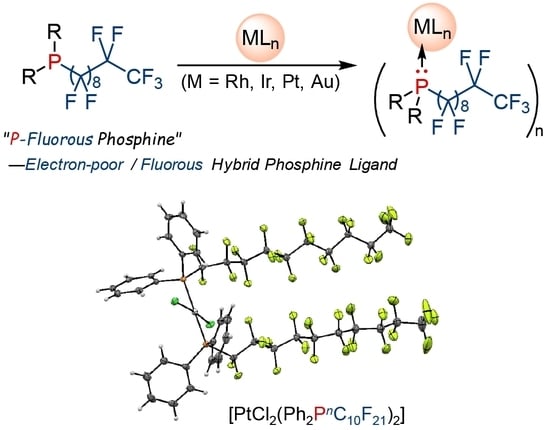
P-Fluorous Phosphines as Electron-Poor/Fluorous Hybrid Functional Ligands for Precious Metal Catalysts: Synthesis of Rh(I), Ir(I), Pt(II), and Au(I) Complexes Bearing P-Fluorous Phosphine Ligands
Abstract
:1. Introduction


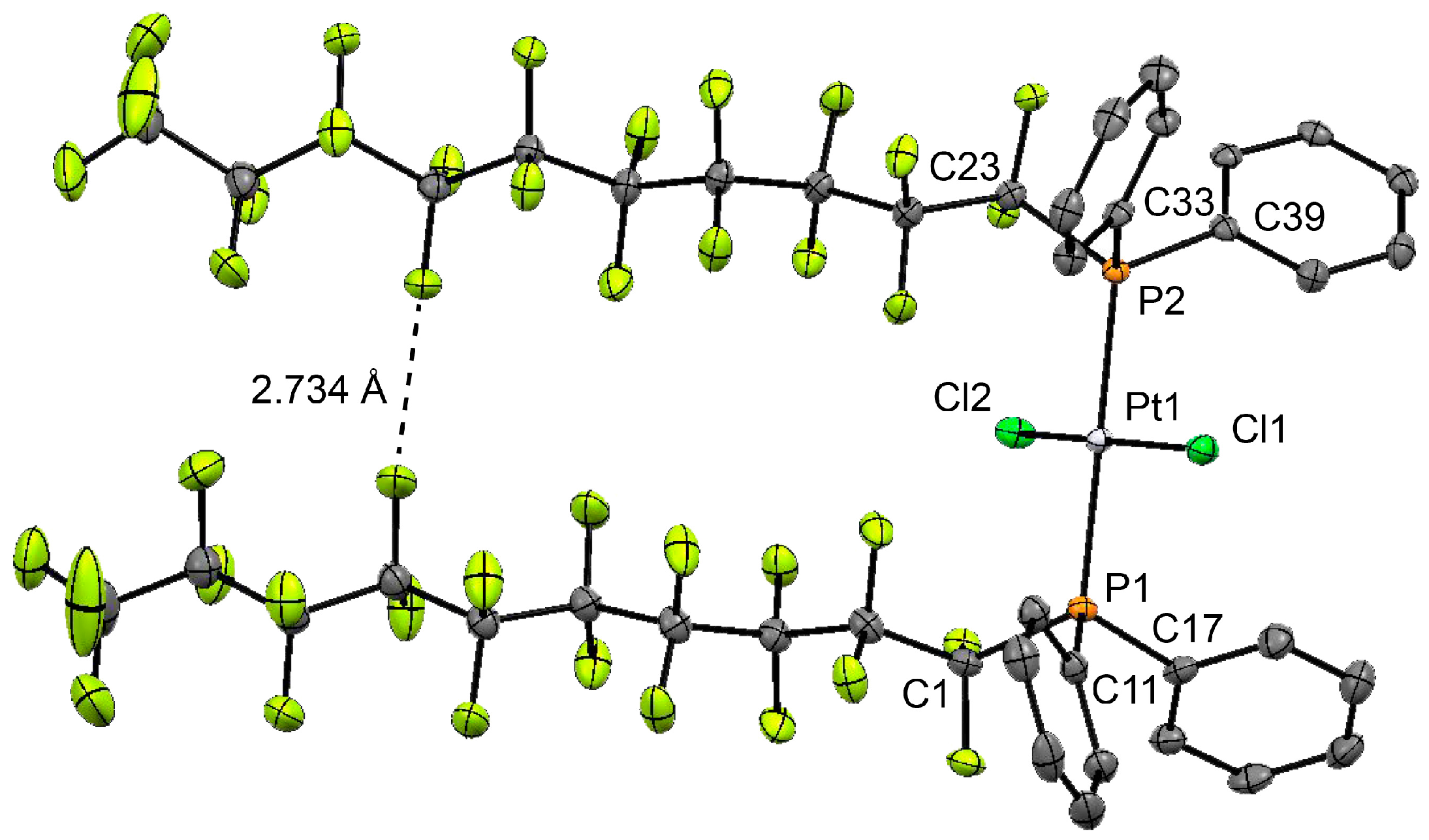
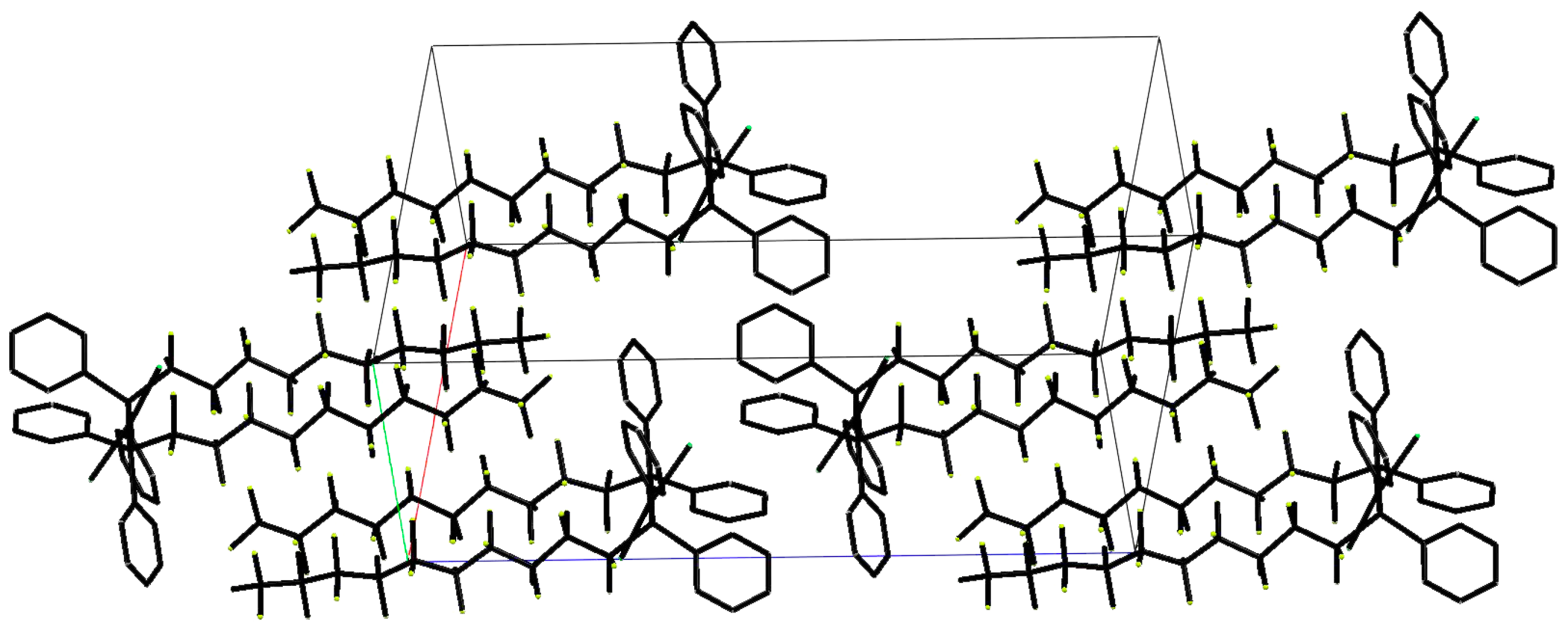
2. Results and Discussion






3. Materials and Methods
3.1. General Comments
3.2. Synthesis of P-Perfluoroalkylated Phosphine Complex with Pt(II)
3.3. Synthesis of P-Perfluoroalkylated Phosphine Complex with Rh(I)
3.4. Synthesis of P-Perfluoroalkylated Phosphine Complex with Ir(I)
3.5. Hydrosilylation Reaction Catalyzed by Iridium Complex (4a)
3.6. Synthesis of P-Perfluoroalkylated Phosphine Complex with Au(I)
3.7. Au Complex-Catalyzed Addition Reaction of 2-Chloroethanol to 1-Octene
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banger, K.K.; Brisdon, A.K.; Herbert, C.J.; Ghaba, H.A.; Tidmarsh, I.S. Fluoroalkenyl, fluoroalkynyl and fluoroalkyl phosphines. J. Fluor. Chem. 2009, 130, 1117–1129. [Google Scholar] [CrossRef]
- Gládysz, J.A.; Curran, D.P.; Horváth, I.T. Handbook of Fluorous Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Betzemeier, B.; Knochel, P. Palladium-catalyzed cross-coupling of organozinc bromides with aryl iodides in perfluorinated solvents. Angew. Chem. Int. Ed. 1997, 36, 2623–2624. [Google Scholar] [CrossRef]
- Horváth, I.T. Fluorous biphase chemistry. Acc. Chem. Res. 1998, 31, 641–650. [Google Scholar] [CrossRef]
- Rábai, J.; Szabó, D.; Borbás, E.K.; Kövesi, I.; Kövsedi, I.; Csámpai, A.; Gömöry, A.; Pashinnik, V.E.; Shermolovich, Y.G. Practice of fluorous biphase chemistry: Convenient synthesis of novel fluorophilic ethers via a mitsunobu reaction. J. Fluor. Chem. 2002, 114, 199–207. [Google Scholar] [CrossRef]
- Moineau, J.; Pozzi, G.; Quici, S.; Sinou, D. Palladium-catalyzed Heck reaction in perfluorinated solvents. Tetrahedron Lett. 1999, 40, 7683–7686. [Google Scholar] [CrossRef]
- Tzschucke, C.C.; Markert, C.; Glatz, H.; Bannwarth, W. Fluorous biphasic catalysis without perfluorinated solvents: Application to Pd-mediated Suzuki and Sonogashira couplings. Angew. Chem. Int. Ed. 2002, 41, 4500–4503. [Google Scholar] [CrossRef]
- Horváth, I.T.; Rábai, J. Facile catalyst separation without water—Fluorous biphase hydroformylation of olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, T.; Ko, A.; Uotani, K.; Tanaka, Y.; Sakai, T. Synthesis and application of 2,6-bis(trifluoromethyl)-4-pyridyl phosphanes: The most electron-poor aryl phosphanes with moderate bulkiness. Angew. Chem. Int. Ed. 2011, 50, 10703–10707. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, T.; Abe, K.; Ko, A.; Maenishi, R.; Sakai, T. Ligand electronic effect on reductive elimination of biphenyl from cis-[Pt(Ph)2(diphosphine)] complexes bearing electron-poor diphosphine: Correlation study between experimental and theoretical results. Organometallics 2010, 29, 4025–4035. [Google Scholar] [CrossRef]
- Korenaga, T.; Osaki, K.; Maenishi, R.; Sakai, T. Electron-poor chiral diphosphine ligands: High performance for Rh-catalyzed asymmetric 1,4-addition of arylboronic acids at room temperature. Org. Lett. 2009, 11, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Dong, H.-Q.; Lin, G.-Q. Rhodium-catalyzed asymmetric arylation. ACS Catal. 2012, 2, 95–119. [Google Scholar] [CrossRef]
- Kawaguchi, S-i.; Minamida, Y.; Okuda, T.; Sato, Y.; Saeki, T.; Yoshimura, A.; Nomoto, A.; Ogawa, A. Photoinduced synthesis of P-perfluoroalkylated phosphines from triarylphosphines and their application in the copper-free cross-coupling of acid chlorides and terminal alkynes. Adv. Synth. Catal. 2015, 357, 2509–2519. [Google Scholar] [CrossRef]
- Kawaguchi, S-i.; Minamida, Y.; Ohe, T.; Nomoto, A.; Sonoda, M.; Ogawa, A. Synthesis and properties of perfluoroalkyl phosphine ligands: Photoinduced reaction of diphosphines with perfluoroalkyl iodides. Angew. Chem. Int. Ed. 2013, 52, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawaguchi, S-i.; Ogawa, A. Photoinduced reductive perfluoroalkylation of phosphine oxides: Synthesis of P-perfluoroalkylated phosphines using TMDPO and perfluoroalkyl iodides. Chem. Commun. 2015, 51, 10385–10388. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.C.; Roddick, D.M. (Fluoroalkyl)phosphine complexes of rhodium and iridium—Synthesis and reactivity properties of [(dfepe)Ir(μ-Cl)]2. Inorg. Chem. 1993, 32, 1513–1518. [Google Scholar] [CrossRef]
- Banger, K.K.; Banham, R.P.; Brisdon, A.K.; Cross, W.I.; Damant, G.; Parsons, S.; Pritchard, R.G.; Sousa-Pedrares, A. Synthesis and coordination chemistry of perfluorovinyl phosphine derivatives. Single crystal structures of PPh(CF=CF2)2, cis-[PtCl2{PPh2(CF=CF2)}2] and [{AuCl[PPh2(CF=CF2)]}2]. J. Chem. Soc. Dalton Trans. 1999, 427–434. [Google Scholar] [CrossRef]
- Palcic, J.D.; Kapoor, P.N.; Roddick, D.M.; Peters, R.G. Perfluoroalkylphosphine coordination chemistry of platinum: Synthesis of (C2F5)2PPh and (C2F5)PPh2 complexes of platinum(II). Dalton Trans. 2004, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Alleyne, L.C.; Murphy-Jolly, M.B.; Le Goff, X.F.; Caffyn, A.J.M. Synthesis and complexation of heptafluoroisopropyldiphenylphosphine. Dalton Trans. 2010, 39, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.A.; Brisdon, A.K.; William Brown, F.R.; Cross, W.I.; Crossley, I.R.; Fish, C.; Herbert, C.J.; Pritchard, R.G.; Warren, J.E. Synthesis of gold(I) fluoroalkyl and fluoroalkenyl-substituted phosphine complexes and factors affecting their crystal packing. Dalton Trans. 2011, 40, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.H.; Otto, S. trans-Dichlorobis(triphenylphosphine-P)platinum(II). Acta Crystallogr. Sect. C 2000, 56, e12–e15. [Google Scholar] [CrossRef]
- Clarke, M.L.; Ellis, D.; Mason, K.L.; Orpen, A.G.; Pringle, P.G.; Wingad, R.L.; Zaher, D.A.; Baker, R.T. The electron-poor phosphines P{C6H3(CF3)2-3,5}3 and P(C6F5)3 do not mimic phosphites as ligands for hydroformylation. A comparison of the coordination chemistry of P{C6H3(CF3)2-3,5}3 and P(C6F5)3 and the unexpectedly low hydroformylation activity of their rhodium complexes. Dalton Trans. 2005, 1294–1300. [Google Scholar] [CrossRef]
- Yajima, T.; Tabuchi, E.; Nogami, E.; Yamagishi, A.; Sato, H. Perfluorinated gelators for solidifying fluorous solvents: Effects of chain length and molecular chirality. RSC Adv. 2015, 5, 80542–80547. [Google Scholar] [CrossRef]
- Skalická, V.; Rybáčková, M.; Skalický, M.; Kvíčalová, M.; Cvačka, J.; Březinová, A.; Čejka, J.; Kvíčala, J. Polyfluoroalkylated tripyrazolylmethane ligands: Synthesis and complexes. J. Fluor. Chem. 2011, 132, 434–440. [Google Scholar] [CrossRef]
- Haar, C.M.; Huang, J.; Nolan, S.P.; Petersen, J.L. Synthetic, thermochemical, and catalytic studies involving novel R2P(ORf) [R = alkyl or aryl; Rf = CH2CH2(CF2)5CF3] ligands. Organometallics 1998, 17, 5018–5024. [Google Scholar] [CrossRef]
- Roodt, A.; Otto, S.; Steyl, G. Structure and solution behaviour of rhodium(I) Vaska-type complexes for correlation of steric and electronic properties of tertiary phosphine ligands. Coord. Chem. Rev. 2003, 245, 121–137. [Google Scholar] [CrossRef]
- Vaska, L.; DiLuzio, J.W. Carbonyl and hydrido-carbonyl complexes of iridium by reaction with alcohols. Hydrido complexes by reaction with acid. J. Am. Chem. Soc. 1961, 83, 2784–2785. [Google Scholar] [CrossRef]
- Rappoli, B.J.; Churchill, M.R.; Janik, T.S.; Rees, W.M.; Atwood, J.D. Crystal structure of the quasitetrahedral iridium(I) complex, Ir(COCH2CMe3)[P(p-tolyl)3]2[C2(CO2Me)2]. An intermediate in cyclotrimerization of activated alkynes by 16-electron alkyl complexes of iridium, trans-RIr(CO)L2 [R = Me, CH2CMe3; L = PPh3, P(p-tolyl)3]. J. Am. Chem. Soc. 1987, 109, 5145–5149. [Google Scholar]
- Vrieze, K.; Collman, J.P.; Sears, C.T.; Kubota, M.; Davison, A.; Shawl, E.T. Trans-chlorocarbonylbis(tri-phenylphosphine)iridium. In Inorganic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1968; pp. 101–104. [Google Scholar]
- Tanke, R.S.; Crabtree, R.H. Unusual activity and selectivity in alkyne hydrosilylation with an iridium catalyst stabilized by an oxygen-donor ligand. J. Am. Chem. Soc. 1990, 112, 7984–7989. [Google Scholar] [CrossRef]
- Apple, D.C.; Brady, K.A.; Chance, J.M.; Heard, N.E.; Nile, T.A. Iridium complexes as hydrosilylation catalysts. J. Mol. Catal. 1985, 29, 55–64. [Google Scholar] [CrossRef]
- Hesp, K.D.; Wechsler, D.; Cipot, J.; Myers, A.; McDonald, R.; Ferguson, M.J.; Schatte, G.; Stradiotto, M. Exploring the utility of neutral rhodium and iridium κ2-P,O and κ2-P(S),O complexes as catalysts for alkene hydrogenation and hydrosilylation. Organometallics 2007, 26, 5430–5437. [Google Scholar] [CrossRef]
- Murai, T.; Nagaya, E.; Shibahara, F.; Maruyama, T.; Nakazawa, H. Rhodium(I) and iridium(I) imidazo[1,5-a]pyridine-1-ylalkylalkoxy complexes: Synthesis, characterization and application as catalysts for hydrosilylation of alkynes. J. Organomet. Chem. 2015, 794, 76–80. [Google Scholar] [CrossRef]
- Shin, S. Tris-(pentafluorophenyl)phosphine gold(I) complexes as new highly efficient catalysts for the oxycarbonylation of homopropargyl carbonates. Bull. Korean Chem. Soc. 2005, 26, 1925–1926. [Google Scholar] [CrossRef]
- Hirai, T.; Hamasaki, A.; Nakamura, A.; Tokunaga, M. Enhancement of reaction efficiency by functionalized alcohols on gold(I)-catalyzed intermolecular hydroalkoxylation of unactivated olefins. Org. Lett. 2009, 11, 5510–5513. [Google Scholar] [CrossRef]
- Takeuchi, R.; Tanouchi, N. Solvent-controlled stereoselectivity in the hydrosilylation of alk-1-ynes catalysed by rhodium complexes. J. Chem. Soc. Perkin Trans. 1 1994, 2909–2913. [Google Scholar] [CrossRef]

| Entry | RhCl(CO)(PR3)2 | ν(CO) (cm−1) |
|---|---|---|
| 1 | RhCl(CO)(Ph2PnC10F21)2 | 2010 |
| 2 | RhCl(CO)(nBu2PnC10F21)2 | 1987 |
| 3 | RhCl(CO)(PPh3)2 | 1967 |
| 4 | RhCl(CO)(P(4-CF3C6H4)3)2 | 1990 |
| 5 | RhCl(CO)(P(C6F5)3)2 | 2008 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, S.-i.; Saga, Y.; Sato, Y.; Minamida, Y.; Nomoto, A.; Ogawa, A. P-Fluorous Phosphines as Electron-Poor/Fluorous Hybrid Functional Ligands for Precious Metal Catalysts: Synthesis of Rh(I), Ir(I), Pt(II), and Au(I) Complexes Bearing P-Fluorous Phosphine Ligands. Inorganics 2017, 5, 5. https://doi.org/10.3390/inorganics5010005
Kawaguchi S-i, Saga Y, Sato Y, Minamida Y, Nomoto A, Ogawa A. P-Fluorous Phosphines as Electron-Poor/Fluorous Hybrid Functional Ligands for Precious Metal Catalysts: Synthesis of Rh(I), Ir(I), Pt(II), and Au(I) Complexes Bearing P-Fluorous Phosphine Ligands. Inorganics. 2017; 5(1):5. https://doi.org/10.3390/inorganics5010005
Chicago/Turabian StyleKawaguchi, Shin-ichi, Yuta Saga, Yuki Sato, Yoshiaki Minamida, Akihiro Nomoto, and Akiya Ogawa. 2017. "P-Fluorous Phosphines as Electron-Poor/Fluorous Hybrid Functional Ligands for Precious Metal Catalysts: Synthesis of Rh(I), Ir(I), Pt(II), and Au(I) Complexes Bearing P-Fluorous Phosphine Ligands" Inorganics 5, no. 1: 5. https://doi.org/10.3390/inorganics5010005