Microbial Profile of Soil-Free versus In-Soil Grown Lettuce and Intervention Methodologies to Combat Pathogen Surrogates and Spoilage Microorganisms on Lettuce
Abstract
:1. Introduction
1.1. Leafy Greens
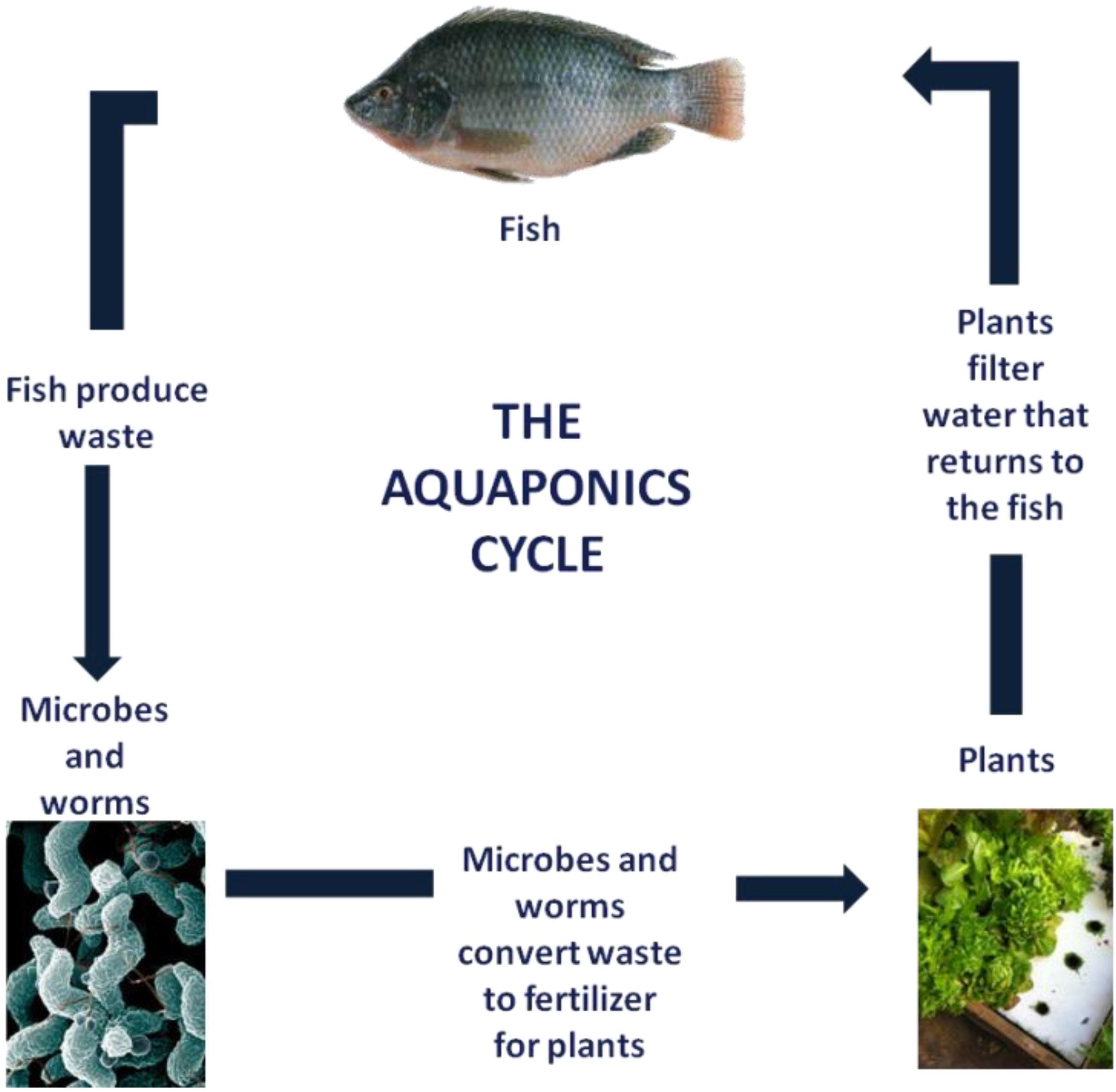
1.2. Aquaponics
1.3. Interventions
2. Experimental Section
2.1. Sampling
2.1.1. Obtaining Leafy Green Samples
2.1.2. Processing Samples for Microbial Quality Analysis
2.1.3. Microbial Analysis
2.2. Intervention
2.2.1. Pathogen Surrogate Growth Conditions and Lettuce Inoculation
2.2.2. Intervention Study
2.3. Statistical Analysis
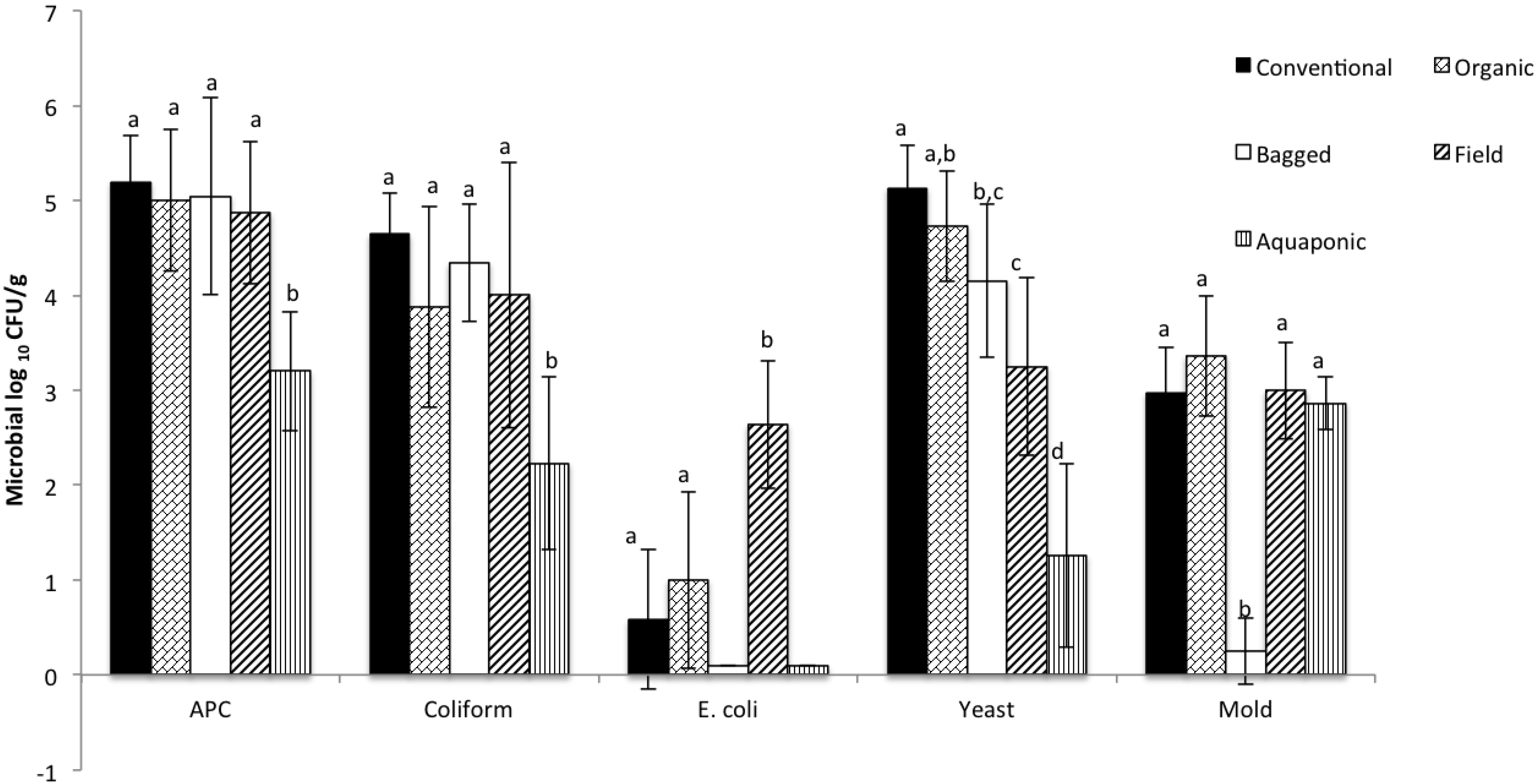
3. Results and Discussion
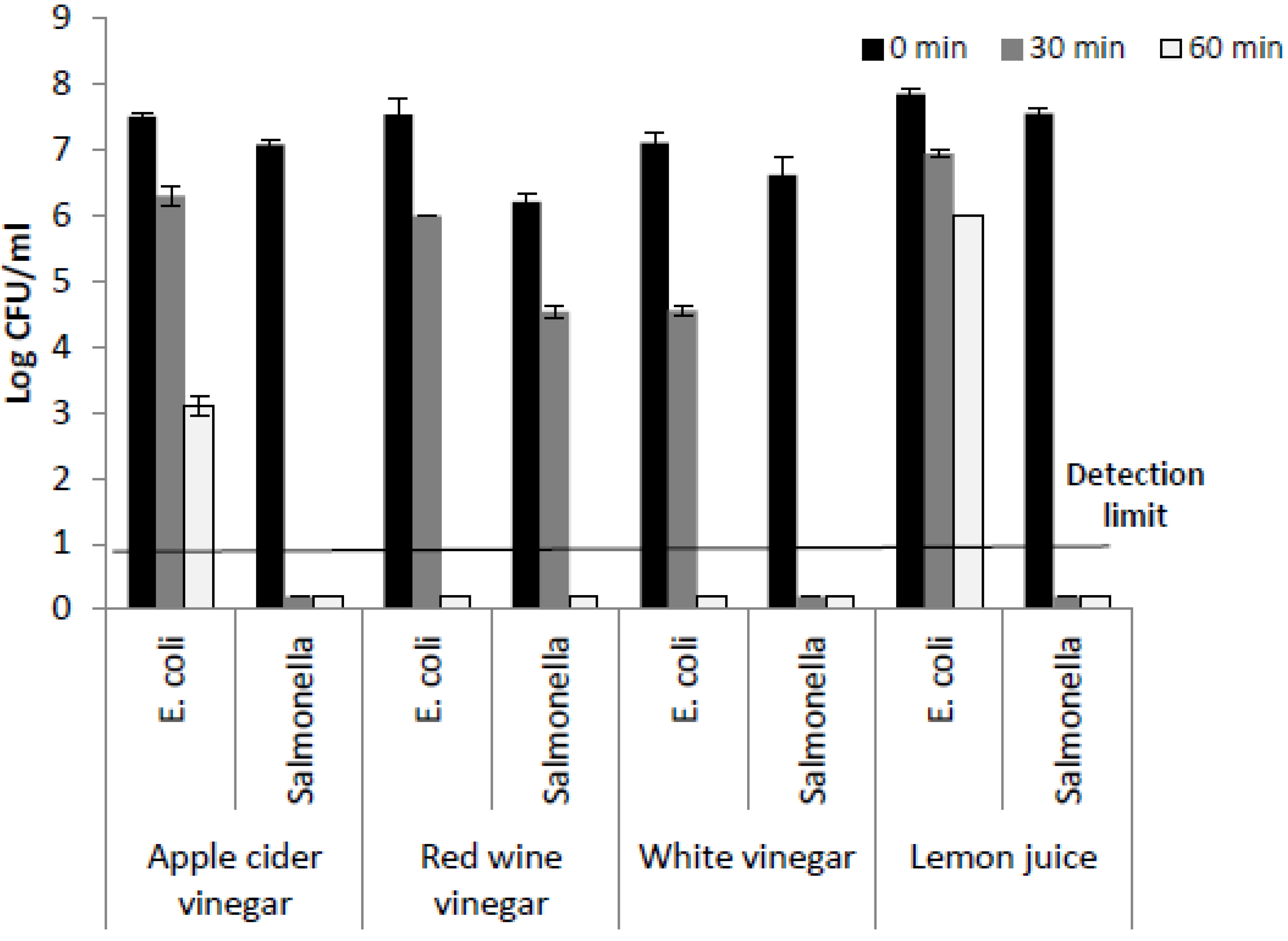
| 1.25 % Acetic Acid | 2.5% Acetic Acid | 5% Acetic Acid | |||
|---|---|---|---|---|---|
| E. coli | Salmonella | E. coli | Salmonella | E. coli | Salmonella |
| 0.73 ± 0.23 | 1.55 ± 0.23 | 1.43 ± 0.15 | 1.36 ± 0.33 | 0.13 ± 0.15 | 0.36 ± 0.05 |
| Aerobic Plate Counts | Coliforms | E. coli | Yeast | Mold |
|---|---|---|---|---|
| 2.18 ± 0.18 | 2.5 ± 0.51 | 1.46 ± 1.51 | 3.7 ± 1.4 | 2.9 ± 0.4 |
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Investigation Update: Multistate Outbreak of E. coli O157:H7 Infections Linked to Romaine Lettuce. Available online: http://www.cdc.gov/ecoli/2011/ecoliO157/romainelettuce/032312/index.html (accessed on 11 August 2013).
- Investigation Update: Multistate Outbreak of Human E. coli O145 Infections Linked to Shredded Romaine Lettuce from a Single Processing Facility. Available online: http://www.cdc.gov/ecoli/2010/ecoli_o145/index.html (accessed on 11 August 2013).
- Lienemann, T.; Niskanen, T.; Guedes, S.; Siitonen, A.; Kuusi, M.; Rimhanen-Finne, R. Iceberg lettuce as suggested source of a nationwide outbreak caused by two Salmonella serotypes, Newport and Reading, in Finland in 2008. J. Food Prot. 2011, 74, 1035–1040. [Google Scholar] [CrossRef]
- Outbreaks of Gastroenteritis Linked to Lettuce. Available online: http://www.eurosurveillance.org/images/dynamic/EE/V15N06/art19484.pdf (accessed on 14 July 2013).
- Oliveira, M.; Usall, J.; Viñas, I.; Anguera, M.; Gatius, F.; Abadias, M. Microbiological quality of fresh lettuce from organic and conventional production. Food Microbiol. 2010, 27, 679–684. [Google Scholar] [CrossRef]
- National Organic Program. Available online: http://www.ams.usda.gov/AMSv1.0/ams.fetchTemplateData.do?template=TemplateA&navID=NationalOrganicProgram&leftNav=NationalOrganicProgram&page=NOPNationalOrganicProgramHome&acct=AMSPW (accessed on 11 July 2013).
- Food Safety Modernization Act. Available online: http://www.fda.gov/Food/FoodSafety/FSMA/default.htm (accessed on 11 July 2013).
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; University of California: Davis, CA, USA, 2002. [Google Scholar]
- Stenstrom, T.A. Bacterial hydrophobicity, an overall parameter for the measurement of adhesion potential to soil particles. Appl. Environ. Microbiol. 1989, 55, 142–147. [Google Scholar]
- Wei, J.; Jin, Y.; Sims, T.; Kniel, K.E. Murine norovirus-1 internalization into Lactuca sativa during irrigation. Appl. Environ. Microbiol. 2011, 77, 2508–2512. [Google Scholar] [CrossRef]
- Jiang, X.; Morgan, J.; Doyle, M.P. Fate of Escherichia coli O157:H7 in manure-amended soil. Appl. Environ. Microbiol. 2002, 68, 2605–2609. [Google Scholar] [CrossRef]
- Guo, X.; Chen, J.; Brackett, R.E.; Beuchat, L.R. Survival of Salmonella on tomatoes stored at high relative humidity, in soil, and on tomatoes in contact with soil. J. Food Prot. 2002, 65, 274–279. [Google Scholar]
- A Preliminary Study of Microbial Water Quality Related to Food Safety in Recirculating Aquaponic Fish and Vegetable Production Systems. Available online: http://www.ctahr.hawaii.edu/oc/freepubs/pdf/FST-51.pdf (accessed on 21 October 2013).
- On-Farm Food Safety: Aquaponics. Available online: http://www.ctahr.hawaii.edu/oc/freepubs/pdf/FST-38.pdf (accessed on 21 October 2013).
- Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture. Available online: http://www2.ca.uky.edu/wkrec/454fs.PDF (accessed on 21 October 2013).
- Goodman, E.R. Aquaponics: Community and Economic Development. Masters Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2011. [Google Scholar]
- Zerio-Egli, C. Economical Post-Harvest Practices for Leafy Greens Grown on Texas Small Farms. Master’s Thesis, University of Houston, Houston, TX, USA, 2012. [Google Scholar]
- Guidance for Industry. Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. Available online: http://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ProduceandPlanProducts/UCM169112.pdf (accessed on 11 August 2013).
- Entani, E.; Asai, M.; Tsujihata, S.; Tsukamoto, Y.; Ohta, M. Antibacterial action of vinegar against food-borne pathogenic bacteria including Escherichia coli O157:H7. J. Food Prot. 1998, 174, 953–959. [Google Scholar]
- Wu, F.; Doyle, M.; Beuchat, L.; Wells, J.; Mintz, E.; Swaminathan, B. Fate of Shigella sonnei on parsley and methods of disinfection. J. Food Prot. 2000, 174, 568–572. [Google Scholar]
- Rutala, W.A.; Barbee, S.L.; Aguiar, N.C.; Sobsey, M.D.; Weber, D.J. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infect. Control Hosp. Epidemiol. 2000, 21, 33–38. [Google Scholar]
- FDA Survey of Domestic Fresh Produce-Domestic Produce Assignment. Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm118297.htm (accessed on 3 October 2013).
- Ailes, E.C.; Leon, J.S.; Jaykus, L.A.; Johnston, L.M.; Clayton, H.A.; Blanding, S.; Kleinbaum, D.G.; Backer, L.C.; Moe, C.L. Microbial concentrations on fresh produce are affected by postharvest processing, importation, and season. J. Food Prot. 2008, 71, 2389–2397. [Google Scholar]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory quality, bioactive constituents and microbiological quality of green and red fresh-cut lettuces (Lactuca sativa L.) are influenced by soil and soilless agricultural production systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Maffei, D.F.; de Arruda Silveira, N.F.; Catanozi, M.P.L.M. Microbiological quality of organic and conventional vegetables sold in Brazil. Food Control 2012, 29, 226–230. [Google Scholar]
- Valentin-Bon, I.; Jacobson, A.; Monday, S.R.; Feng, P.C.H. Microbiological quality of bagged cut spinach and lettuce mixes. Appl. Environ. Microbiol. 2008, 74, 1240–1242. [Google Scholar] [CrossRef]
- Kase, J.A.; Borenstein, S.; Blodgett, R.J.; Feng, P.C.H. Microbial quality of bagged baby spinach and romaine lettuce: Effects of top versus bottom sampling. J. Food Prot. 2012, 75, 132–136. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Brenes, M.; de Castro, A. Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sirsat, S.A.; Neal, J.A. Microbial Profile of Soil-Free versus In-Soil Grown Lettuce and Intervention Methodologies to Combat Pathogen Surrogates and Spoilage Microorganisms on Lettuce. Foods 2013, 2, 488-498. https://doi.org/10.3390/foods2040488
Sirsat SA, Neal JA. Microbial Profile of Soil-Free versus In-Soil Grown Lettuce and Intervention Methodologies to Combat Pathogen Surrogates and Spoilage Microorganisms on Lettuce. Foods. 2013; 2(4):488-498. https://doi.org/10.3390/foods2040488
Chicago/Turabian StyleSirsat, Sujata A., and Jack A. Neal. 2013. "Microbial Profile of Soil-Free versus In-Soil Grown Lettuce and Intervention Methodologies to Combat Pathogen Surrogates and Spoilage Microorganisms on Lettuce" Foods 2, no. 4: 488-498. https://doi.org/10.3390/foods2040488




