in conjugated linoleic acid and trans-vaccenic acid. Int. Dairy J. 2015, 43, 70–77. [Google Scholar] [CrossRef]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A.; Torres, J.A.; Kennelly, J.J. Effect of pressure-assisted thermal sterilization on conjugated linoleic acid (CLA) content in CLA-enriched milk. Innov. Food Sci. Emerg. Tech. 2012, 16, 291–297. [Google Scholar] [CrossRef]
- Bell, J.A.; Griinari, J.M.; Kennelly, J.J. Effect of safflower oil, flaxseed oil, monensin, and vitamin E on concentration of conjugated linoleic acid in bovine milk fat. J. Dairy Sci. 2006, 89, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Rasanavagam, V.; Balasubramaniam, V.M.; Ting, E.; Sizer, C.E.; Bush, C.; Anderson, C. Compression heating of selected fatty food materials during high pressure processing. J. Food Sci. 2003, 68, 2544–2559. [Google Scholar]
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A.; Kennelly, J.J. Kinetics of non-isothermal oxidation of anhydrous milk fat rich in conjugated linoleic acid using differential scanning calorimetry. J. Therm. Anal. Calorim. 2012, 107, 973–981. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Gänzle, M.G.; Saldaña, M.D.A. High-pressure and temperature effects on the inactivation of Bacillus amyloliquefaciens, alkaline phosphatase and storage stability of conjugated linoleic acid in milk. Innov. Food Sci. Emerg. Tech. 2014, 16, 291–297. [Google Scholar] [CrossRef]
- Neuman, R.C. Pressure effects as mechanistic probes of organic radical reactions. Accounts Chem. Res. 1972, 5, 381–387. [Google Scholar] [CrossRef]
- Neuman, R.C.; Amrich, M.J. High pressure studies. X. Activation volumes for homolysis of single bonds. J. Am. Chem. Soc. 1972, 98, 2730–2733. [Google Scholar] [CrossRef]
- Destaillats, F.; Angers, P. Thermally induced formation of conjugated isomers of linoleic acid. Eur. J. Lipid Sci. Tech. 2005, 107, 167–172. [Google Scholar] [CrossRef]
- Destaillats, F.; Angers, P. Evidence for [1,5] Sigmatropic rearrangements of CLA in heated oils. Lipids 2002, 37, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, N.S. Liquid Phase High Pressure Chemistry; John Wiley: Toronto, Canada, 1981. [Google Scholar]
- Lindmark-Mansson, H.; Akesson, B. Antioxidative factors in milk. Br. J. Nutr. 2000, 84, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Effect of high-pressure-moderate-temperature processing on the volatile profile of milk. J. Agr. Food Chem. 2006, 54, 9184–9192. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr. 2006, 46, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Chimi, H.; Cillard, J.; Cillard, P.; Rahmani, M. Peroxyl and hydroxyl radical scavenging activity of some natural phenolic antioxidants. J. Am. Oil Chem. Soc. 1991, 68, 307–312. [Google Scholar] [CrossRef]
- Khan, N.S.; Ahmad, A.; Hadi, S.M. Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem-Biol. Interact. 2000, 125, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Kim, T.J.; Silva, J.L.; Kim, M.K.; Jung, Y.S. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010, 118, 740–746. [Google Scholar] [CrossRef]
- Huppertz, T.; Smiddy, M.A.; Vpadhyay, V.K.; Kelly, A.L. High-pressure-induced changes in bovine milk: A review. Int. J. Dairy Technol. 2006, 59, 58–66. [Google Scholar] [CrossRef]
- Hamann, S.D.; Linton, M. Influence of pressure on the ionization of substituted phenols. J. Chem. Soc. Faraday Trans. 1 1974, 70, 2239–2249. [Google Scholar] [CrossRef]
- Ohara, K.; Ichimura, Y.; Nagaoka, S. Kinetic study of singlet-oxygen quenching by caffeic acid and related phenols. Bull. Chem. Soc. Jpn. 2009, 82, 689–691. [Google Scholar] [CrossRef]
- Schamberger, G.P.; Labuza, T.P. Effect of green tea flavonoids on Maillard browning in UHT milk. LWT Food Sci. Technol. 2007, 40, 1410–1417. [Google Scholar] [CrossRef]
- Garro-Galvez, J.M.; Fechtal, M.; Riedl, B. Gallic acid as a model of tannins in condensation with formaldehyde. Thermochim. Acta 1996, 274, 149–163. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Combined Effect of Pressure-Assisted Thermal Processing and Antioxidants on the Retention of Conjugated Linoleic Acid in Milk. Foods 2015, 4, 65-79. https://doi.org/10.3390/foods4020065
Martinez-Monteagudo SI, Saldaña MDA. Combined Effect of Pressure-Assisted Thermal Processing and Antioxidants on the Retention of Conjugated Linoleic Acid in Milk. Foods. 2015; 4(2):65-79. https://doi.org/10.3390/foods4020065
Chicago/Turabian StyleMartinez-Monteagudo, Sergio I., and Marleny D.A. Saldaña. 2015. "Combined Effect of Pressure-Assisted Thermal Processing and Antioxidants on the Retention of Conjugated Linoleic Acid in Milk" Foods 4, no. 2: 65-79. https://doi.org/10.3390/foods4020065





