Imaging in Patients with Bisphosphonate-Associated Osteonecrosis of the Jaws (MRONJ)
Abstract
:1. Introduction
2. Anatomical Imaging
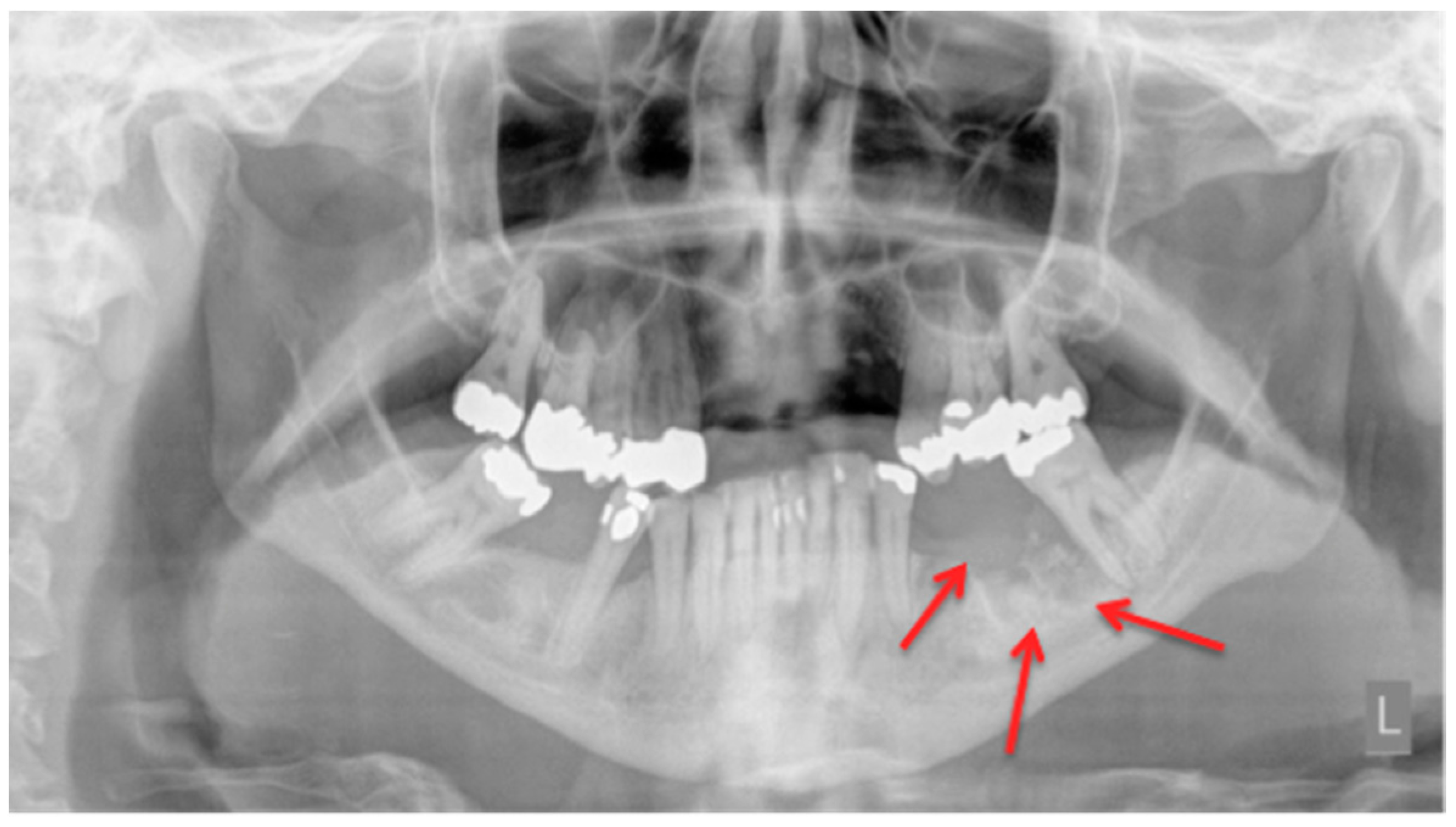
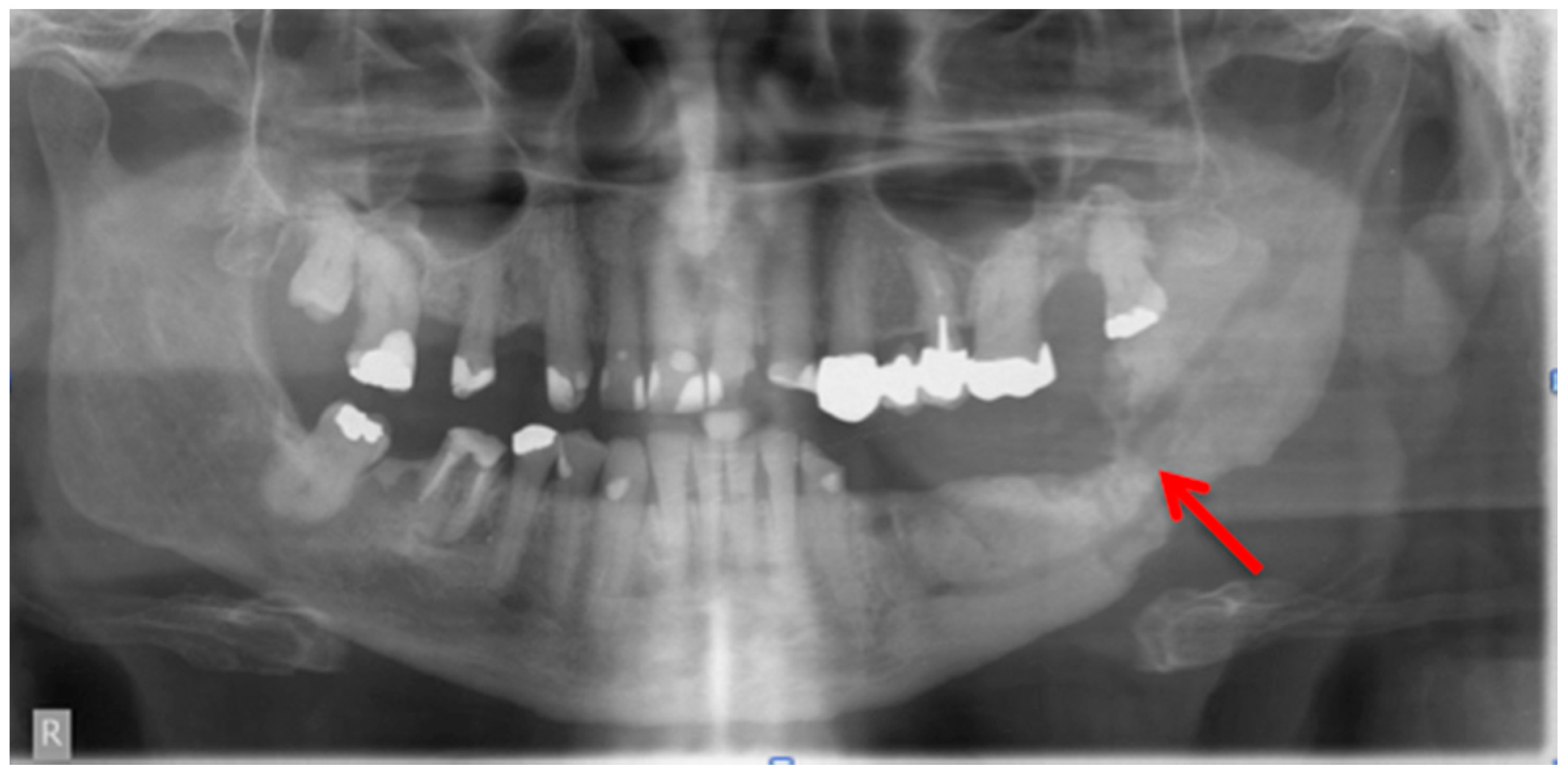
2.1. Panoramic Radiographs
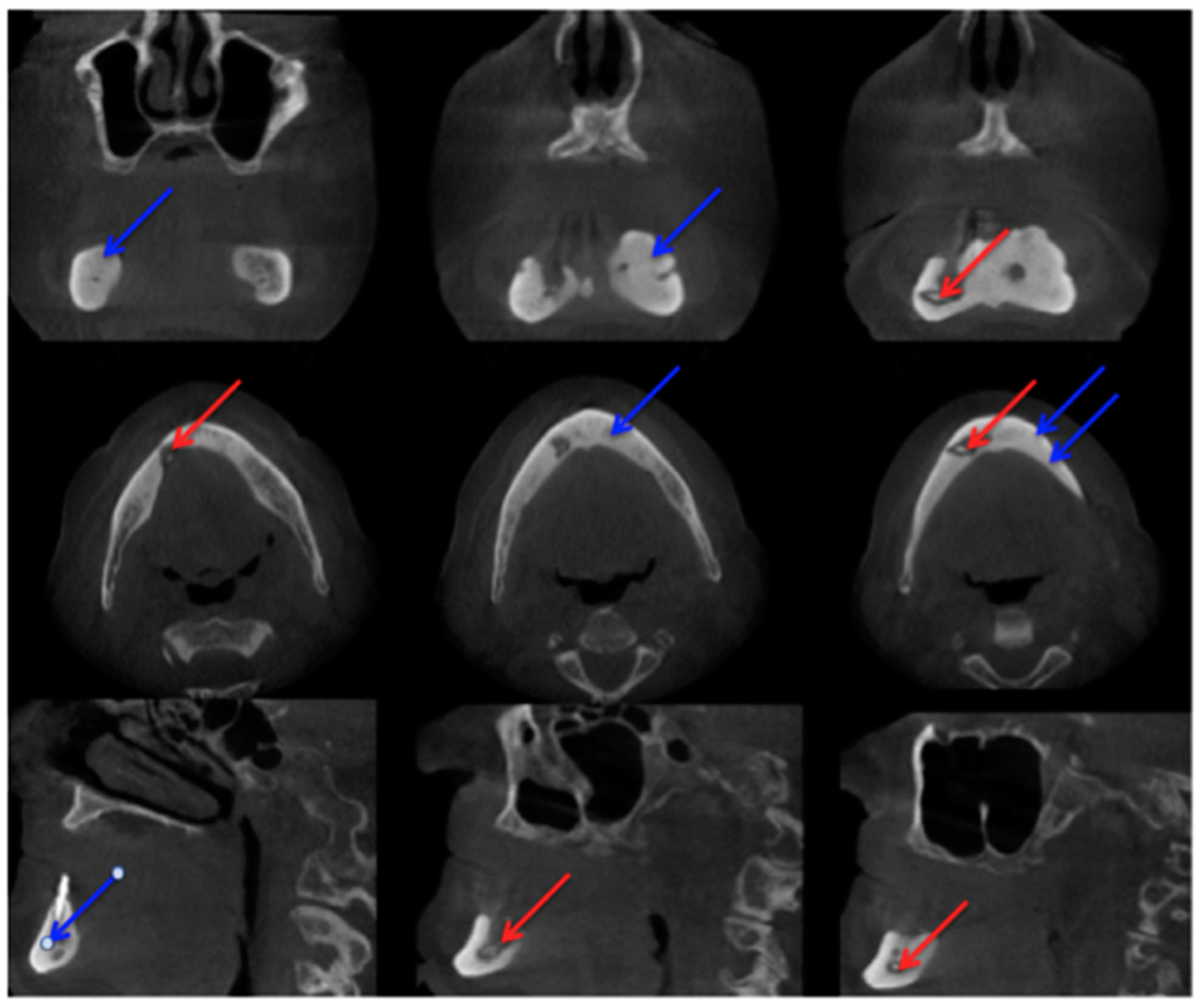
2.2. Cone-Beam Computed Tomography
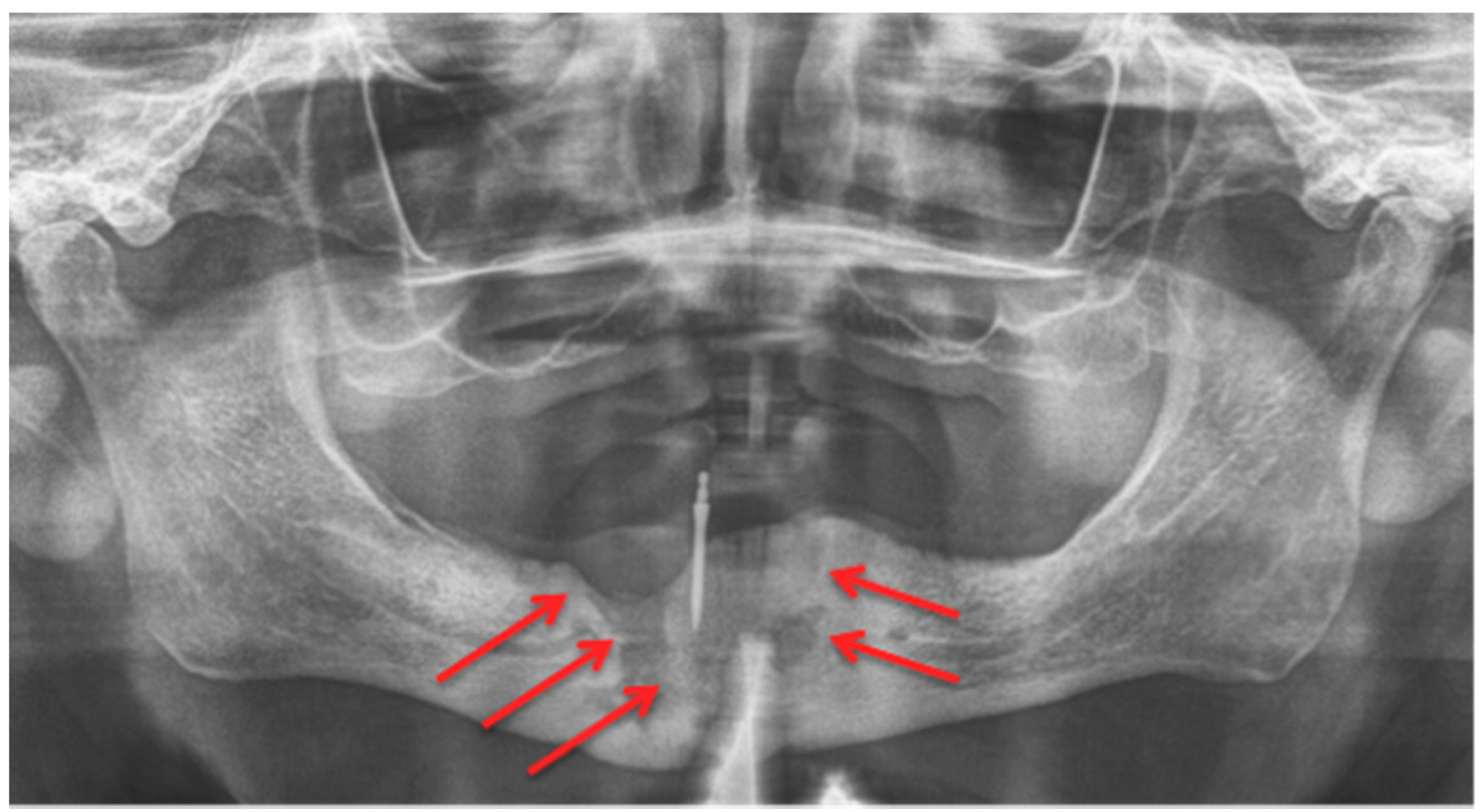
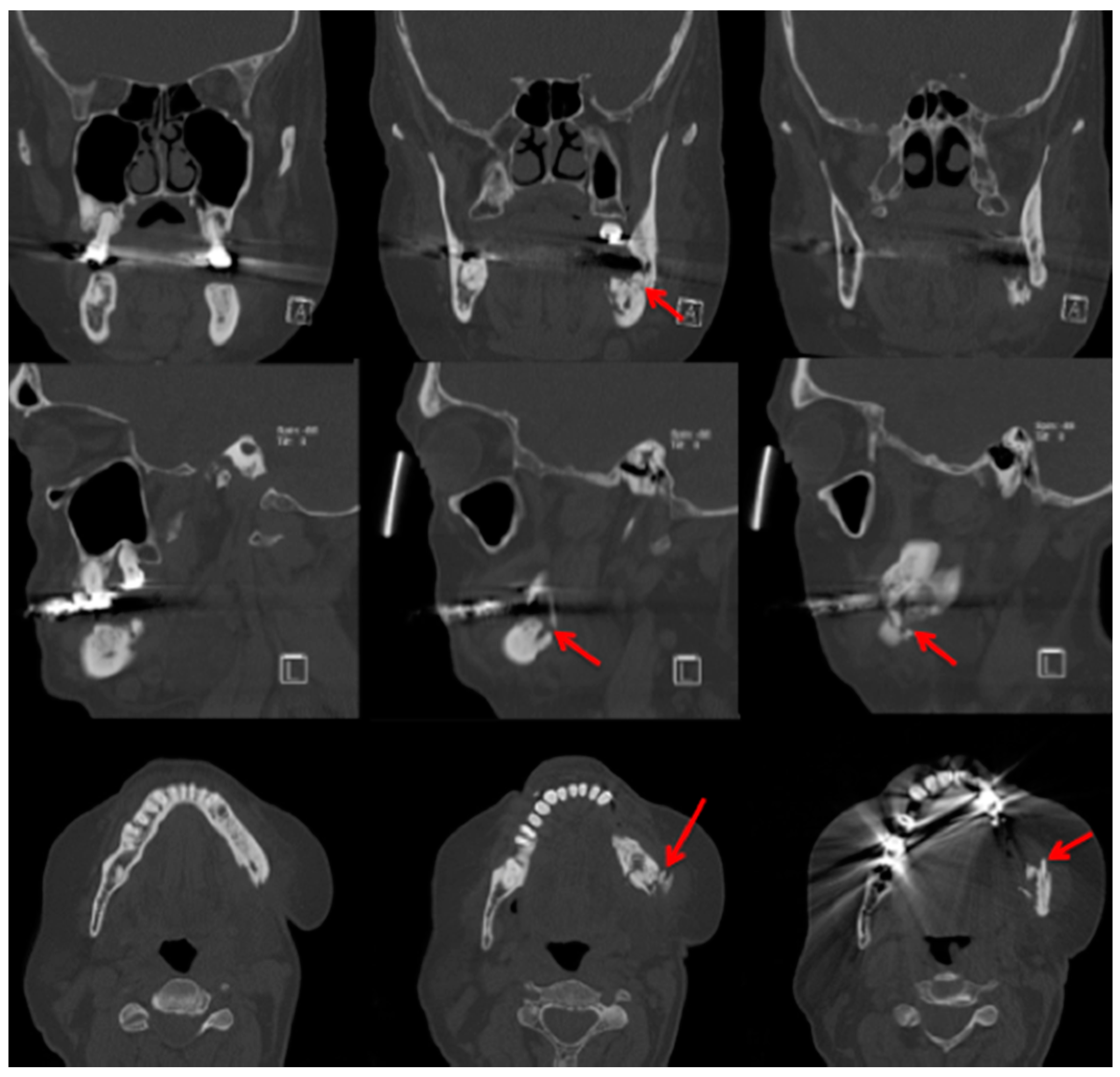
2.3. Computed Tomography
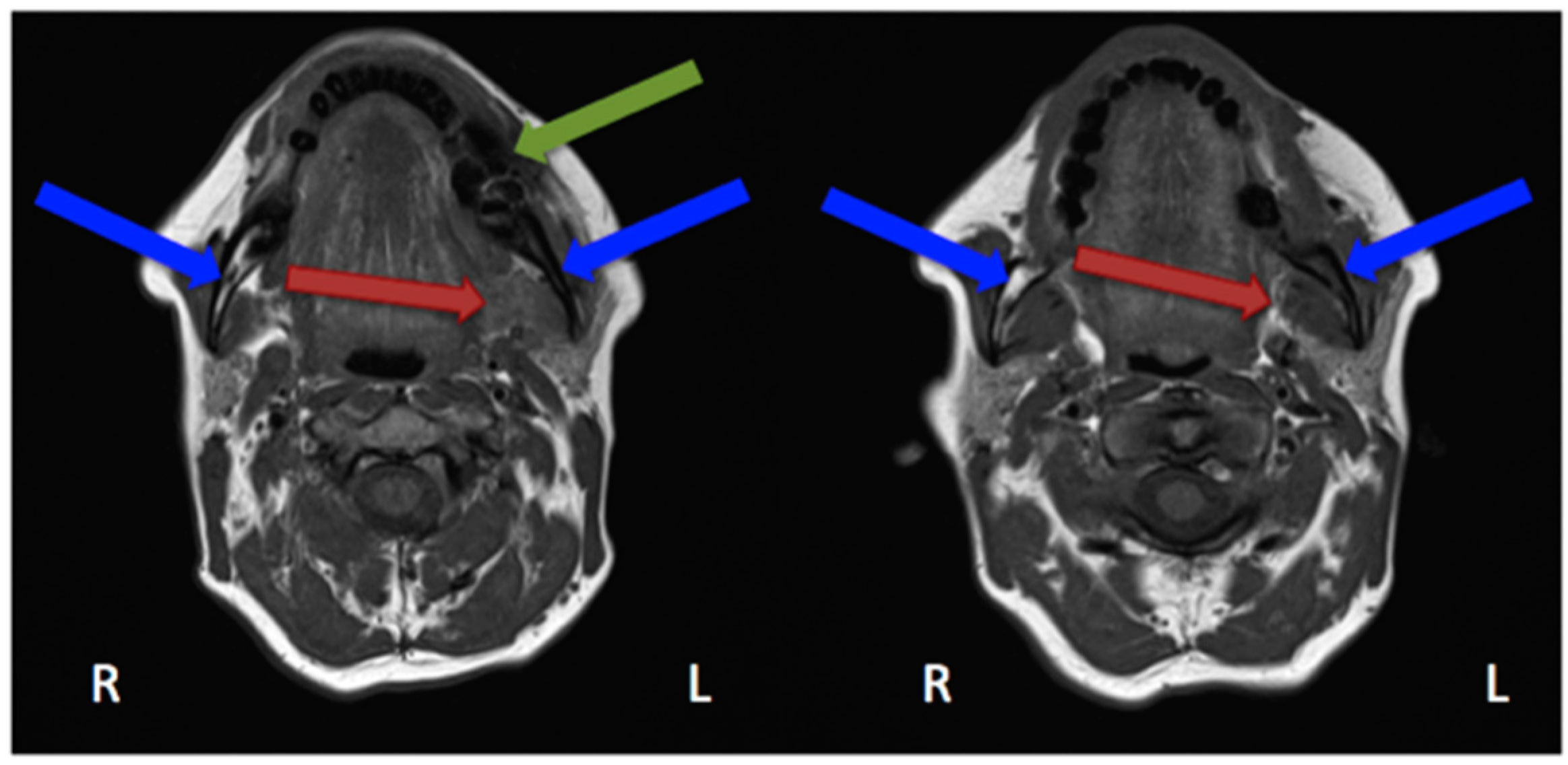
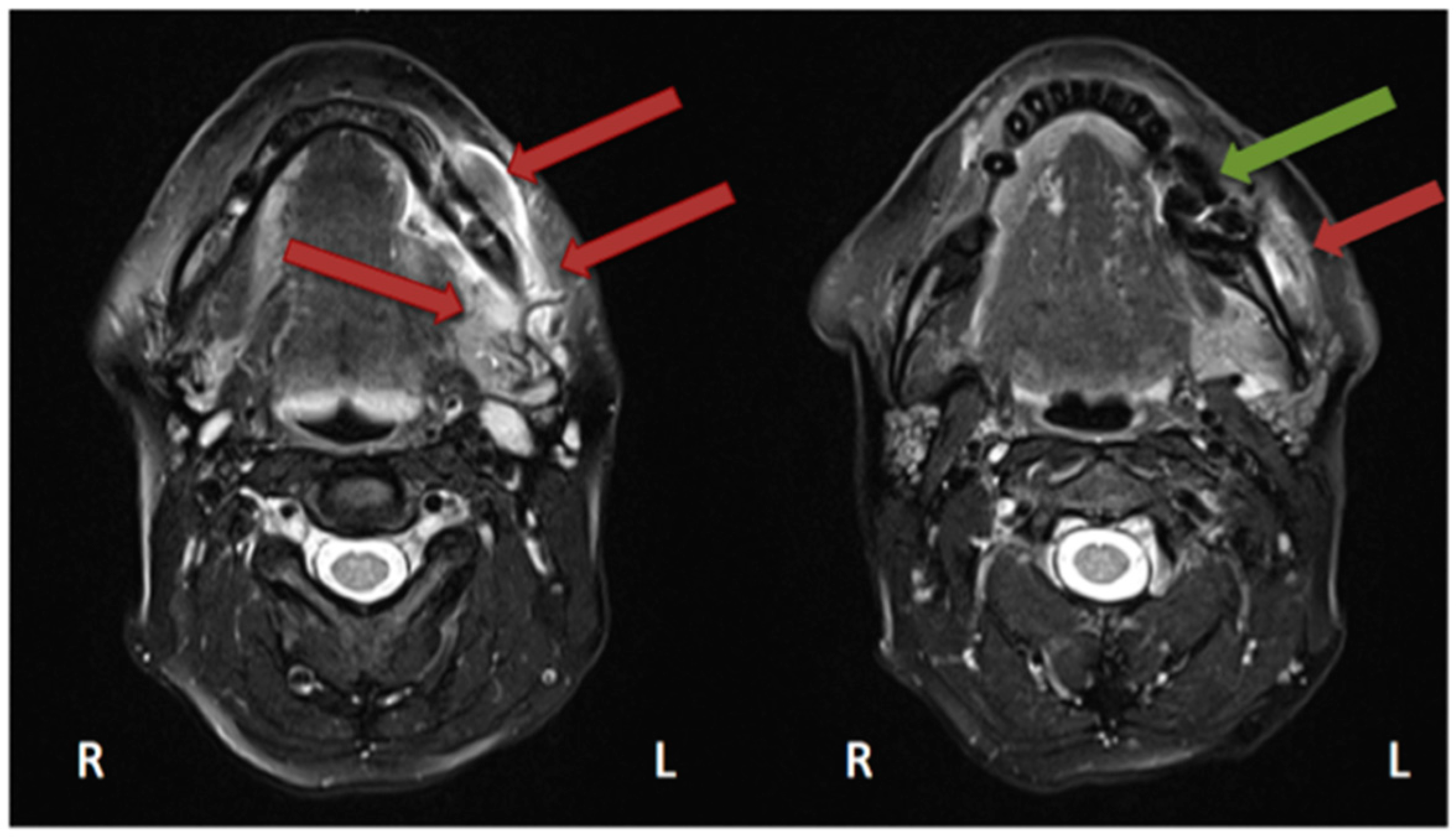
2.4. Magnetic Resonance Imaging
3. Functional Imaging
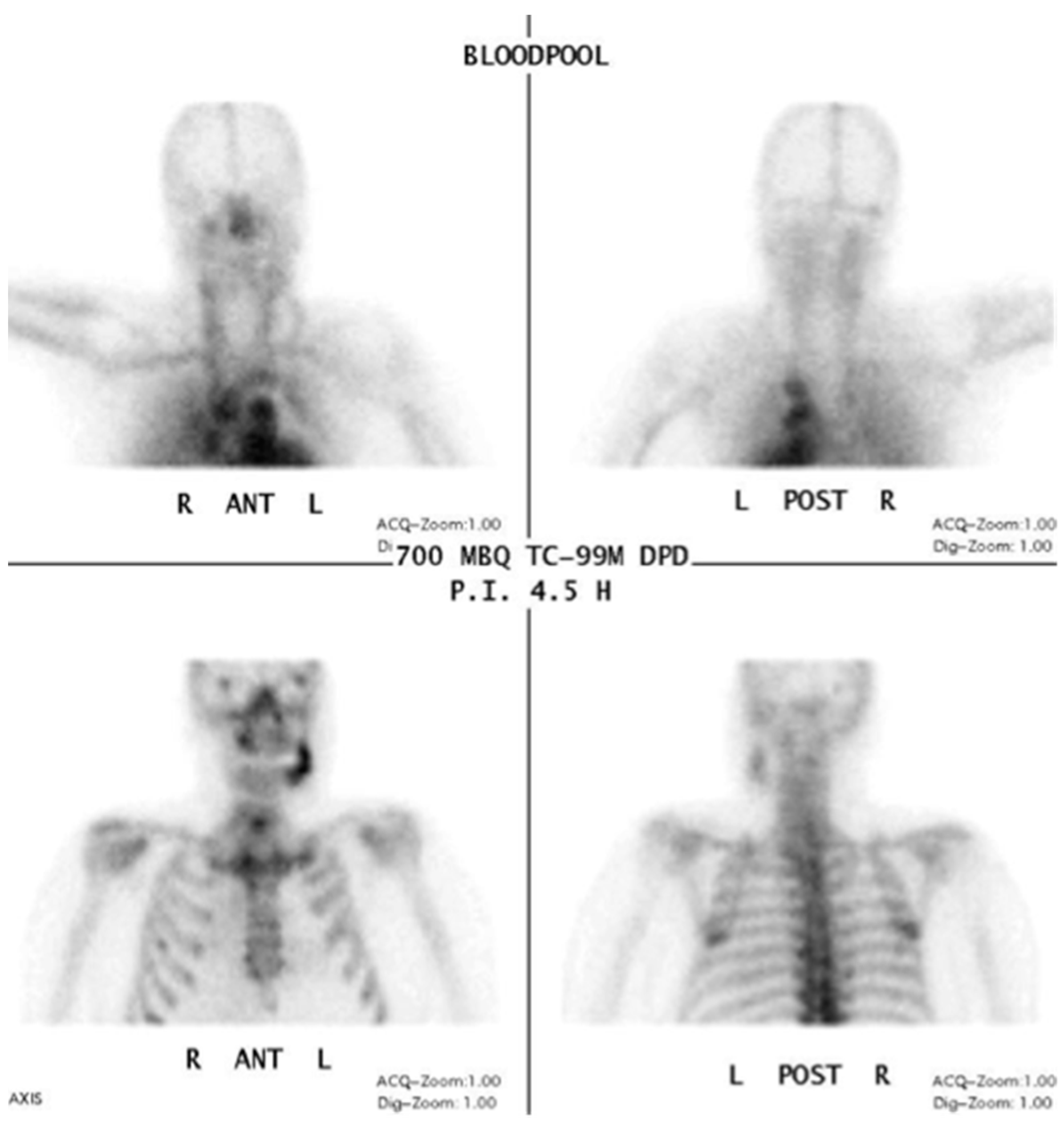
3.1. 99Tcm-DPD/99Tcm-MDP Bone Scan/Scintigraphy/Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT)
3.2. 18F-FDG Positron Emission Tomography/Computed Tomography (PET/CT)
3.3. Fluorescence-Guided Bone Resection/Visually Enhanced Lesion Scope (VELscope®)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; du Bois, A.; Buch, L.; Harter, P.; Grötz, K.A. Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 2009, 115, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; Frickhofen, N.; Gamm, H.; Beck, J.; Reinsch, L.; Blum, C.; Grötz, K.A.; Wagner, W. Prevalence of bisphosphonate associated osteonecrosis of the jaws in multiple myeloma patients. Head Face Med. 2010, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Al-Nawas, B.; Grötz, K.A.; Thomas, C.; Thuroff, J.W.; Zinser, V.; Gamm, H.; Beck, J.; Wagner, W. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur. Urol. 2008, 54, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Laux, C.; Sagheb, K. Radiologic bone loss in patients with bisphosphonate-associated osteonecrosis of the jaws: A case-control study. Clin. Oral Investig. 2014, 18, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, 2698–2699. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Vassiliou, V.; Tselis, N.; Kardamakis, D. Osteonecrosis of the jaws: Clinicopathologic and radiologic characteristics, preventive and therapeutic strategies. Strahlenther. Onkol. 2010, 186, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.S.; Katayama, E.; Bombana, A.C.; Marques, M.M. Cytotoxicity analysis of alendronate on cultured endothelial cells and subcutaneous tissue. A pilot study. Dent. Traumatol. 2005, 21, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar]
- Phal, P.M.; Myall, R.W.; Assael, L.A.; Weissman, J.L. Imaging findings of bisphosphonate-associated osteonecrosis of the jaws. AJNR Am. J. Neuroradiol. 2007, 28, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone osteonecrosis/osteopetrosis of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.C.; Jaguar, G.C.; Moreira, C.R.; Neves, E.G.; Fonseca, F.P.; Pedreira, E.N. Radiographic evaluation of maxillofacial region in oncology patients treated with bisphosphonates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Arce, K.; Assael, L.A.; Weissman, J.L.; Markiewicz, M.R. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J. Oral Maxillofac. Surg. 2009, 67, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.R.; Chen, C.S.; Leroux, B.G.; Lee, P.P.; Hollender, L.G.; Lloid, M.; Drew, S.P.; Schubert, M.M. Mandibular inferior cortical bone thickness on panoramic radiographs in patients using bisphosphonates. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Watkins, B.A.; Lyng, G.D.; Lerman, M.A.; Anderson, K.C. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009, 45, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Hinkmann, F.M.; Lell, M.M.; Fenner, M.; Vairaktaris, E.; Neukam, F.W.; Nkenke, E. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin. Oral Investig. 2010, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.; Sheehy, N.; Bae, E.H.; Friedland, B.; Lerman, M.; Woo, S. Dental panoramic radiographic evaluation in bisphosphonate-associated osteonecrosis of the jaws. Oral Dis. 2009, 15, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.D.; Scoletta, M.; Cassione, F.B.; Migliaretti, G.; Mozzati, M. Computerized tomographic findings in bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Saia, G.; Bettini, G.; Tronchet, A.; Totola, A.; Bedogni, G.; Ferronato, G.; Nocini, P.F.; Blandamura, S. Long-term outcomes of surgical resection of the jaws in cancer patients with bisphosphonate-related osteonecrosis. Oral Oncol. 2011, 47, 420–424. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Radiation Protection no 136: European Guidelines on Radiation Protection in Dental Radiology. The Safe Use of Radiographs in Dental Practice. 2004. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/136.pdf (accessed on 10 May 2016).
- Yalcin, E.D.; Gungormus, M. Cone-beam Computed Tomography Imaging Findings of Bisphosphonate-related Osteonecrosis of the Jaws (BRONJ): A Review Article. Int. J. Dent. Sci. Res. 2015, 3, 111–115. [Google Scholar]
- Torres, S.R.; Chen, C.S.; Leroux, B.G.; Lee, P.P.; Hollender, L.G.; Santos, E.C.; Drew, S.P.; Hung, K.C.; Schubert, M.M. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, R.; Koral, E.; Zemann, W.; Jacobsen, C.; Andreisek, G.; Metzler, P. Cone beam computed tomography for diagnosis of bisphosphonate-related osteonecrosis of the jaw: Evaluation of quantitative and qualitative image parameters. Skelet. Radiol. 2014, 43, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Heufelder, M.; Lorenz, K.; Liese, S.; Liese, J.; Helmrich, J.; Schramm, A.; Hemprich, A.; Hirsch, E.; Winter, K. Prevalence of cone beam computed tomography imaging findings according to the clinical stage of bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Olutayo, J.; Olubanwo Agbaje, J.; Jacobs, R.; Verhaeghe, V.; Vande Velde, F.; Vinckier, F. Bisphosphonate-related osteonecrosis of the jaw bone: Radiological pattern and the potential role of CBCT in early diagnosis. J. Oral Maxillofac. Res. 2010, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Treister, N.S.; Friedland, B.; Woo, S.B. Use of cone-beam computerized tomography for evaluation of bisphosphonate-associated osteonecrosis of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, R.; Fischer, D.R.; Metzler, P.; Andreisek, G.; Nanz, D.; Jacobsen, C.; Schmid, D.T. Bisphosphonate-induced osteonecrosis of the jaw: Comparison of disease extent on contrast-enhanced MR imaging, [18F] fluoride PET/CT, and conebeam CT imaging. AJNR Am. J. Neuroradiol. 2013, 34, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Adjemian, C.; Lausten, L.; Ang, D.B.; Johnson, M.; Katz, J.; Bonewald, L.F. Bisphosphonate-related osteonecrosis of the jaw: Model and diagnosis with cone beam computerized tomography. Cells Tissues Organs 2009, 189, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, B.A.; Erdem, M.A.; Isler, S.C.; Demircan, S.; Soluk, M.; Kasapoglu, C.; Korhan, C. Use of Cone-Beam Computerized Tomography for Evaluation of Bisphosphonate-Associated Osteonecrosis of the Jaws in an Experimental Rat Model. Int. J. Med. Sci. 2011, 8, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Carestream. Based on studies conducted by John B. Ludlow, University of North Carolina, School of Dentistry: Dosimetry of CS 8100 CBCT Unit and CS 9300 Low-Dose Protocol. August 2014. Available online: http://mediaroom.carestreamdental.com/press-releases/press-release-independent-study-recognizes-extreme-low-dose-capabilities-of-carestream-dentals-cs-9300-low-dose-mode (accessed on 19 April 2016).
- Sanna, G.; Preda, L.; Bruschini, R.; Cossu Rocca, M.; Ferretti, S.; Adamoli, L.; Verri, E.; Franceschelli, L.; Goldhirsch, A.; Nolè, F. Bisphosphonates and jaw osteonecrosis in patients with advanced breast cancer. Ann. Oncol. 2006, 17, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Gomori, M.J.; Ben-Ami, N.; Friedlander-Barenboim, S.; Regev, E.; Lazarovici, T.S.; Yarom, N. Bisphosphonate-related osteonecrosis of the jaw: Clinical correlations with computerized tomography presentation. Clin. Oral Investig. 2010, 14, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.S.; Zen Filho, E.V.; de Oliveira, T.F.; Tinôco-Araújo, J.E.; Sampieri, M.B.; Antunes, H.S.; Santos, P.S. Clinical and image findings in bisphosphonate-related osteonecrosis of the jaws. J. Craniofac. Surg. 2013, 24, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Junquera, L.; Pelaz, A.; García-Consuegra, L.; Alvarez-Arenal, A.; Costilla, S. Sinus mucosal thickening in bisphosphonate-related osteonecrosis of the jaws: A case-control study. ORL J. Otorhinolaryngol. Relat. Spec. 2014, 76, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.; O’Ryan, F.; Chavez, V.; Lathon, P.V.; Sanchez, G.; Hatcher, D.C.; Indresano, A.T.; Lo, J.C. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J. Oral Maxillofac. Surg. 2010, 68, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Matsuo, A.; Koizumi, T.; Chikazu, S.T. A simple evaluation method for early detection of bisphosphonate-related osteonecrosis of the mandible using computed tomography. J. Craniomaxillofac. Surg. 2014, 42, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Wutzl, A.; Eisenmenger, G.; Hoffmann, M.; Czerny, C.; Moser, D.; Pietschmann, P.; Ewers, R.; Baumann, A. Osteonecrosis of the jaws and bisphosphonate treatment in cancer patients. Wutzl. Wien Klin. Wochenschr. 2006, 118, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Bisdas, S.; Chambron Pinho, N.; Smolarz, A.; Sader, R.; Vogl, T.J.; Mack, M.G. Biphosphonate-induced osteonecrosis of the jaws: CT and MRI spectrum of findings in 32 patients. Clin. Radiol. 2008, 63, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Blandamura, S.; Lokmic, Z.; Palumbo, C.; Ragazzo, M.; Ferrari, F.; Tregnaghi, A.; Pietrogrande, F.; Procopio, O.; Saia, G.; et al. Bisphosphonate-associated jawbone osteonecrosis: A correlation between imaging techniques and histopathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Arslanoglu, A.; Yildirm, N.; Silbergleit, R.; Aygun, N. Imaging findings of bisphosphonate-related osteonecrosis of the jaw with emphasis on early magnetic resonance imaging findings. J. Comput. Assist. Tomogr. 2009, 33, 298–304. [Google Scholar] [CrossRef] [PubMed]
- García-Ferrer, L.; Bagán, J.V.; Martínez-Sanjuan, V.; Hernandez-Bazan, S.; García, R.; Jiménez-Soriano, Y.; Hervas, V. MRI of mandibular osteonecrosis secondary to bisphosphonates. AJR Am. J. Roentgenol. 2008, 190, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Chiandussi, S.; Biasotto, M.; Dore, F.; Cavalli, F.; Cova, M.A.; Di Lenarda, R. Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac. Radiol. 2006, 35, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.T.; Chiang, W.F.; Chuang, C.Y.; Chang, S.W. Resolution of oral bisphosphonate and steroid-related osteonecrosis of the jaw—A serial case analysis. J. Oral Maxillofac. Surg. 2010, 68, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Lantto, T.; Vorne, M.; Mokka, R.; Vähätalo, S. 99Tcm-MDP and 99Tcm-DPD in pathologic bone lesions. A visual and quantitative comparison. Acta Radiol. 1987, 28, 631–633. [Google Scholar] [CrossRef] [PubMed]
- O’Ryan, F.S.; Khoury, S.; Liao, W.; Han, M.M.; Hui, R.L.; Baer, D.; Martin, D.; Liberty, D.; Lo, J.C. Intravenous bisphosphonate-related osteonecrosis of the jaw: Bone scintigraphy as an early indicator. J. Oral Maxillofac. Surg. 2009, 67, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, V.D.; Zakavi, S.R. Age-related normal variants of sternal uptake on bone scintigraphy. Clin. Nucl. Med. 2006, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Spanidis, M.; Engel, C.; Roos, F.C.; Frees, S.; Neisius, A.; Hampel, C.; Rubenwolf, P.; Thüroff, J.W.; Walter, C.; et al. Bone scintigraphy predicts bisphosphonate-induced osteonecrosis of the jaw (BRONJ) in patients with metastatic castration-resistant prostate cancer (mCRPC). Clin. Oral Investig. 2016, 20, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Gerngroβ, C.; Schwaiger, M.; Hohlweg-Majert, B.; Kehl, V.; Jansen, H.; Hahnefeld, L.; Koerdt, S.; Otto, S.; Pautke, C. Effect of antiresorptive drugs on bony turnover in the jaw: Denosumab compared with bisphosphonates. Br. J. Oral Maxillofac. Surg. 2014, 52, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Belcher, R.; Boyette, J.; Pierson, T.; Siegel, E.; Bartel, T.B.; Aniasse, E.; Stack, B. What is the role of positron emission tomography in osteonecrosis of the jaws? J. Oral Maxillofac. Surg. 2014, 72, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, K.E.; Raad, R.A.; Rakheja, R.; Gupta, V.; Chan, K.C.; Friedman, K.P.; Mourtzikos, K.A.; Janal, M.; Glickman, R.S. Fluorodeoxyglucose positron emission tomography with computed tomography detects greater metabolic changes that are not represented by plain radiography for patients with osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2014, 72, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Bauer, F.; Tischer, T.; Kreutzer, K.; Weitz, J.; Kesting, M.; Hölzle, F.; Kolk, A.; Stürzenbaum, S.R.; Wolff, K.D. Fluorescence-guided bone resection in bisphosphonate-associated osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009, 67, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Bauer, F.; Otto, S.; Tischer, T.; Steiner, T.; Weitz, J.; Kreutzer, K.; Hohlweg-Majert, B.; Wolff, K.D.; Hafner, S.; et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: First clinical results of a prospective pilot study. J. Oral Maxillofac. Surg. 2011, 69, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.T.; Zrnc, T.A.; Riecke, B.; Wikner, J.; Zustin, J.; Friedrich, R.E.; Heiland, M.; Smeets, R.; Gröbe, A. Intraoperative efficiency of fluorescence imaging by Visually Enhanced Lesion Scope (VELscope) in patients with bisphosphonate related osteonecrosis of the jaw (BRONJ). J. Craniomaxillofac. Surg. 2014, 42, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ristow, O.; Pautke, C. Auto-fluorescence of the bone and its use for delineation of bone necrosis. Int. J. Oral Maxillofac. Surg. 2014, 43, 1391–1393. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg, B.-I.; Mueller, A.A.; Augello, M.; Berg, S.; Jaquiéry, C. Imaging in Patients with Bisphosphonate-Associated Osteonecrosis of the Jaws (MRONJ). Dent. J. 2016, 4, 29. https://doi.org/10.3390/dj4030029
Berg B-I, Mueller AA, Augello M, Berg S, Jaquiéry C. Imaging in Patients with Bisphosphonate-Associated Osteonecrosis of the Jaws (MRONJ). Dentistry Journal. 2016; 4(3):29. https://doi.org/10.3390/dj4030029
Chicago/Turabian StyleBerg, Britt-Isabelle, Andreas A. Mueller, Marcello Augello, Scott Berg, and Claude Jaquiéry. 2016. "Imaging in Patients with Bisphosphonate-Associated Osteonecrosis of the Jaws (MRONJ)" Dentistry Journal 4, no. 3: 29. https://doi.org/10.3390/dj4030029





