Synthesis of LiAlH4 Nanoparticles Leading to a Single Hydrogen Release Step upon Ti Coating
Abstract
:1. Introduction
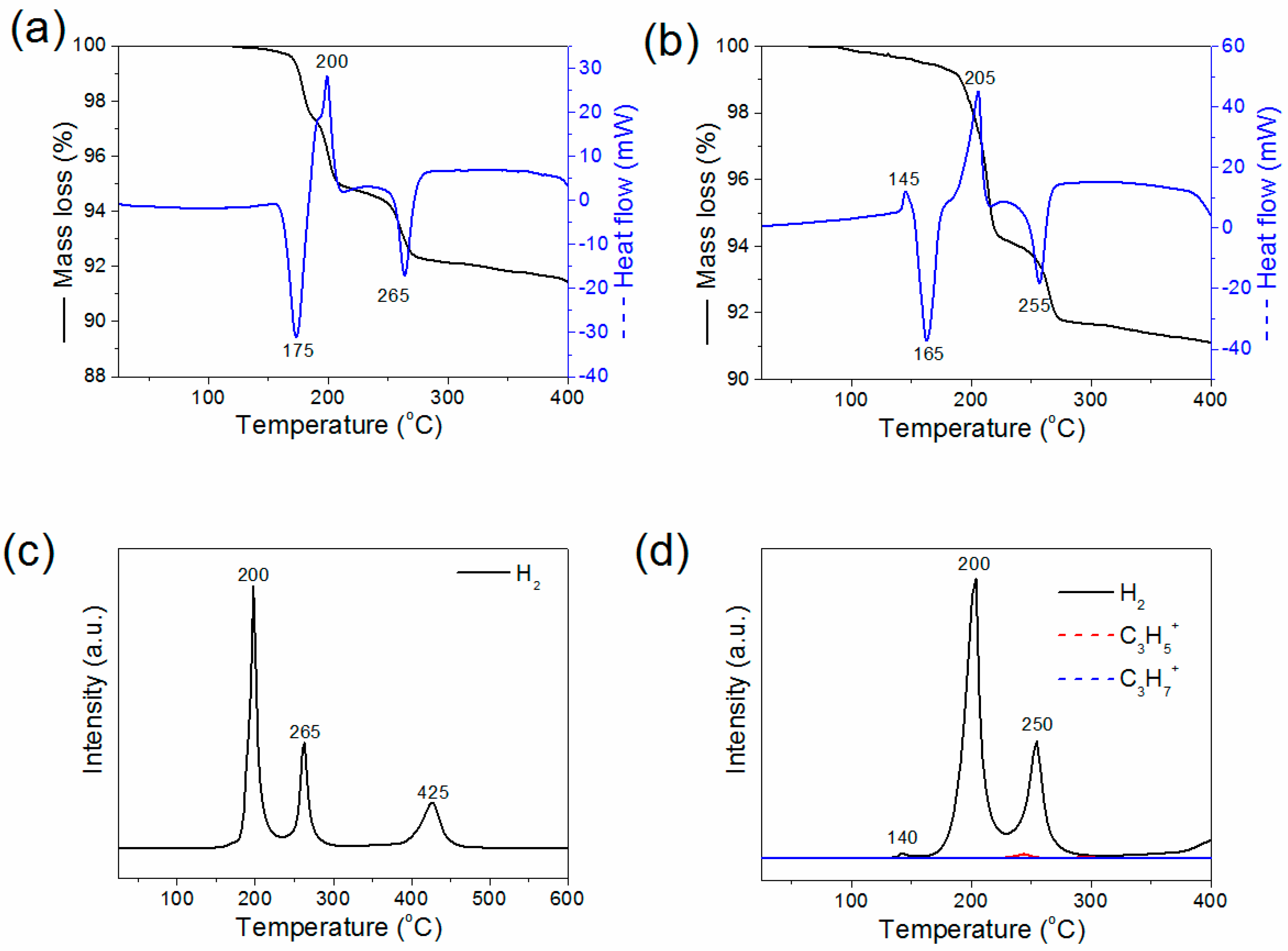
| Step 1a | 150–175 °C | |
| LiAlH4(solid)→ LiAlH4(liquid) | endothermic | |
| Step 1b | 150–200 °C | |
| LiAlH4(liquid) → 1/3 Li3AlH6(solid) + 2/3 Al + H2↑ | exothermic | 5.3 mass % H2 |
| Step 2 | 200–270 °C | |
| Li3AlH6(solid) → 3 LiH + Al + 3/2 H2↑ | endothermic | 2.6 mass % H2 |
| Step 3 | 400–440 °C | |
| LiH + Al → LiAl + 1/2 H2 ↑ | endothermic | 2.6 mass % H2 |
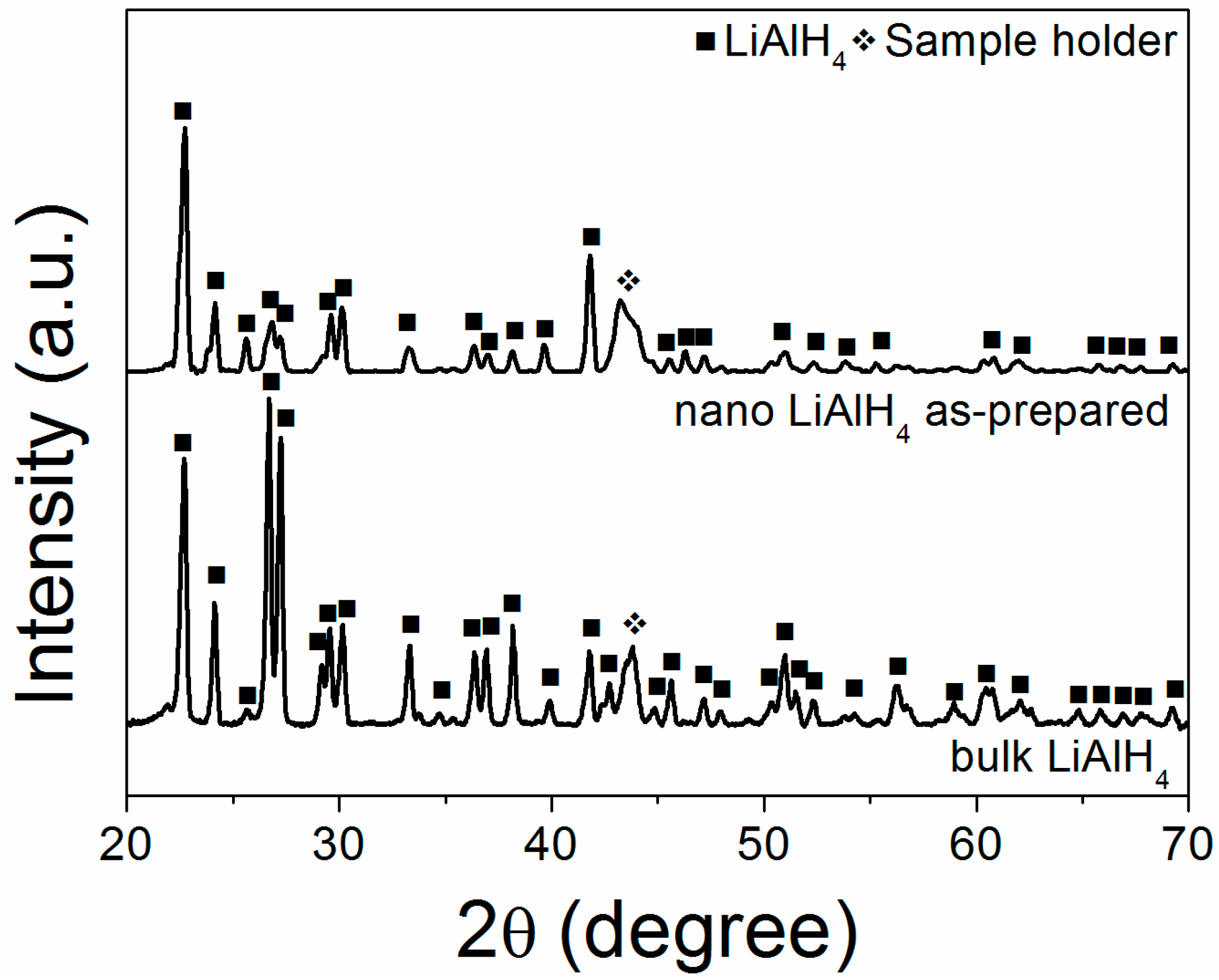
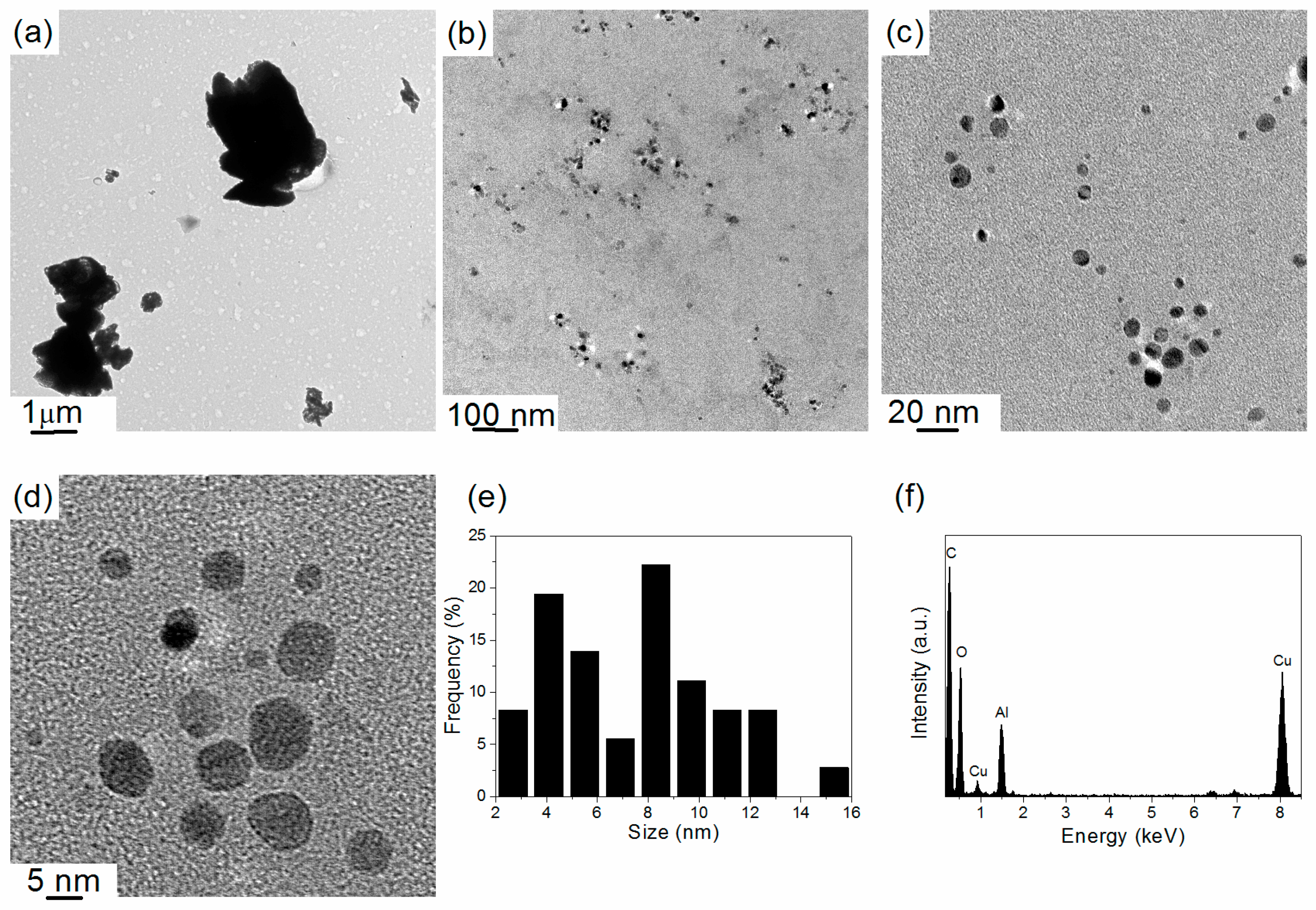
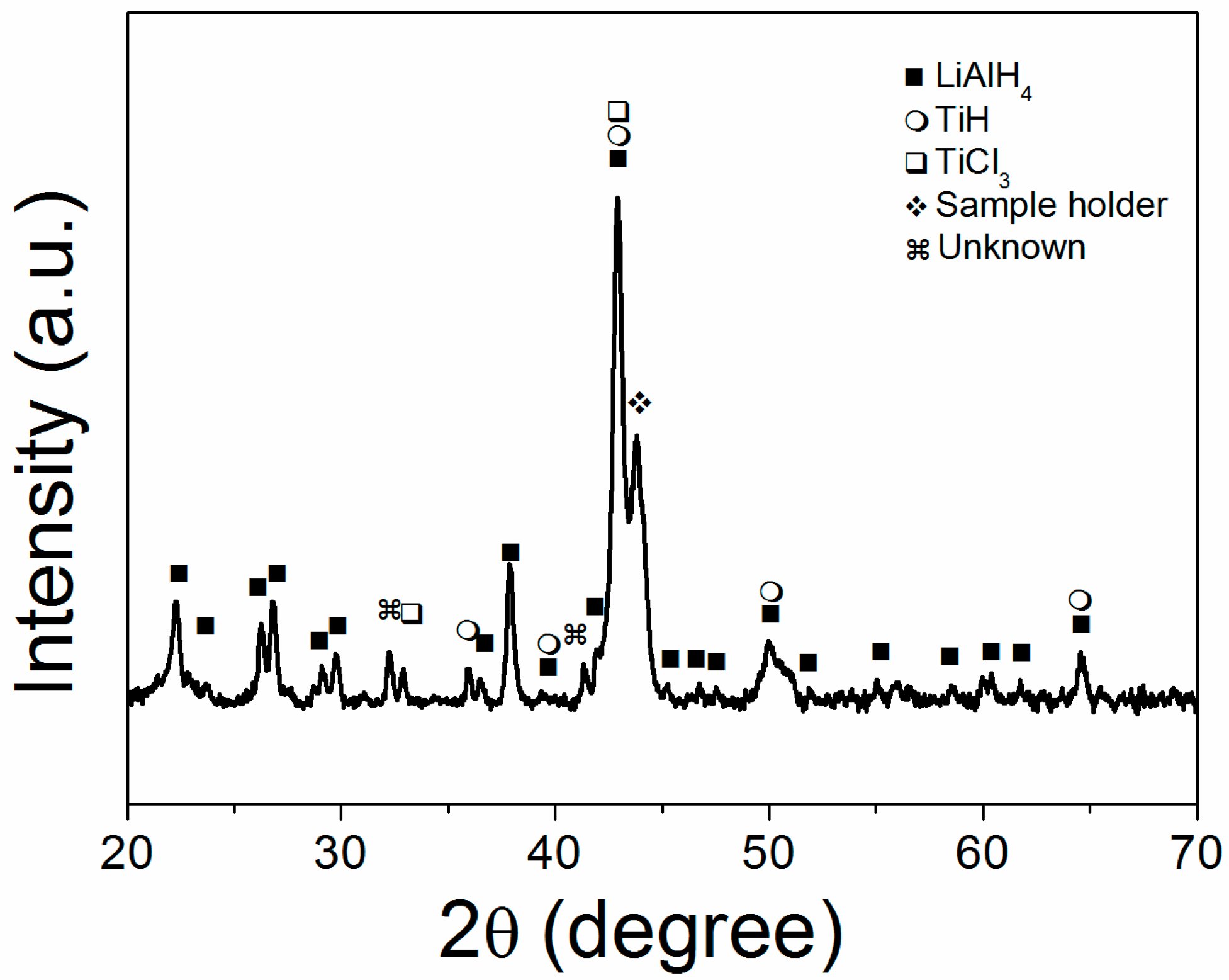
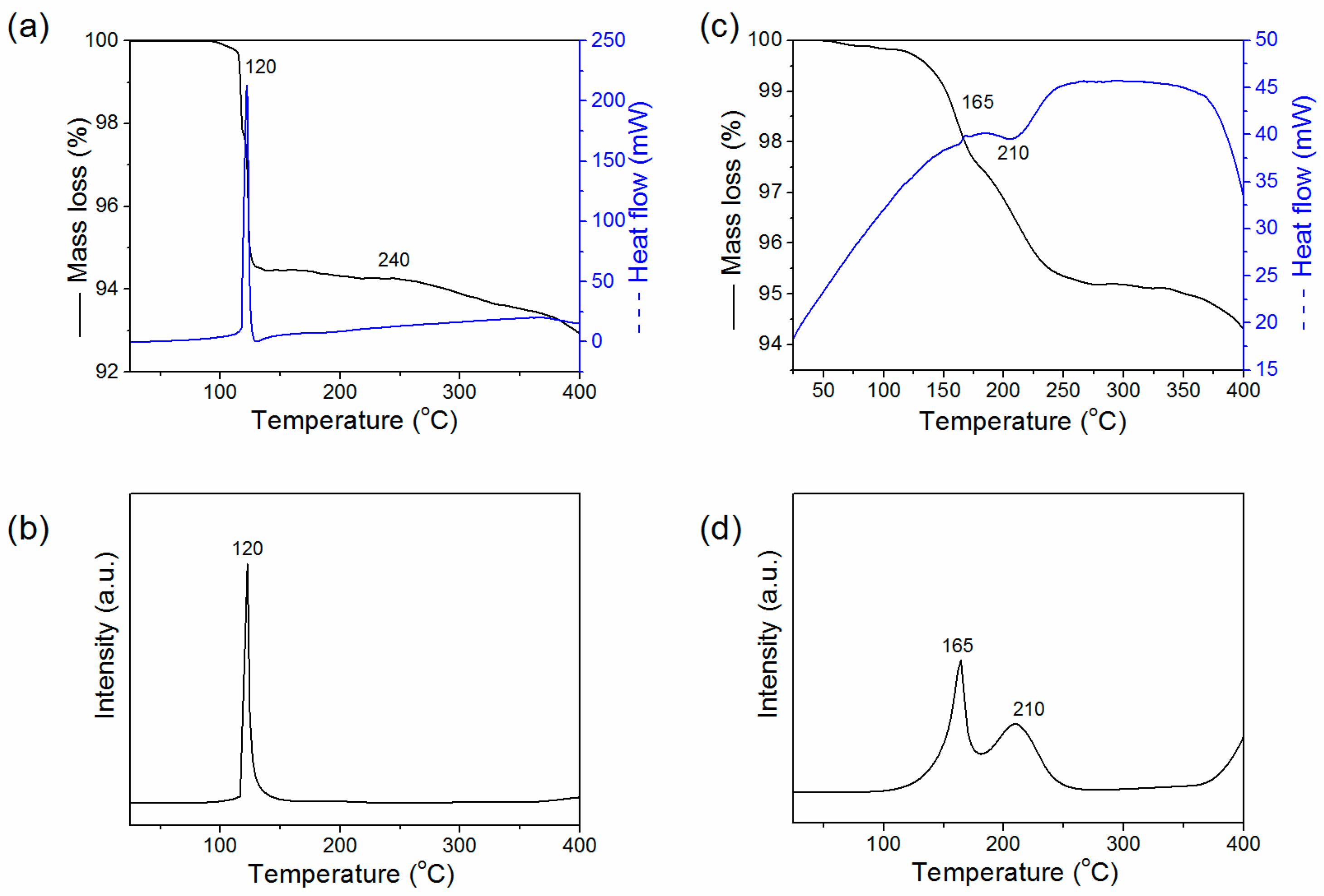
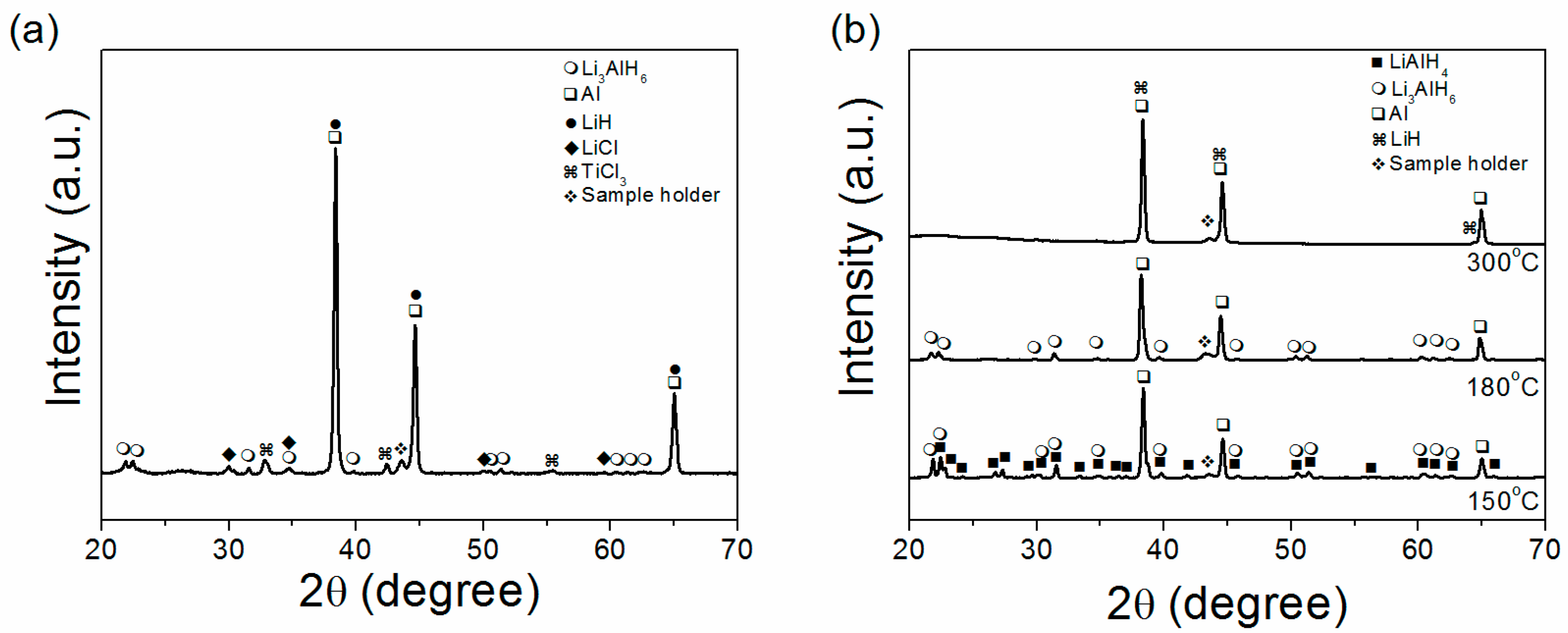
2. Results
3. Materials and Methods
3.1. Synthesis of LiAlH4 Nanoparticles
3.2. Coating of the LiAlH4 Nanoparticles with Ti
3.3. Preparation of Reference Material Ball Milled LiAlH4 with TiCl3
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials; In Tech: Rijeka, Croatia, 2011. [Google Scholar]
- Jang, J.W.; Shim, J.H.; Cho, Y.W.; Lee, B.J. Thermodynamic calculation of LiH ↔ Li3AlH6 ↔ LiAlH4 reactions. J. Alloys Compd. 2006, 420, 286–290. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer: Berlin, Germany, 2009. [Google Scholar]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Lacina, D.; Yang, L.; Chopra, I.; Muckerman, J.; Chabal, Y.; Graetz, J. Investigation of LiAlH4–THF formation by direct hydrogenation of catalyzed Al and LiH. Phys. Chem. Chem. Phys. 2012, 14, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.A.; Zbroniec, L. The effects of nanometric nickel (n-Ni) catalyst on the dehydrogenation and rehydrogenation behavior of ball milled lithium alanate (LiAlH4). J. Alloy. Compd. 2010, 506, 928–939. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinsky, A.A.; Qu, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef]
- Amama, P.B.; Grant, J.T.; Shamberger, P.J.; Voevodin, A.A.; Fisher, T.S. Improved Dehydrogenation Properties of Ti-Doped LiAlH4: Role of Ti Precursors. J. Phys. Chem. 2012, 116, 21886–21894. [Google Scholar] [CrossRef]
- Easton, D.S.; Schneibel, J.H.; Speakman, S.A. Factors affecting hydrogen release from lithium alanate (LiAlH4). J. Alloy Compd. 2005, 398, 245–248. [Google Scholar] [CrossRef]
- Fu, J.; Tegel, M.; Kieback, B.; Röntzsch, L. Dehydrogenation properties of doped LiAlH4 compacts for hydrogen generator applications. Int. J. Hydrog. Energy 2014, 39, 16362–16371. [Google Scholar] [CrossRef]
- Liu, X.; McGrady, G.S.; Langmi, H.W.; Jensen, C.M. Facile cycling of Ti-doped LiAlH4 for high performance hydrogen storage. J. Am. Chem. Soc. 2009, 131, 5032–5033. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Wegrzyn, J.; Reilly, J.J. Regeneration of lithium aluminum hydride. Chem. Inf. 2008, 130, 17790–17794. [Google Scholar] [CrossRef] [PubMed]
- Ashby, E.C.; Brendel, G.J.; Redman, H.E. Direct Synthesis of Complex Metal Hydrides. Inorg. Chem. 1963, 2, 499–504. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Ritter, J.A. Physiochemical Pathway for Cyclic Dehydrogenation and Rehydrogenation of LiAlH4. J. Am. Chem. Soc. 2006, 128, 5949–5954. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ebner, A.D.; Ritter, J.A. Synthesis of metal complex hydrides for hydrogen storage. J. Phys. Chem. 2007, 111, 14917–14924. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Xu, Q.; Takeshita, H.T.; Sakai, T. Reversible hydrogen storage via titanium-catalyzed LiAlH4 and Li3AlH6. J. Phys. Chem. 2001, 105, 11214–11220. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J Hydrog. Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Wang, L.; Quadir, M.Z.; Aguey-Zinsou, K.F. Ni coated LiH nanoparticles for reversible hydrogen storage. Int. J Hydrog. Energy 2016, 41, 6376–6386. [Google Scholar] [CrossRef]
- Christian, M.L.; Aguey-Zinsou, K.F. Core–shell strategy leading to high reversible hydrogen storage capacity for NaBH4. ACS Nano 2012, 6, 7739–7751. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, B.; Schwickardi, M. Ti-doped NaAlH4 as a hydrogen-storage material—Preparation by Ti-catalyzed hydrogenation of aluminum powder in conjunction with sodium hydride. Appl. Phys. 2001, 72, 221–223. [Google Scholar] [CrossRef]
- Blanchard, D.; Brinks, H.W.; Hauback, B.C.; Norby, P. Desorption of LiAlH4 with Ti- and V-based additives. Mat. Sci. Eng. Solid 2004, 108, 54–59. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Haga, T.; Matsumoto, M.; Koiwai, A. Direct formation of LiAlH4 by a mechanochemical reaction. J. Alloy. Compd. 2007, 441, 189–191. [Google Scholar] [CrossRef]
- Liu, X.; Langmi, H.W.; Beattie, S.D.; Azenwi, F.F.; McGrady, G.S.; Jensen, C.M. High-yield direct synthesis of LiAlH4 from LiH and Al in the Presence of TiCl3 and Me2O. J. Am. Chem. Soc. 2011. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Walker, G. Solid-State Hydrogen Storage: Materials and Chemistry; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kojima, Y.; Kawai, Y.; Matsumoto, M.; Haga, T. Hydrogen release of catalyzed lithium aluminum hydride by a mechanochemical reaction. J. Alloy Compd. 2008, 462, 275–278. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.W.; Aguey-Zinsou, K.F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2017. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Shaw, L.L. Novel dehydrogenation properties derived from nanoscale LiBH4. Acta Mater. 2011, 59, 4606–4615. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Bensebaa, F. Chapter 2—Wet production methods. In Interface Science and Technology; Farid, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 85–146. [Google Scholar]
- Wang, L.S.; Hong, R.Y. Synthesis, surface modification and characterisation of nanoparticles. Adv. Nanocompos. 2011, 18, 7533–7548. [Google Scholar]
- Kühnle, A.; Vollmer, S.; Linderoth, T.R.; Witte, G.; Besenbacher, F. Adsorption of dodecanethiol on Cu (110): Structural ordering upon thiolate formation. Langmuir 2002, 18, 5558–5565. [Google Scholar] [CrossRef]
- Stein, S.E. “Mass Spectra” in NIST Chemistry WebBook. In NIST Standard Reference Database Number 69; NIST Mass Spec Data Center: Gaithersburg, MD, USA, 2016.
- Chaudhuri, S.; Muckerman, J.T. First-principles study of Ti-catalyzed hydrogen chemisorption on an al surface: A critical first step for reversible hydrogen storage in NaAlH4. J. Phys. Chem. 2005, 109, 6952–6957. [Google Scholar] [CrossRef] [PubMed]
- Wipf, H.; Kappesser, B.; Werner, R. Hydrogen diffusion in titanium and zirconium hydrides. J. Alloy Compd. 2000, 310, 190–195. [Google Scholar] [CrossRef]
- Livanov, V.A.; Bukhanova, A.A.; Kolachev, B. Hydrogen in Titanium; Israel Program for Scientific Translations: Jerusalem, Israel, 1965. [Google Scholar]
- Gu, J.; Zhang, Y.-W.; Tao, F. Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches. Chem. Soc. Rev. 2012, 41, 8050–8065. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Rittmeyer, P.; Wietelmann, U. Hydrides. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co: Berlin, Germany, 2000. [Google Scholar]
- Liu, X.; Beattie, S.D.; Langmi, H.W.; McGrady, G.S.; Jensen, C.M. Ti-doped LiAlH4 for hydrogen storage: Rehydrogenation process, reaction conditions and microstructure evolution during cycling. Int. J. Hydrog. Energy 2012, 37, 10215–10221. [Google Scholar] [CrossRef]
- Resan, M.; Hampton, M.D.; Lomness, J.K.; Slattery, D.K. Effects of various catalysts on hydrogen release and uptake characteristics of LiAlH. Int. J. Hydrog. Energy 2005, 30, 1413–1416. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of TiH2. Mat. Sci. Eng. A Struct. 2003, 356, 190–199. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Aguey-Zinsou, K.-F. Synthesis of LiAlH4 Nanoparticles Leading to a Single Hydrogen Release Step upon Ti Coating. Inorganics 2017, 5, 38. https://doi.org/10.3390/inorganics5020038
Wang L, Aguey-Zinsou K-F. Synthesis of LiAlH4 Nanoparticles Leading to a Single Hydrogen Release Step upon Ti Coating. Inorganics. 2017; 5(2):38. https://doi.org/10.3390/inorganics5020038
Chicago/Turabian StyleWang, Lei, and Kondo-Francois Aguey-Zinsou. 2017. "Synthesis of LiAlH4 Nanoparticles Leading to a Single Hydrogen Release Step upon Ti Coating" Inorganics 5, no. 2: 38. https://doi.org/10.3390/inorganics5020038





