Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions
Abstract
:1. Introduction
2. Results and Discussion
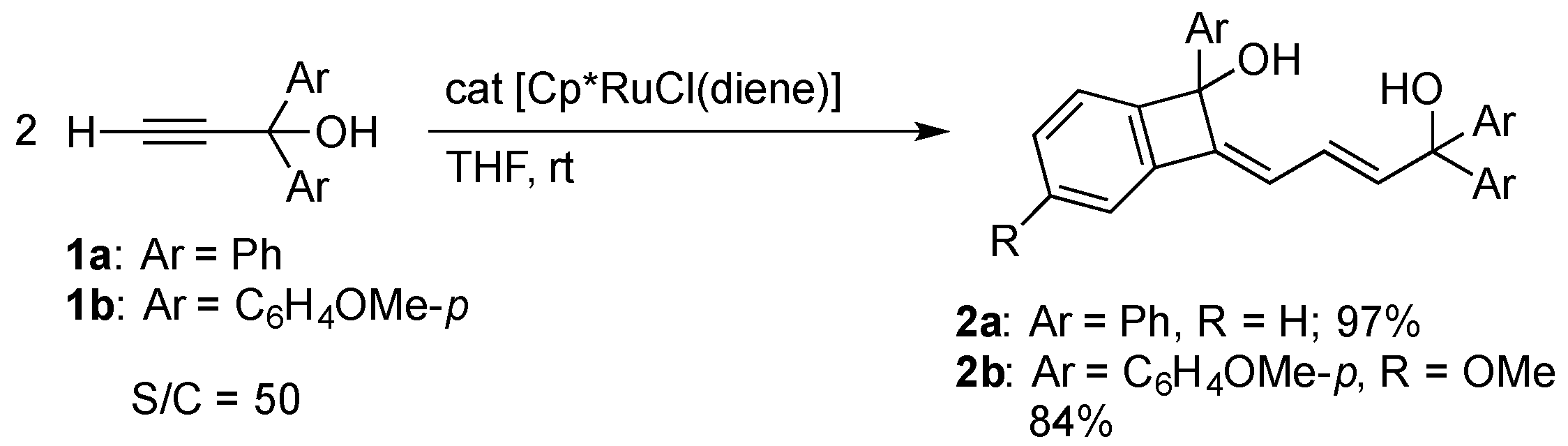
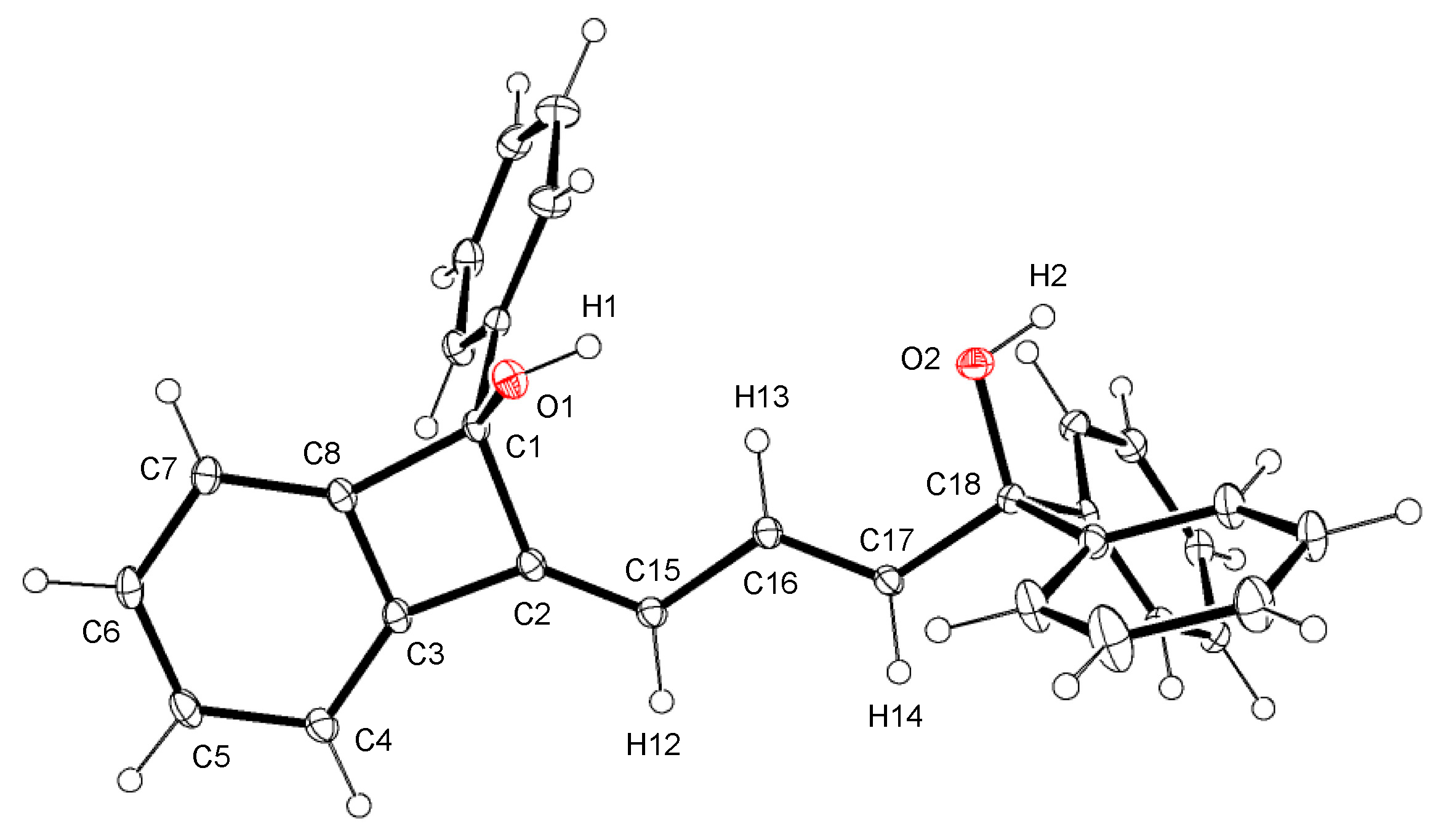
2.1. Ruthenium-Catalytic Dimerization of 1,1-Arylpropargyl Alcohol
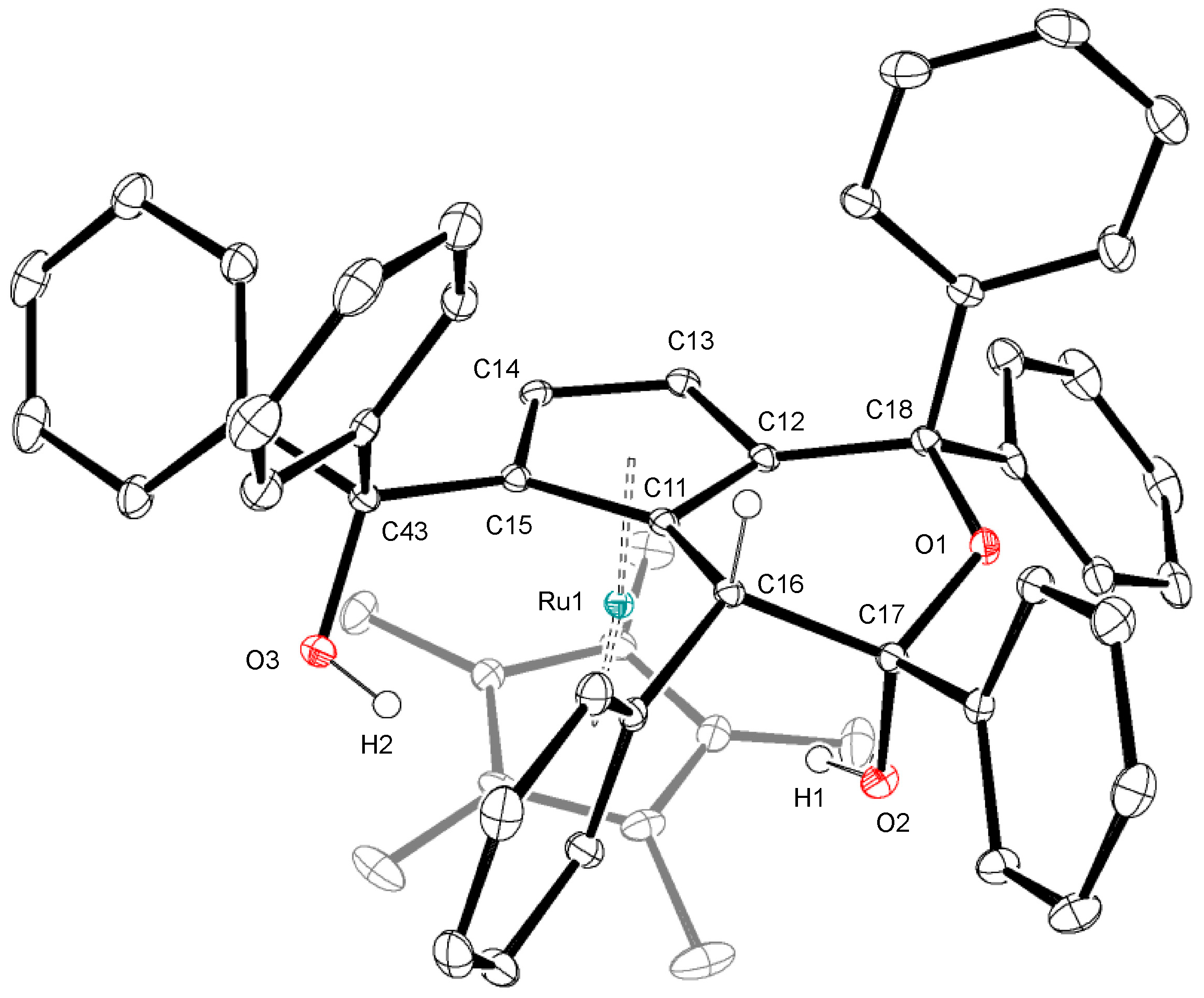
2.2. Reaction of a (Hydrido)ruthenium Complex with 1,1-Diphenylpropargyl Alcohol
3. Experimental
3.1. General
3.2. Catalytic Dimerization of 1,1-Diarylprop-2-yn-1-ols to Give 2
3.3. Synthesis of 4
3.4. Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.-X.; Tang, Y.-L. Cycloisomerization of Pyridine-Substituted Propargylic Alcohols or Esters to Construct Indolizines and Indolizinones. Eur. J. Org. Chem. 2017, 2207–2213. [Google Scholar] [CrossRef]
- Rintjema, J.; Kleij, A. Substrate-Assisted Carbon Dioxide Activation as a Versatile Approach for Heterocyclic Synthesis. Synthesis 2016, 48, 3863–3878. [Google Scholar]
- Ayers, B.; Chan, P. Harnessing the Versatile Reactivity of Propargyl Alcohols and Their Derivatives for Sustainable Complex Molecule Synthesis. Synlett 2015, 26, 1305–1339. [Google Scholar] [CrossRef]
- Hu, X.-H.; Liu, Z.-T.; Shao, L.; Hu, X.-P. Recent Advances in Catalytic Stereocontrolled Cycloaddition with Terminal Propargylic Compounds. Synthesis 2015, 47, 913–923. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Lu, P.; Wang, Y. Recent Advances on the Lewis Acid-Catalyzed Cascade Rearrangements of Propargylic Alcohols and Their Derivatives. ACS Catal. 2014, 4, 1911–1925. [Google Scholar] [CrossRef]
- Bauer, E. Transition-Metal-Catalyzed Functionalization of Propargylic Alcohols and Their Derivatives. Synthesis 2012, 44, 1131–1151. [Google Scholar] [CrossRef]
- Ding, C.-H.; Hou, X.-L. Catalytic Asymmetric Propargylation. Chem. Rev. 2011, 111, 1914–1937. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Itoh, K. Carbon–Carbon Bond Formations via Ruthenacycle Intermediates. In Ruthenium in Organic Synthesis; Murahashi, S.-I., Ed.; Wiley-VCH: Weinheim, Germany, 2004; pp. 95–128. ISBN 3-527-30692-7. [Google Scholar]
- Derien, S.; Dixneuf, P.H. The Versatility of Molecular Ruthenium Catalyst RuCl(COD)(C5Me5). J. Organomet. Chem. 2004, 689, 1382–1392. [Google Scholar] [CrossRef]
- Nishibayashi, Y.; Wakiji, I.; Hidai, M. Novel Propargylic Substitution Reactions Catalyzed by Thiolate-Bridged Diruthenium Complexes via Allenylidene Intermediates. J. Am. Chem. Soc. 2000, 122, 11019–11020. [Google Scholar] [CrossRef]
- Hidai, M.; Mizobe, Y. Research Inspired by the Chemistry of Nitrogenase—Novel Metal Complexes and Their Reactivity Toward Dinitrogen, Nitriles, and Alkynes. Can. J. Chem. 2005, 83, 358–374. [Google Scholar] [CrossRef]
- Nishibayashi, Y. Transition-Metal-Catalyzed Enantioselective Propargylic Substitution Reactions of Propargylic Alcohol Derivatives with Nucleophiles. Synthesis 2012, 44, 489–503. [Google Scholar] [CrossRef]
- Roşca, D.-A.; Radkowski, K.; Wolf, L.M.; Wagh, M.; Goddard, R.; Thiel, W.; Fürstner, A. Ruthenium-Catalyzed Alkyne trans-Hydrometalation: Mechanistic Insights and Preparative Implications. J. Am. Chem. Soc. 2017, 139, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Rummelt, S.M.; Radkowski, K.; Roşca, D.-A.; Fürstner, A. Interligand Interactions Dictate the Regioselectivity of trans-Hydrometalations and Related Reactions Catalyzed by [Cp*RuCl]. Hydrogen Bonding to a Chloride Ligand as a Steering Principle in Catalysis. J. Am. Chem. Soc. 2015, 137, 5506–5519. [Google Scholar] [CrossRef] [PubMed]
- Rummelt, S.M.; Cheng, G.-J.; Gupta, P.; Thiel, W.; Fürstner, A. Hydroxy-Directed Ruthenium-Catalyzed Alkene/Alkyne Coupling: Increased Scope, Stereochemical Implications, and Mechanistic Rationale. Angew. Chem. Int. Ed. 2017, 56, 3599–3604. [Google Scholar] [CrossRef] [PubMed]
- Hase, S.; Kayaki, Y.; Ikariya, T. Mechanistic Aspects of the Carboxylative Cyclization of Propargylamines and Carbon Dioxide Catalyzed by Gold(I) Complexes Bearing an N-Heterocyclic Carbene Ligand. ACS Catal. 2015, 5, 5135–5140. [Google Scholar] [CrossRef]
- Kayaki, Y.; Yamamoto, M.; Ikariya, T. N-Heterocyclic Carbenes as Efficient Organocatalysts for CO2 Fixation Reactions. Angew. Chem. Int. Ed. 2009, 48, 4194–4197. [Google Scholar] [CrossRef] [PubMed]
- Kayaki, Y.; Koda, T.; Ikariya, T. Halide-Free Dehydrative Allylation Using Allylic Alcohols Promoted by a Palladium–Triphenyl Phosphite Catalyst. J. Org. Chem. 2004, 69, 2595–2597. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Kuwata, S.; Ikariya, T. Isolation and Interconversion of Protic N-Heterocyclic Carbene and Imidazolyl Complexes: Application to Catalytic Dehydrative Condensation of N-(2-Pyridyl)benzimidazole and Allyl Alcohol. Organometallics 2008, 27, 2176–2178. [Google Scholar] [CrossRef]
- Cadierno, V.; Gimeno, J. Allenylidene and Higher Cumulenylidene Complexes. Chem. Rev. 2009, 109, 3512–3560. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.J.; Singer, H. Die Dimerisierung von Endständigen α-Hydroxyacetylenen mit Rhodiumkomplexkatalysatoren. J. Organomet. Chem. 1978, 153, 165–179. [Google Scholar] [CrossRef]
- Katayama, H.; Ozawa, F. Vinylideneruthenium Complexes in Catalysis. Coord. Chem. Rev. 2004, 248, 1703–1715. [Google Scholar] [CrossRef]
- Yi, C.S.; Liu, N. Ruthenium-Catalyzed Coupling Reactions of Alkynes and Alkenes. Synlett 1999, 281–287. [Google Scholar] [CrossRef]
- Crochet, P.; Esteruelas, M.; Gutierrez-Puebla, E. Unusual Activation of 1,1-Diphenyl-2-propyn-1-ol Mediated by the Os(η5-C5H5) Unit. Organometallics 1998, 17, 3141–3142. [Google Scholar] [CrossRef]
- Dutta, B.; Curchod, B.F.E.; Campomanes, P.; Solari, E.; Scopelliti, R.; Rothlisberger, U.; Severin, K. Reactions of Alkynes with [RuCl(cyclopentadienyl)] Complexes: The Important First Steps. Chem. Eur. J. 2010, 16, 8400–8409. [Google Scholar] [CrossRef] [PubMed]
- Le Paih, J.; Derien, S.; Bruneau, C.; Demerseman, B.; Toupet, L.; Dixneuf, P.H. Ruthenium-Catalyzed One-Step Transformation of Propargylic Alcohols Into Alkylidene Cyclobutenes: X-ray Characterization of an Ru(η3-cyclobutenyl) Intermediate. Angew. Chem. Int. Ed. 2001, 40, 2912–2915. [Google Scholar] [CrossRef]
- Trost, B.M.; Rudd, M.T. An Unusual Ruthenium-Catalyzed Dimerization of Propargyl Alcohols. J. Am. Chem. Soc. 2001, 123, 8862–8863. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Rudd, M.T. An Unusual Ruthenium-Catalyzed Cycloisomerization of Alkynes and Propargyl Alcohols. J. Am. Chem. Soc. 2002, 124, 4178–4179. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Chan, P.W.H. Unexpected Iron(III) Chloride-Catalysed Dimerisation of 1,1,3-Trisubstituted-prop-2-yn-1-ols as an Expedient Route to Highly Conjugated Indenes. Org. Biomol. Chem. 2010, 8, 4016–4025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, C.; Lin, Y.; He, X.; Zhang, Y.; Wang, J.; Xia, H. 1,2-Migration in the Reactions of Ruthenium Vinyl Carbene with Propargyl Alcohols. Org. Chem. Front. 2014, 1, 1077–1082. [Google Scholar] [CrossRef]
- Fagan, P.J.; Mahoney, W.S.; Calabrese, J.C.; Williams, I.D. Structure and Chemistry of the Complex Tetrakis(η5-pentamethylcyclopentadienyl)tetrakis(μ3-chloro)tetraruthenium(II): A Useful Precursor to (Pentamethylcyclopentadienyl)ruthenium(0), -(II), and -(IV) Complexes. Organometallics 1990, 9, 1843–1852. [Google Scholar] [CrossRef]
- Radkowski, K.; Sundararaju, B.; Fürstner, A. A Functional-Group-Tolerant Catalytic trans Hydrogenation of Alkynes. Angew. Chem. Int. Ed. 2012, 52, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Crystal Structure 4.1: Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2000–2015.
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camallli, M. SIR92—A Program for Automatic Solution of Crystal Structures by Direct Methods. J. Appl. Crystallogr. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Serron, S.A.; Luo, L.; Stevens, E.D.; Nolan, S.P.; Jones, N.L.; Fagan, P.J. Organoruthenium Thermochemistry. Enthalpies of Reaction of Cp’Ru(COD)Cl (Cp’ = η5-C5H5 and η5-C5Me5) with Tertiary Phosphite Ligands. Organometallics 1996, 15, 5209–5215. [Google Scholar] [CrossRef]
- Bosson, J.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanism of Racemization of Chiral Alcohols Mediated by 16-Electron Ruthenium Complexes. J. Am. Chem. Soc. 2010, 132, 13146–13149. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.N.; Tashima, N.; Ikariya, T.; Kuwata, S. Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions. Inorganics 2017, 5, 80. https://doi.org/10.3390/inorganics5040080
Nguyen HN, Tashima N, Ikariya T, Kuwata S. Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions. Inorganics. 2017; 5(4):80. https://doi.org/10.3390/inorganics5040080
Chicago/Turabian StyleNguyen, Hoang Ngan, Naoto Tashima, Takao Ikariya, and Shigeki Kuwata. 2017. "Ruthenium-Catalyzed Dimerization of 1,1-Diphenylpropargyl Alcohol to a Hydroxybenzocyclobutene and Related Reactions" Inorganics 5, no. 4: 80. https://doi.org/10.3390/inorganics5040080




