Synthesis, Structural, and Magnetic Characterization of a Mixed 3d/4f 12-Metallacrown-4 Family of Complexes
Abstract
:1. Introduction
2. Results and Discussion
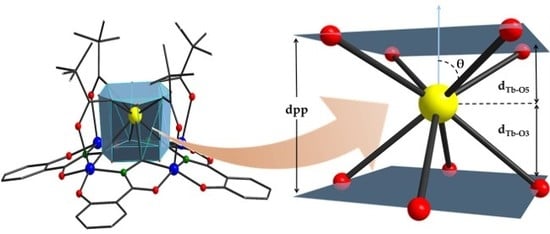
2.1. Crystal Structures of Compounds 1–3
2.2. Magnetic Studies of Complexes 1–3
3. Materials and Methods
3.1. General Information
3.2. X-ray Crystallography
3.3. Magnetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chow, C.Y.; Trivedi, E.R.; Pecoraro, V.; Zaleski, C.M. Heterometallic Mixed 3d-4f Metallacrowns: Structural Versatility, Luminescence, and Molecular Magnetism. Comments Inorg. Chem. 2015, 35, 214–253. [Google Scholar] [CrossRef]
- Jia, R.; Li, H.-F.; Chen, P.; Gao, T.; Sun, W.-B.; Li, G.-M.; Yan, P.-F. Synthesis, Structure, and Tunable White Light Emission of Heteronuclear Zn2 Ln2 Arrays Using a Zinc Complex as Ligand. CrystEngComm 2016, 18, 917–923. [Google Scholar] [CrossRef]
- Evangelisti, F.; Moré, R.; Hodel, F.; Luber, S.; Patzke, G.R. 3d–4f {CoII3Ln(OR)4} Cubanes as Bio-Inspired Water Oxidation Catalysts. J. Am. Chem. Soc. 2015, 137, 11076–11084. [Google Scholar] [CrossRef] [PubMed]
- Rosado Piquer, L.; Sañudo, E.C. Heterometallic 3d–4f Single-Molecule Magnets. Dalton Trans. 2015, 44, 8771–8780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.; Gatteschi, D.; Hendrickson, D.N.; Sessoli, R. Single-Molecule Magnets. MRS Bull. 2000, 25, 66–71. [Google Scholar] [CrossRef]
- Bagai, R.; Christou, G. The Drosophila of Single-Molecule Magnetism: [Mn12O12(O2CR)16(H2O)4]. Chem. Soc. Rev. 2009, 38, 1011. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Sarachik, M.P.; Tejada, J.; Ziolo, R. Macroscopic Measurement of Resonant Magnetization Tunneling in High-Spin Molecules. Phys. Rev. Lett. 1996, 76, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Lionti, F.; Ballou, R.; Gatteschi, D.; Sessoli, R.; Barbara, B. Macroscopic Quantum Tunnelling of Magnetization in a Single Crystal of Nanomagnets. Nature 1996, 383, 145–147. [Google Scholar] [CrossRef]
- Bogani, L.; Wernsdorfer, W. Molecular Spintronics Using Single-Molecule Magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, M.; Klyatskaya, S.; Cleuziou, J.-P.; Ruben, M.; Wernsdorfer, W. Supramolecular Spin Valves. Nat. Mater. 2011, 10, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Lah, M.S.; Kirk, M.L.; Hatfield, W.; Pecoraro, V.L. The Tetranuclear Cluster FeIII[FeIII (Salicylhydroximato)(MeOH)(Acetate)]3 Is an Analogue of M3+(9-Crown-3). J. Chem. Soc. Chem. Commun. 1989, 1606–1608. [Google Scholar] [CrossRef]
- Lah, M.S.; Pecoraro, V.L. Isolation and Characterization of {MnII[MnIII(Salicylhydroximate)]4(Acetate)2(DMF)6}·2DMF: An Inorganic Analog of M2+(12-Crown-4). J. Am. Chem. Soc. 1989, 111, 7258–7259. [Google Scholar] [CrossRef]
- Dutton, J.C.; Murray, K.S.; Tiekink, E.R.T. Magnetism of Oxovanadium(IV) Complexes of Binucleating Ligands. Oxidation to and Structure of a Mononuclear Oxovanadium(V) Complex of N,N′-(Pentan-3-Ol) Bis(Salicylaldimine). Inorg. Chim. Acta 1989, 166, 5–8. [Google Scholar] [CrossRef]
- Mezei, G.; Zaleski, C.M.; Pecoraro, V.L. Structural and Functional Evolution of Metallacrowns. Chem. Rev. 2007, 107, 4933–5003. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Furlotti, M.; Tropiano, M.; Lim, C.S.; Pecoraro, V.L. Thermodynamics of Core Metal Replacement and Self-Assembly of Ca2+ 15-Metallacrown-5†. Inorg. Chem. 2010, 49, 5190–5201. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.F.; Kilner, C.A.; Halcrow, M.A. A Cobalt Metallacrown Anion Host with Guest-Dependent Redox Activity. Chem. Eur. J. 2009, 15, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.F.; Barrett, S.A.; Kilner, C.A.; Halcrow, M.A. Ammonium, Alkylammonium, and Amino Acid Complexes of a Hexacopper Fluoro-Metallacrown Cavitand. Chem. Eur. J. 2008, 14, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.Y.; Eliseeva, S.V.; Trivedi, E.R.; Nguyen, T.N.; Kampf, J.W.; Petoud, S.; Pecoraro, V.L. Ga3+/Ln3+ Metallacrowns: A Promising Family of Highly Luminescent Lanthanide Complexes That Covers Visible and Near-Infrared Domains. J. Am. Chem. Soc. 2016, 138, 5100–5109. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, E.R.; Eliseeva, S.V.; Jankolovits, J.; Olmstead, M.M.; Petoud, S.; Pecoraro, V.L. Highly Emitting Near-Infrared Lanthanide “Encapsulated Sandwich” Metallacrown Complexes with Excitation Shifted Toward Lower Energy. J. Am. Chem. Soc. 2014, 136, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, M.; Fritsky, I.O.; Gumienna-Kontecka, E.; Pavlishchuk, A.V. Metallacrown-Based Compounds: Applications in Catalysis, Luminescence, Molecular Magnetism, and Adsorption. Coord. Chem. Rev. 2016, 327–328, 304–332. [Google Scholar] [CrossRef]
- Athanasopoulou, A.A.; Gamer, C.; Völker, L.; Rentschler, E. Novel Magnetic Nanostructures: Unique Properties and Applications; ELSEVIER: New York, NY, USA, 2018. [Google Scholar]
- Qin, Y.; Gao, Q.; Chen, Y.; Liu, W.; Lin, F.; Zhang, X.; Dong, Y.; Li, Y. Four Mixed 3d-4f 12-Metallacrown-4 Complexes: Syntheses, Structures and Magnetic Properties. J. Clust. Sci. 2017, 28, 891–903. [Google Scholar] [CrossRef]
- Cao, F.; Wang, S.; Li, D.; Zeng, S.; Niu, M.; Song, Y.; Dou, J. Family of Mixed 3d–4f Dimeric 14-Metallacrown-5 Compounds: Syntheses, Structures, and Magnetic Properties. Inorg. Chem. 2013, 52, 10747–10755. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Yang, H.; Zeng, S.; Li, D.; Dou, J. A New Family of Heterometallic LnIII[12-MCFeIIIN(Shi)-4] Complexes: Syntheses, Structures and Magnetic Properties. Crystals 2018, 8, 229. [Google Scholar] [CrossRef]
- Azar, M.R.; Boron, T.T.; Lutter, J.C.; Daly, C.I.; Zegalia, K.A.; Nimthong, R.; Ferrence, G.M.; Zeller, M.; Kampf, J.W.; Pecoraro, V.L.; et al. Controllable Formation of Heterotrimetallic Coordination Compounds: Systematically Incorporating Lanthanide and Alkali Metal Ions into the Manganese 12-Metallacrown-4 Framework. Inorg. Chem. 2014, 53, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.R.; Zeller, M.; Zaleski, C.M. Facile Carboxylate Ligand Variation of Heterotrimetallic 12-Metallacrown-4 Complexes. Polyhedron 2016, 114, 29–36. [Google Scholar] [CrossRef]
- Boron, T.T.; Lutter, J.C.; Daly, C.I.; Chow, C.Y.; Davis, A.H.; Nimthong-Roldán, A.; Zeller, M.; Kampf, J.W.; Zaleski, C.M.; Pecoraro, V.L. The Nature of the Bridging Anion Controls the Single-Molecule Magnetic Properties of DyX4M 12-Metallacrown-4 Complexes. Inorg. Chem. 2016, 55, 10597–10607. [Google Scholar] [CrossRef] [PubMed]
- Happ, P.; Rentschler, E. Enforcement of a High-Spin Ground State for the First 3d Heterometallic 12-Metallacrown-4 Complex. Dalton Trans. 2014, 43, 15308–15312. [Google Scholar] [CrossRef] [PubMed]
- Happ, P.; Plenk, C.; Rentschler, E. 12-MC-4 Metallacrowns as Versatile Tools for SMM Research. Coord. Chem. Rev. 2015, 289–290, 238–260. [Google Scholar] [CrossRef]
- Plenk, C.; Krause, J.; Beck, M.; Rentschler, E. Rational Linkage of Magnetic Molecules Using Click Chemistry. Chem. Commun. 2015, 51, 6524–6527. [Google Scholar] [CrossRef] [PubMed]
- Happ, P.; Sapozhnik, A.; Klanke, J.; Czaja, P.; Chernenkaya, A.; Medjanik, K.; Schuppler, S.; Nagel, P.; Merz, M.; Rentschler, E.; et al. Analyzing the Enforcement of a High-Spin Ground State for a Metallacrown Single-Molecule Magnet. Phys. Rev. B 2016, 93, 174404. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Liu, W.; Thorp, H.H. Bond Valence Sum Analysis of Metal-Ligand Bond Lengths in Metalloenzymes and Model Complexes. 2. Refined Distances and Other Enzymes. Inorg. Chem. 1993, 32, 4102–4105. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Koumousi, E.S.; Cunha-Silva, L.; McCormick, L.J.; Teat, S.J.; Psycharis, V.; Raptopoulou, C.P.; Mukherjee, S.; Li, C.; Gupta, S.D.; et al. Structural Diversities in Heterometallic Mn–Ca Cluster Chemistry from the Use of Salicylhydroxamic Acid: {MnIII4Ca2}, {MnII/III6Ca2}, {MnIII/IV8Ca}, and {MnIII8Ca2} Complexes with Relevance to Both High- and Low-Valent States of the Oxygen-Evolving Complex. Inorg. Chem. 2017, 56, 10760–10774. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Gass, I.A.; Milios, C.J.; Lalioti, N.; Terzis, A.; Aromí, G.; Teat, S.J.; Brechin, E.K.; Perlepes, S.P. A MnII4 Cubane and a Novel MnII10MnIII4 Cluster from the Use of Di-2-Pyridyl Ketone in Manganese Acetate Chemistry. Dalton Trans. 2009, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Sorace, L.; Benelli, C.; Gatteschi, D. Lanthanides in Molecular Magnetism: Old Tools in a New Field. Chem. Soc. Rev. 2011, 40, 3092. [Google Scholar] [CrossRef] [PubMed]
- Baldoví, J.J.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Palii, A. Rational Design of Single-Ion Magnets and Spin Qubits Based on Mononuclear Lanthanoid Complexes. Inorg. Chem. 2012, 51, 12565–12574. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Weihe, H.; Vinum, M.G.; Mortensen, J.S.; Doerrer, L.H.; Bendix, J. Imposing High-Symmetry and Tuneable Geometry on Lanthanide Centres with Chelating Pt and Pd Metalloligands. Chem. Sci. 2017, 8, 3566–3575. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Milios, C.J.; Piligkos, S.; Brechin, E.K. Ground State Spin-Switching via Targeted Structural Distortion: Twisted Single-Molecule Magnets from Derivatised Salicylaldoximes. Dalton Trans. 2008, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Papatriantafyllopoulou, C.; Abboud, K.A.; Christou, G. Carboxylate-Free MnIII2LnIII2 (Ln = Lanthanide) and MnIII2YIII2 Complexes from the Use of (2-Hydroxymethyl)pyridine: Analysis of Spin Frustration Effects. Inorg. Chem. 2011, 50, 8959–8966. [Google Scholar] [CrossRef] [PubMed]
- Savva, M.; Skordi, K.; Fournet, A.D.; Thuijs, A.E.; Christou, G.; Perlepes, S.P.; Papatriantafyllopoulou, C.; Tasiopoulos, A.J. Heterometallic MnIII4Ln2 (Ln = Dy, Gd, Tb) Cross-Shaped Clusters and Their Homometallic MnIII4MnII2 Analogues. Inorg. Chem. 2017, 56, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-J.; Xie, X.-R.; Zheng, T.-F.; Bao, J.; Liao, J.-S.; Chen, J.-L.; Wen, H.-R. Three-Dimensional Two-Fold Interpenetrated CrIII–GdIII Heterometallic Framework as an Attractive Cryogenic Magnetorefrigerant. CrystEngComm 2015, 17, 7270–7275. [Google Scholar] [CrossRef]
- Gómez, V.; Vendier, L.; Corbella, M.; Costes, J.-P. Tetranuclear [Co–Gd]2 Complexes: Aiming at a Better Understanding of the 3d-Gd Magnetic Interaction. Inorg. Chem. 2012, 51, 6396–6404. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.B.; Lippard, S.J.; Shweky, I.; Bino, A. Dinuclear Manganese(II) Complexes with Water and Carboxylate Bridges. Inorg. Chem. 1992, 31, 3502–3504. [Google Scholar] [CrossRef]
- Bruker Apex 3; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Sheldrick, G.M. SADABS-2016/2; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Stoe & Cie X-RED; Stoe & Cie: Darmstadt, Germany, 2002.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasopoulou, A.A.; Carrella, L.M.; Rentschler, E. Synthesis, Structural, and Magnetic Characterization of a Mixed 3d/4f 12-Metallacrown-4 Family of Complexes. Inorganics 2018, 6, 66. https://doi.org/10.3390/inorganics6030066
Athanasopoulou AA, Carrella LM, Rentschler E. Synthesis, Structural, and Magnetic Characterization of a Mixed 3d/4f 12-Metallacrown-4 Family of Complexes. Inorganics. 2018; 6(3):66. https://doi.org/10.3390/inorganics6030066
Chicago/Turabian StyleAthanasopoulou, Angeliki A., Luca M. Carrella, and Eva Rentschler. 2018. "Synthesis, Structural, and Magnetic Characterization of a Mixed 3d/4f 12-Metallacrown-4 Family of Complexes" Inorganics 6, no. 3: 66. https://doi.org/10.3390/inorganics6030066