Construction of a Planar Tetrapalladium Cluster by the Reaction of Palladium(0) Bis(isocyanide) with Cyclic Tetrasilane
Abstract
:1. Introduction
2. Results and Discussion
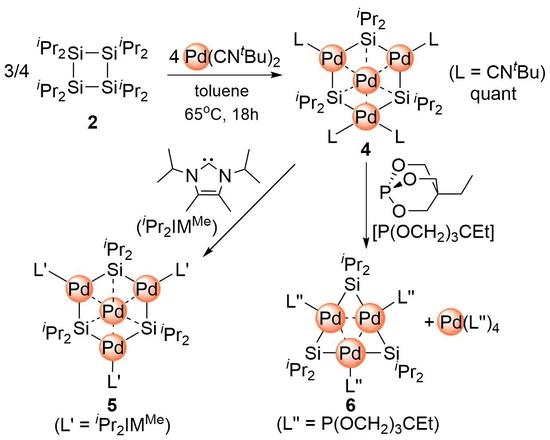
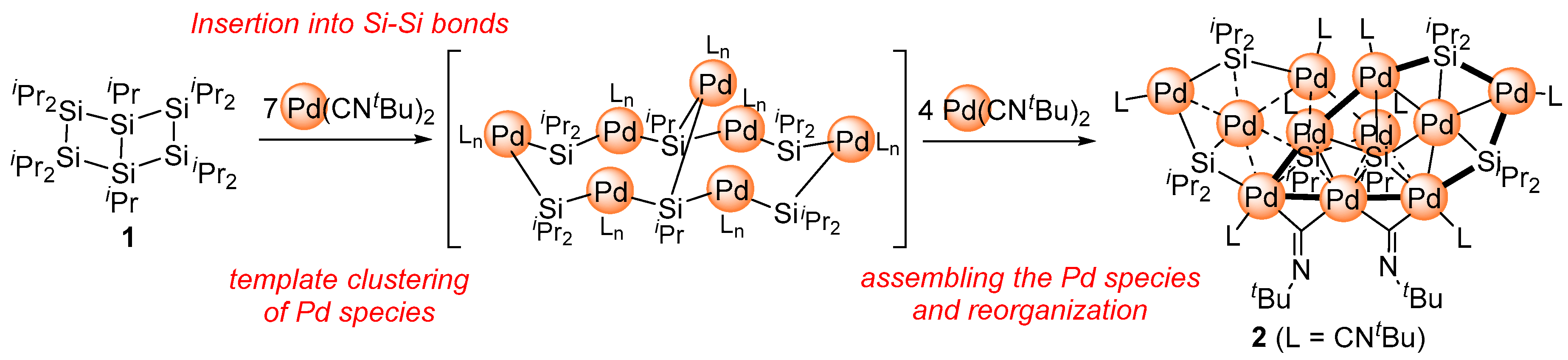
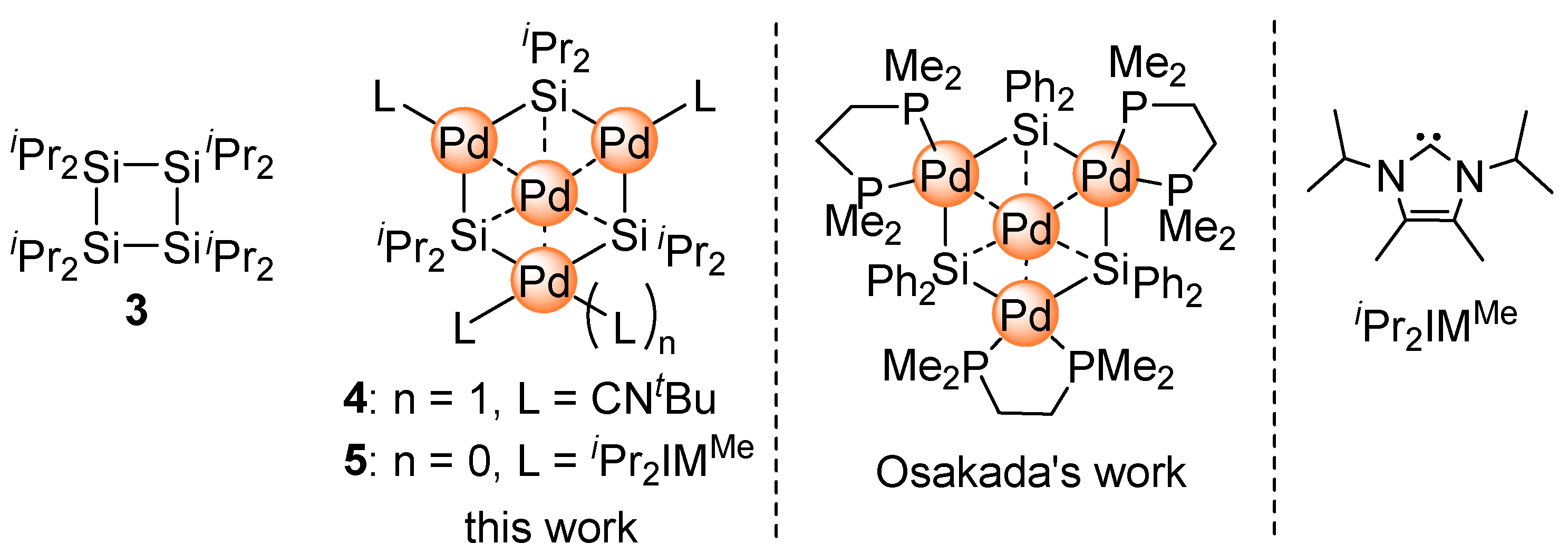
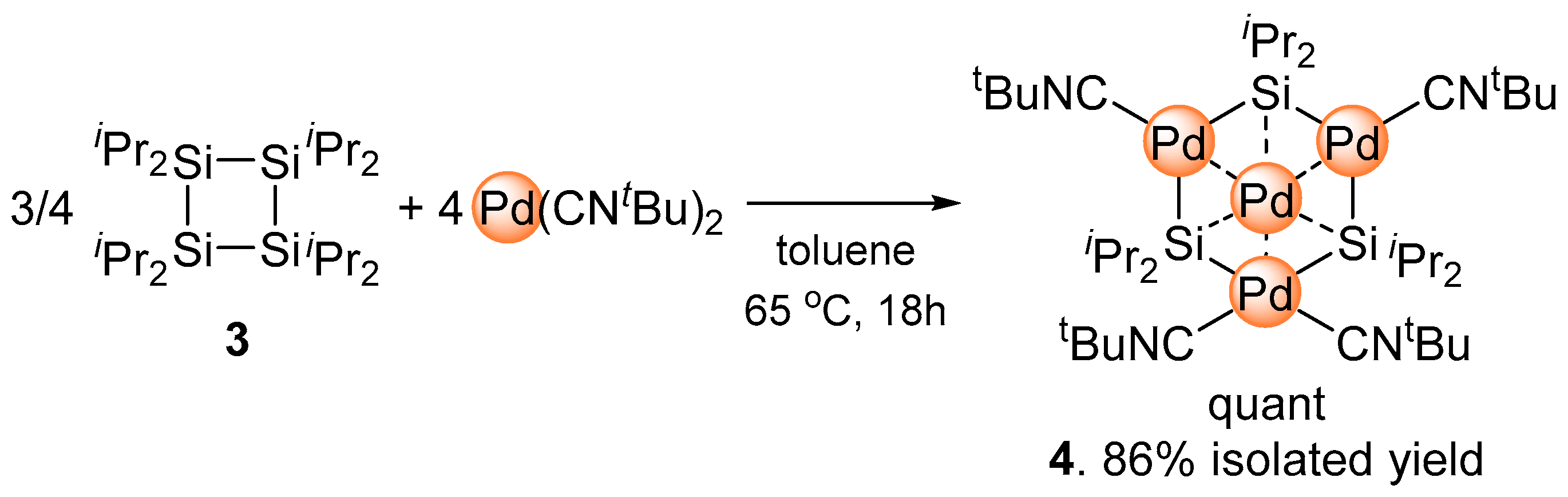
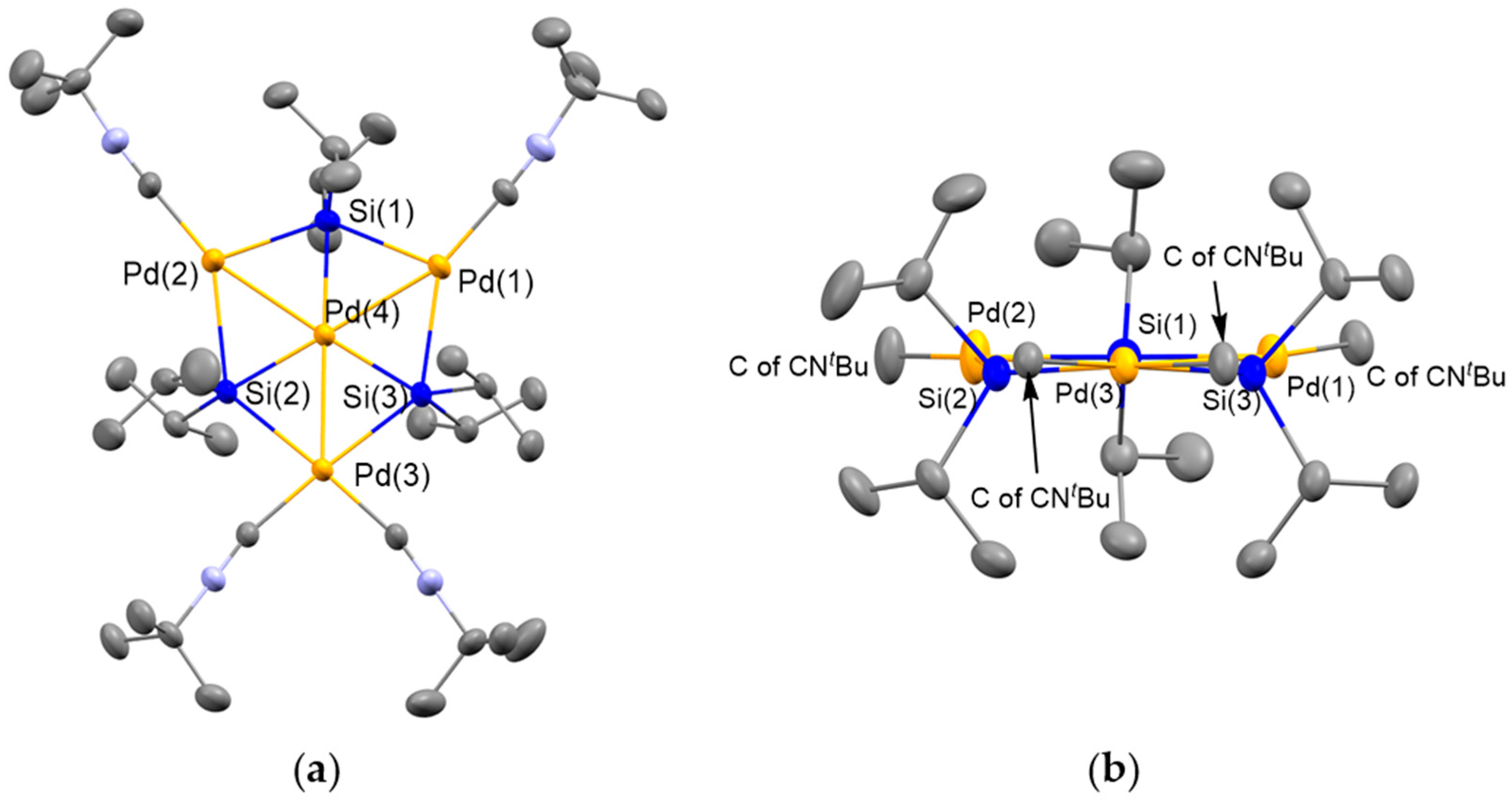
2.1. Synthesis of Planar Pd4 Cluster 4 by Reaction of Pd(CNtBu)2 with Cyclotetrasilane 3
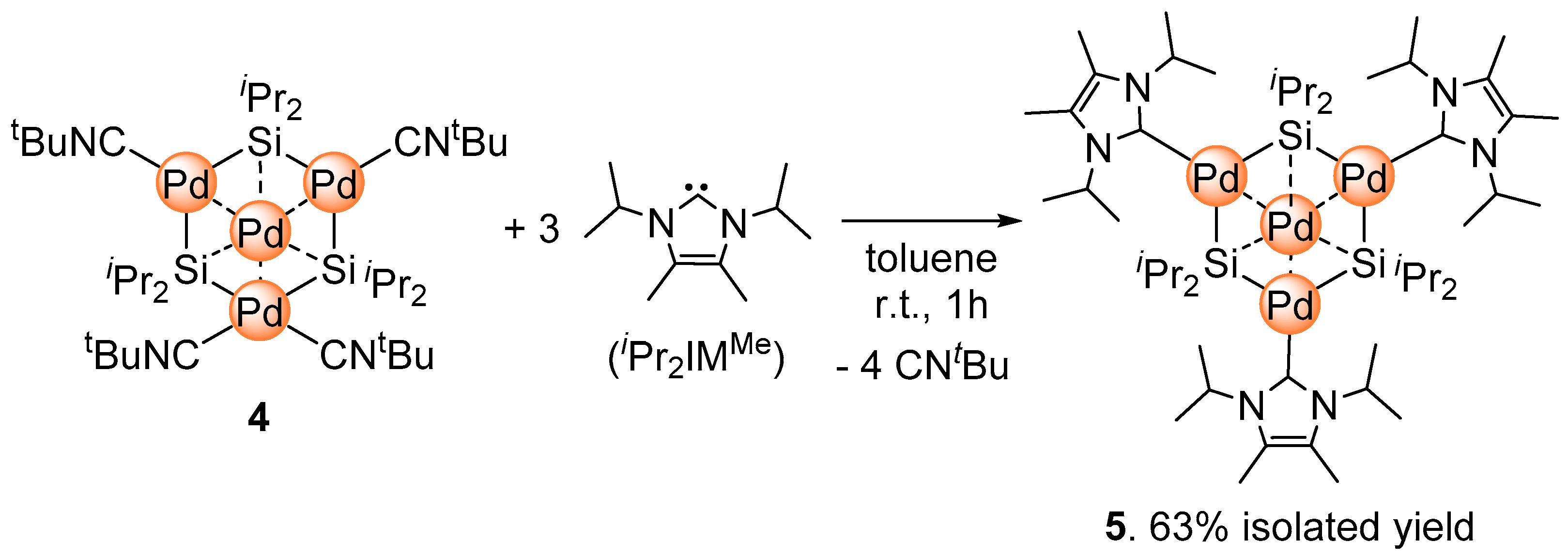
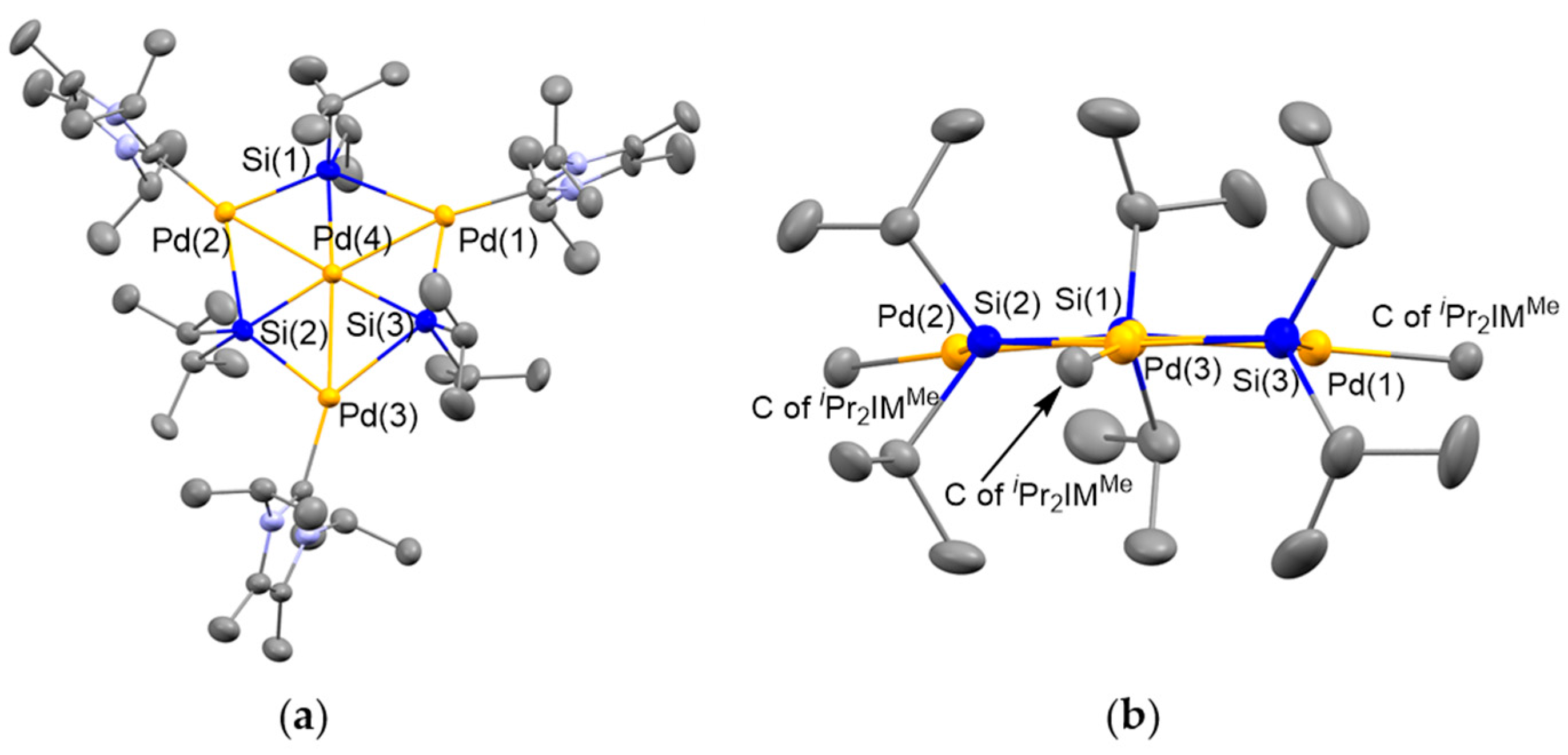
2.2. Ligand Exchange of 4 with N-Heterocyclic Carbene
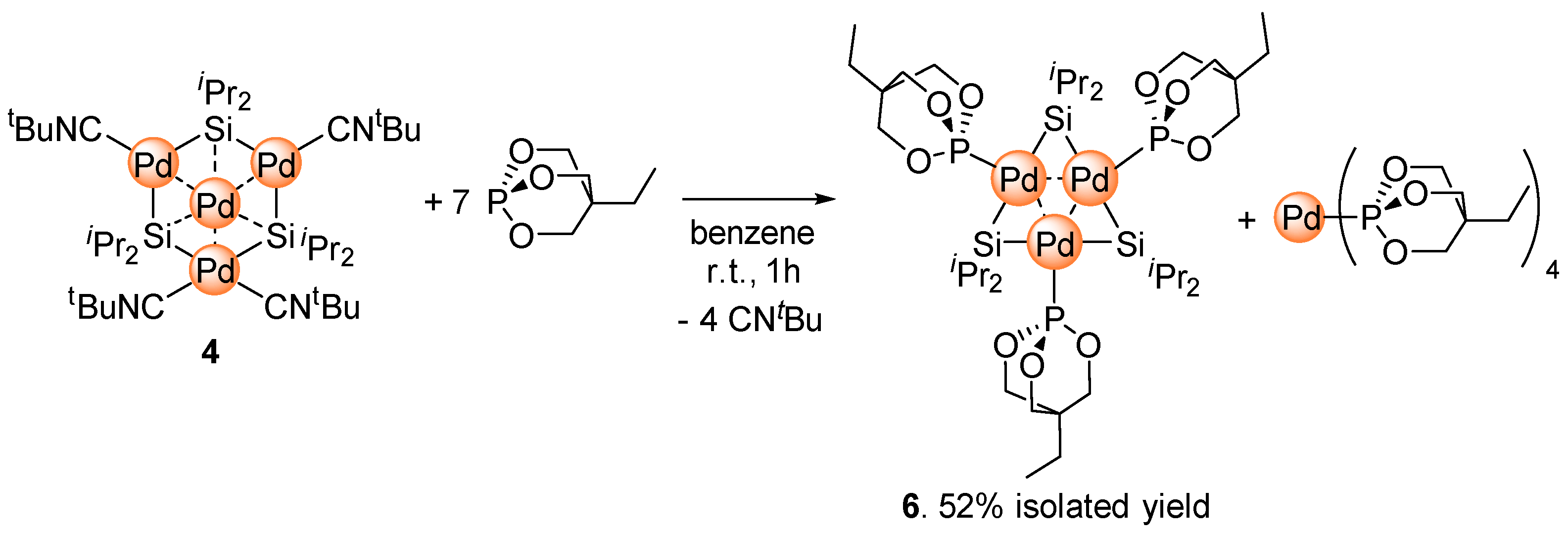
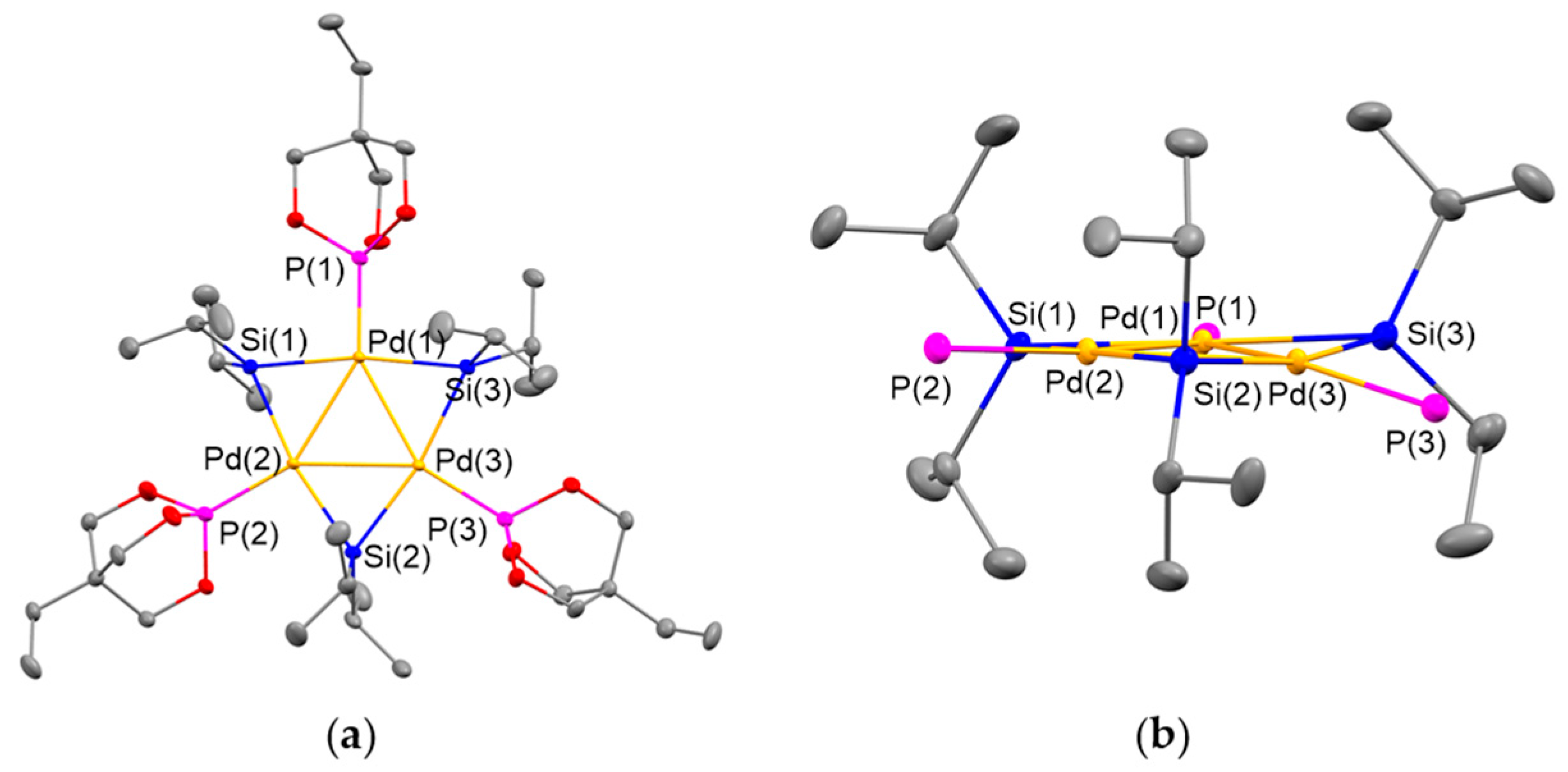
2.3. Ligand Exchange of 4 with Trimethylolpropane Phosphite to Afford the Planar Pd3 Cluster 6
3. Materials and Methods
3.1. Synthesis of Pd4(Si(iPr)2)3(CNtBu)4 (4)
3.2. Synthesis of Pd4(Si(iPr)2)3(iPr2IMMe)4 (5)
3.3. Synthesis of Pd3{Si(iPr)2}3{P(OCH2)3CEt}3 (6)
3.4. In Situ Preparation of Pd{P(OCH2)3CEt}4
3.5. X-Ray Data Collection and Reduction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Markó, L.; Vizi-Orosz, A. Metal Clusters in Catalysis; Gates, B.C., Guczi, L., Knözinger, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Süss-Fink, G.; Jahncke, M. Catalysis by Di- and Polynuclear Metal Cluster Complexes; Adams, R.D., Cotton, F.A., Eds.; Wiley-VCH: Toronto, ON, Canada, 1998. [Google Scholar]
- Puddephatt, R.J. Metal Clusters in Chemistry; Braunstein, P., Oro, L.A., Raithby, P.R., Eds.; Wiley-VCH: Toronto, ON, Canada, 1999. [Google Scholar]
- Braga, D.; Dyson, F.; Grepioni, F.; Johnson, B.F.G. Arene clusters. Chem. Rev. 1994, 94, 1585–1620. [Google Scholar] [CrossRef]
- Inagaki, A.; Takaya, Y.; Takemori, T.; Suzuki, H.; Tanaka, M.; Haga, M. Trinuclear ruthenium complex with a face-capping benzene ligand. Hapticity change induced by two-electron redox reaction. J. Am. Chem. Soc. 1997, 119, 625–626. [Google Scholar] [CrossRef]
- Holm, R.H.; Lo, W. Structural Conversions of synthetic and protein-bound iron–sulfur clusters. Chem. Rev. 2016, 116, 13685–13713. [Google Scholar] [CrossRef] [PubMed]
- Groysman, S.; Holm, R.H. Biomimetic chemistry of iron, nickel, molybdenum, and tungsten in sulfur-ligated protein sites. Biochemistry 2009, 48, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lo, W.; Holm, R.H. Developments in the biomimetic chemistry of cubane-type and higher nuclearity iron–sulfur cluster. Chem. Rev. 2014, 114, 3579–3600. [Google Scholar] [CrossRef] [PubMed]
- Ohki, Y.; Tatsumi, K. New synthetic routes to metal-sulfur clusters relevant to the nitrogenase metallo-clusters. Z. Anorg. Allg. Chem. 2013, 639, 1340–1349. [Google Scholar] [CrossRef]
- Murahashi, T.; Kurosawa, H. Organopalladium complexes containing palladium–palladium bonds. Coord. Chem. Rev. 2002, 231, 207–228. [Google Scholar] [CrossRef]
- Horiuchi, S.; Tachibana, Y.; Yamashita, M.; Yamamoto, K.; Masai, K.; Takase, K.; Matsutani, T.; Kawamata, S.; Kurashige, Y.; Yanai, T.; et al. Multinuclear metal-binding ability of a carotene. Nat. Commun. 2015, 6, 7742/1–7742/8. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, T.; Mochizuki, E.; Kai, Y.; Kurosawa, H. Organometallic sandwich chains made of conjugated polyenes and metal-metal chains. J. Am. Chem. Soc. 1999, 121, 10660–10661. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Nagai, T.; Nakashima, H.; Murahashi, T.; Kurosawa, H. Stepwise growth of polypalladium chains in 1,4-diphenyl-1,3-butadiene sandwich complexes. Chem. Commun. 2004, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Nakamae, K.; Takeemura, Y.; Kure, B.; Nakajima, T.; Kitagawa, Y.; Tanase, T. Self-alignment of low-valent octanuclear palladium atoms. Angew. Chem. Int. Ed. 2015, 54, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Begum, R.A.; Zhan, S.; Tanase, T.; Tanigaki, K.; Sakai, K. Linear, redox-active Pt6 and Pt2Pd2Pt2 clusters. Angew. Chem. Int. Ed. 2004, 43, 5029–5032. [Google Scholar] [CrossRef] [PubMed]
- Goto, E.; Begum, R.A.; Ueno, C.; Hosokawa, A.; Yamamoto, C.; Nakamae, K.; Liure, B.; Nakajima, T.; Kajiwara, T.; Tanase, T. Electron-deficient Pt2M2Pt2 hexanuclear metal strings (M = Pt, Pd) supported by triphosphine ligands. Organometallics 2014, 33, 1893–1904. [Google Scholar] [CrossRef]
- Takemura, Y.; Takenaka, H.; Nakajima, T.; Tanase, T. Hexa- and octagold chains from flexible tetragold molecular units supported by linear tetraphosphine ligands. Angew. Chem. Int. Ed. 2009, 48, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.-A.; Cheng, M.-C.; Chen, C.-H.; Peng, S.-M. From homonuclear metal string complexes to heteronuclear metal string complexes. Eur. J. Inorg. Chem. 2015, 2015, 2510–2523. [Google Scholar] [CrossRef]
- Hurley, T.J.; Robinson, M.A. Nickel(II)-2,2′-dipyridylamine system. I. Synthesis and stereochemistry of the complexes. Inorg. Chem. 1968, 7, 33–38. [Google Scholar] [CrossRef]
- Wu, L.P.; Field, P.; Morrissey, T.; Murphy, C.; Nagle, P.; Hathaway, B.; Simmons, C.; Thornton, P. Crystal structure and electronic properties of dibromo- and dichloro-tetrakis[µ3-bis(2-pyridyl)amido]tricopper(II) hydrate. J. Chem. Soc. Dalton Trans. 1990, 3835–3840. [Google Scholar] [CrossRef]
- Liu, I.P.-O.; Bénard, M.; Hasanov, H.; Chen, I.-W.P.; Tseng, W.-H.; Fu, M.-D.; Rohmer, M.-M.; Chen, C.-H.; Lee, G.-H.; Peng, S.-M. A new generation of metal string complexes: Structure, magnetism, spectroscopy, theoretical analysis, and single molecular conductance of an unusual mixed-valence linear [Ni5]8+ complex. Chem. Eur. J. 2007, 13, 8667–8677. [Google Scholar] [CrossRef] [PubMed]
- Ismayilov, R.-H.; Wang, W.-Z.; Lee, G.-H.; Yeh, C.-Y.; Hua, S.-A.; Song, Y.; Rohmer, M.-M.; Bénard, M.; Peng, S.-M. Two linear undecanickel mixed-valence complexes: Increasing the size and the scope of the electronic properties of nickel metal strings. Angew. Chem. Int. Ed. 2011, 50, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, T.; Fujimoto, M.; Oka, M.; Hashimoto, Y.; Uemura, T.; Tatsumi, Y.; Nakao, Y.; Ikeda, A.; Sakaki, S.; Kurosawa, H. Discrete sandwich compounds of monolayer palladium sheets. Science 2006, 313, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, T.; Inoue, R.; Usui, K.; Ogoshi, S. Square tetrapalladium sheet sandwich complexes: Cyclononatetraenyl as a versatile face-capping ligand. J. Am. Chem. Soc. 2009, 131, 9888–9889. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, T.; Usui, K.; Inoue, R.; Ogoshi, S.; Kurosawa, H. Metallocenoids of platinum: Syntheses and structures of triangular triplatinum sandwich complexes of cycloheptatrienyl. Chem. Sci. 2011, 2, 117–122. [Google Scholar] [CrossRef]
- Murahashi, T.; Kato, N.; Uemura, T.; Kurosawa, H. Rearrangement of a Pd4 skeleton from a 1D Chain to a 2D sheet on the face of a perylene or fluoranthene ligand caused by exchange of the binder molecule. Angew. Chem. Int. Ed. 2007, 46, 3509–3512. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kimura, S.; Yamamoto, K.; Murahashi, T. Bridging coordination of vinylarenes to Pd3- or Pd4 cluster sites. Chem. Eur. J. 2017, 23, 14149–14152. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, T.; Uemura, T.; Kurosawa, H. Perylene—Tetrapalladium sandwich complexes. J. Am. Chem. Soc. 2003, 125, 8436–8437. [Google Scholar] [CrossRef] [PubMed]
- Sunada, Y.; Haige, R.; Otsuka, K.; Kyushin, S.; Nagashima, H. A ladder polysilane as a template for folding palladium nanosheets. Nat. Commun. 2013, 4, 3014/1–3014/7. [Google Scholar] [CrossRef] [PubMed]
- Suginome, M.; Kato, Y.; Takeda, N.; Oike, H.; Ito, Y. Reactions of a spiro trisilane with palladium complexes: synthesis and structure of Tris(organosilyl)CpPdIV and Bis(organosilyl)(μ-organosilylene)PdII2 complexes. Organometallics 1998, 17, 495–497. [Google Scholar] [CrossRef]
- Ansell, M.B.; Navarro, O.; Spencer, J. Transition metal catalyzed element–element’ additions to alkynes. Coord. Chem. Rev. 2017, 336, 54–77. [Google Scholar] [CrossRef]
- Ansell, M.B.; Roberts, D.E.; Cloke, F.G.N.; Navarro, O.; Spencer, J. Synthesis of an [(NHC)2Pd(SiMe3)2] complex and catalytic cis-bis(silyl)ations of alkynes with unactivated disilanes. Angew. Chem. Int. Ed. 2015, 54, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Suginome, M.; Oike, H.; Park, S.-S.; Ito, Y. Reactions of Si–Si σ-bonds with Bis(t-alkyl isocyanide)palladium(0) complexes, synthesis and reactions of cyclic Bis(organosilyl)palladium complexes. Bull. Chem. Soc. Jpn. 1996, 69, 289–299. [Google Scholar] [CrossRef]
- Chen, Y.; Sunada, Y.; Nagashima, H.; Sakaki, S. Theoretical study of Pd11Si6 nanosheet compounds including seven-coordinated Si species and its Ge analogues. Chem. Eur. J. 2016, 22, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mawatari, A.; Tanabe, M.; Osakada, K.; Tanase, T. Planar tetranuclear and dumbbell-shaped octanuclear palladium complexes with bridging silylene ligands. Angew. Chem. Int. Ed. 2009, 48, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Yumoto, R.; Yamada, T.; Fukuta, T.; Hoshino, T.; Osakada, K.; Tanase, T. Planar PtPd3 complexes stabilized by three bridging silylene ligands. Chem. Eur. J. 2017, 23, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Li, Y.-H.; Choe, Y.-K.; Tanaka, M.; Bao, M.; Uchimaru, T. Multinuclear palladium compounds containing palladium centers ligated by five silicon atoms. Proc. Nat. Acad. Sci. USA 2007, 104, 7758–7763. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Ishikawa, N.; Chiba, M.; Ide, T.; Osakada, K.; Tanase, T. Tetrapalladium complex with bridging germylene ligands. Structural change of the planar Pd4Ge3 core. J. Am. Chem. Soc. 2011, 133, 18598–18601. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Johnson, S.A. Structural similarities in dinuclear, tetranuclear, and pentanuclear nickel silyl and silylene complexes obtained via Si–H and Si–C activation. Organometallics 2012, 31, 3599–3609. [Google Scholar] [CrossRef]
- Bradford, A.M.; Kristof, E.; Rashidi, M.; Yang, D.-S.; Payne, N.C.; Puddephatt, R.J. Isocyanide and diisocyanide complexes of a triplatinum cluster: Fluxionality, isomerism, structure, and bonding. Inorg. Chem. 1994, 33, 2355–2363. [Google Scholar] [CrossRef]
- Mann, B.E. Mechanism of the low-energy fluxional process in [Fe3(CO)12−nLn] (n = 0–2): A perspective. J. Chem. Soc. Dalton Trans. 1997, 1457–1471. [Google Scholar] [CrossRef]
- Corey, J.Y. Reactions of hydrosilanes with transition metal complexes. Chem. Rev. 2016, 116, 11291–11435. [Google Scholar] [CrossRef] [PubMed]
- Corey, J.Y. Reactions of hydrosilanes with transition metal complexes and characterization of the products. Chem. Rev. 2011, 111, 863–1071. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Tobita, H. Bridged silylene and germylene complexes. Adv. Organomet. Chem. 1998, 42, 223–290. [Google Scholar] [CrossRef]
- Tanaka, K.; Kamono, M.; Tanabe, M.; Osakada, K. Ring expansion of cyclic triplatinum(0) silylene complexes induced by insertion of alkyne into a Si–Pt bond. Organometallics 2015, 34, 2985–2990. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Corey, J.Y.; Dill, K.; Rath, N.P. Formation and X-ray crystal structure determination of the novel triplatinum cluster [(Ph3P)Pt(μ-SiC12H8)]3 from reaction of silafluorene with (Ph3P)2Pt(η2-C2H4). Organometallics 2002, 21, 5467–5469. [Google Scholar] [CrossRef]
- Osakada, K.; Tanabe, M.; Tanase, T. A triangular triplatinum complex with electron-releasing SiPh2 and PMe3 ligands: [{Pt(μ-SiPh2)(PMe3)}3]. Angew. Chem. Int. Ed. 2000, 39, 4053–4055. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Corey, J.Y.; French, L.M.; Choi, E.; Speedie, V.J.; Rutheeford, M.F.; Yao, S.; Xu, H.; Rath, N.P. Si–H bond activation by (Ph3P)2Pt(η2-C2H4) in dihydrosilicon tricycles that also contain O and N heteroatoms. Organometallics 2006, 25, 3974–3988. [Google Scholar] [CrossRef]
- Watanebe, H.; Muraoka, T.; Kageyama, M.; Yoshizumi, K.; Nagai, Y. Synthesis and some spectral properties of peralkylcyclopolysilanes, [R1R2Si]n (n = 4–7). Organometallics 1984, 3, 141–147. [Google Scholar] [CrossRef]
- Ryan, S.J.; Schimler, S.D.; Bland, D.C.; Sanford, M.S. Acyl azolium fluorides for room temperature nucleophilic aromatic fluorination of chloro- and nitroarenes. Org. Lett. 2015, 17, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna, R. SIR2011: A new package for crystal structure determination and refinement. J. Appl. Cryst. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Beurskens, P.T.; Admiraal, G.; Beurskens, G.; Bosman, W.P.; de Gelder, R.; Israel, R.; Smits, J.M.M. The DIRDIF-99 program system; Technical Report of the Crystallography Laboratory; University of Nijmegen: Nijmegen, The Netherlands, 1999. [Google Scholar]
- Cromer, D.T.; Waber, J.T. International Tables for X-ray Crystallography; Kynoch Press: Birmingham, UK, 1974. [Google Scholar]
- CrystalStructure 4.0, Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2000–2010.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
| 4 | 5 | 6 | |
|---|---|---|---|
| Pd(1)–Pd(4) | 2.6812(6) | 2.7029(6) | - |
| Pd(2)–Pd(4) | 2.6778(6) | 2.6991(8) | - |
| Pd(3)–Pd(4) | 2.7523(5) | 2.7030(7) | - |
| Pd(1)–Pd(2) | - | - | 2.7117(5) |
| Pd(1)–Pd(3) | - | - | 2.7041(6) |
| Pd(2)–Pd(3) | - | - | 2.7050(4) |
| Pd(1)–Si(1) | 2.4252(12) | 2.3668(13) | 2.3557(9) |
| Pd(1)–Si(3) | 2.5827(12) | 2.6359(14) | 2.3602(9) |
| Pd(2)–Si(1) | 2.4339(12) | 2.6518(12) | 2.3707(12) |
| Pd(2)–Si(2) | 2.6401(12) | 2.3769(15) | 2.3754(11) |
| Pd(3)–Si(2) | 2.5094(11) | 2.6659(14) | 2.3658(11) |
| Pd(3)–Si(3) | 2.5341(12) | 2.3693(12) | 2.3691(11) |
| Pd(4)–Si(1) | 2.3106(12) | 2.2638(14) | - |
| Pd(4)–Si(2) | 2.2621(10) | 2.2558(11) | - |
| Pd(4)–Si(3) | 2.2692(12) | 2.2603(13) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunada, Y.; Taniyama, N.; Shimamoto, K.; Kyushin, S.; Nagashima, H. Construction of a Planar Tetrapalladium Cluster by the Reaction of Palladium(0) Bis(isocyanide) with Cyclic Tetrasilane. Inorganics 2017, 5, 84. https://doi.org/10.3390/inorganics5040084
Sunada Y, Taniyama N, Shimamoto K, Kyushin S, Nagashima H. Construction of a Planar Tetrapalladium Cluster by the Reaction of Palladium(0) Bis(isocyanide) with Cyclic Tetrasilane. Inorganics. 2017; 5(4):84. https://doi.org/10.3390/inorganics5040084
Chicago/Turabian StyleSunada, Yusuke, Nobuhiro Taniyama, Kento Shimamoto, Soichiro Kyushin, and Hideo Nagashima. 2017. "Construction of a Planar Tetrapalladium Cluster by the Reaction of Palladium(0) Bis(isocyanide) with Cyclic Tetrasilane" Inorganics 5, no. 4: 84. https://doi.org/10.3390/inorganics5040084