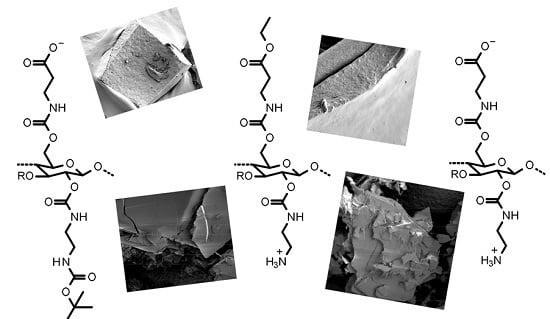
Water-Soluble Cellulose Derivatives Are Sustainable Additives for Biomimetic Calcium Phosphate Mineralization
Abstract
:1. Introduction
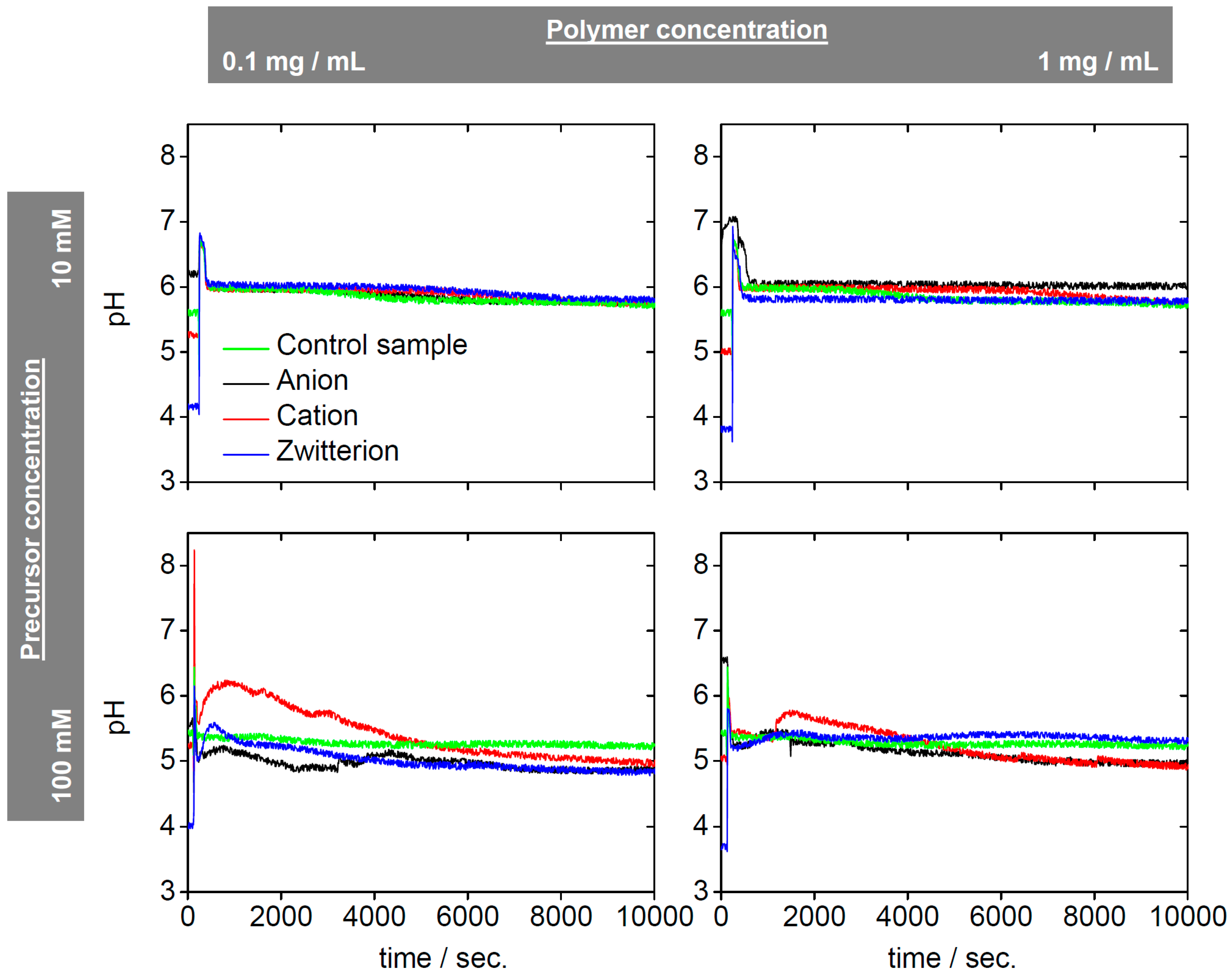
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mineralization Reactions
4.3. Characterization
4.3.1. Infrared (IR) Spectroscopy
4.3.2. Raman Spectroscopy
4.3.3. Scanning Electron Microscopy (SEM)
4.3.4. Energy Dispersive X-ray Spectroscopy (EDXS)
4.3.5. Thermogravimetric Analysis (TGA)
4.3.6. X-ray Diffraction (XRD)
4.3.7. Elemental Analysis (EA)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qiu, K.Y.; Netravali, A.N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Tai, B.C.U. Chitin—A promising biomaterial for tissue engineering and stem cell technologies. Biotechnol. Adv. 2013, 31, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.M.; Pires, R.A.; Mano, J.F.; Reis, R.L. Bionanocomposites from lignocellulosic resources: Properties, applications and future trends for their use in the biomedical field. Prog. Polym. Sci. 2013, 38, 1415–1441. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Taubert, A. Polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Macromol. Biosci. 2007, 7, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Signe, G.H.; Skrydstrup, T. Modification of amino acids, peptides, and carbohydrates through radical chemistry. In Radicals in Synthesis II: Complex Molecules; Springer-Verlag: Berlin, Germany, 2006; Volume 264, pp. 135–162. [Google Scholar]
- Falini, G.; Fermani, S.; Ripamonti, A. Oriented crystallization of octacalcium phosphate into beta-chitin scaffold. J. Inorg. Biochem. 2001, 84, 255–258. [Google Scholar] [CrossRef]
- Vittori, M.; Srot, V.; Zagar, K.; Bussmann, B.; van Aken, P.A.; Ceh, M.; Strus, J. Axially aligned organic fibers and amorphous calcium phosphate form the claws of a terrestrial isopod (Crustacea). J. Struct. Biol. 2016, 195, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Dumont, V.C.; Mansur, A.A.P.; Carvalho, S.M.; Borsagli, F.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands: Effects on the nucleation and growth of hydroxyapatite nanoparticles for producing biocomposite membranes. Mater. Sci. Eng. C 2016, 59, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Kawata, M.; Azuma, K.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Biomineralization of calcium phosphate crystals on chitin nanofiber hydrogel for bone regeneration material. Carbohydr. Polym. 2016, 136, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.; Sun, Z.F.; Ali, A.; Gould, M. A novel squid pen chitosan/hydroxyapatite/β-tricalcium phosphate composite for bone tissue engineering. Mater. Sci. Eng. C 2015, 55, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.Q.; Sun, X.X.; Kishen, A.; Deng, X.L.; Yang, X.P.; Wang, H.J.; Cong, C.H.; Wang, Y.H.; Wu, M.Y. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J. Mater. Sci. Mater. Med. 2014, 25, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Jayakumar, R.; Chennazhi, K.P. Versatile carboxymethyl chitin and chitosan nanomaterials: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 574–598. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Schweizer, S.; Schuster, T.; Junginger, M.; Siekmeyer, G.; Taubert, A. Surface modification of nickel/titanium alloy and titanium surfaces via a polyelectrolyte multilayer/calcium phosphate hybrid coating. Macromol. Mater. Eng. 2010, 295, 535–543. [Google Scholar] [CrossRef]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Gentile, P.; Boccafoschi, F.; Carmagnola, I.; Ninov, M.; Georgieva, V.; Georgiev, G.; Ciardelli, G. Photoactive chitosan switching on bone-like apatite deposition. Biomacromolecules 2010, 11, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yamane, S.; Sugawara, A.; Watanabe, A.; Akiyoshi, K. Hybrid nanoapatite by polysaccharide nanogel-templated mineralization. J. Bioact. Compat. Polym. 2009, 24, 151–168. [Google Scholar] [CrossRef]
- Dogan, O.; Oener, M.; Cinel, O. The inhibitory effects of inulin biopolymers on the seeded growth of hydroxyapatite. J. Ceram. Soc. Jpn 2010, 1118, 579–586. [Google Scholar] [CrossRef]
- Thula, T.T.; Svedlund, F.; Rodriguez, D.E.; Podschun, J.; Pendi, L.; Gower, L.P. Mimicking the nanostructure of bone: Composition of polymeric process-dorecting agents. Polymers 2011, 3, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; Kim, H.J.; Park, C.H.; Kim, C.S. Three-dimensional cellulose sponge: Fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lukasheva, N.V.; Tolmachev, D.A. Cellulose nanofibrils and mechanism of their mineralization in biomimetic synthesis of hydroxyapatite/native bacterial cellulose nanocomposites: Molecular dynamics simulations. Langmuir 2016, 32, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.L.; Xiong, G.Y.; Zhang, C.; Li, D.Y.; Zhu, Y.; Guo, R.S.; Wan, Y.Z. Surface controlled calcium phosphate formation on three-dimensional bacterial cellulose-based nanofibers. Mater. Sci. Eng. C 2015, 49, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, D.A.; Lukasheva, N.V. Study of the process of mineralization of nanofibrils of native bacterial cellulose in solutions of mineral ions: Modeling via the method of molecular dynamics. Polym. Sci. Ser. A 2014, 56, 545–557. [Google Scholar] [CrossRef]
- Petrauskaite, O.; Gomes, P.D.S.; Fernandes, M.H.; Juodzbalys, G.; Stumbras, A.; Maminskas, J.; Liesiene, J.; Cicciu, M. Biomimetic mineralization on a macroporous cellulose-based matrix for bone regeneration. Biomed. Res. Int. 2013, 9, 452750. [Google Scholar] [CrossRef] [PubMed]
- Li, K.N.; Wang, J.N.; Liu, X.Q.; Xiong, X.P.; Liu, H.Q. Biomimetic growth of hydroxyapatite on phosphorylated electrospun cellulose nanofibers. Carbohydr. Polym. 2012, 90, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, D.A.; Lukasheva, N.V. Interactions binding mineral and organic phases in nanocomposites based on bacterial cellulose and calcium phosphates. Langmuir 2012, 28, 13473–13484. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Kondo, T. Nematic ordered cellulose templates mediating order-patterned deposition accompanied with synthesis of calcium phosphates. Cellulose 2012, 19, 81–90. [Google Scholar] [CrossRef]
- Salama, A.; Neumann, M.; Günter, C.; Taubert, A. Ionic liquid-assisted formation of cellulose/calcium phosphate hybrid materials. Beilstein. J. Nanotechnol. 2014, 5, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, S.M.; Zhu, J.F.; Ma, M.G.; Xu, F.; Wang, B.; Sun, R.C. Microwave-assisted synthesis and characterization of cellulose-carbonated hydroxyapatite nanocomposites in NaOH–urea aqueous solution. Mater. Lett. 2010, 64, 2223–2225. [Google Scholar] [CrossRef]
- Jia, N.; Li, S.M.; Ma, M.G.; Sun, R.C.; Zhu, J.F. Hydrothermal synthesis and characterization of cellulose-carbonated hydroxyapatite nanocomposites in NaOH–urea aqueous solution. Sci. Adv. Mater. 2010, 2, 210–214. [Google Scholar] [CrossRef]
- Ma, M.G.; Zhu, J.F.; Jia, N.; Li, S.M.; Sun, R.C.; Cao, S.W.; Chen, F. Rapid microwave-assisted synthesis and characterization of cellulose-hydroxyapatite nanocomposites in N,N-dimethylacetamide solvent. Carbohydr. Res. 2010, 345, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Kozlova, D.; Ganesan, K.; Biewald, C.; Seipold, N.; Gaengler, P.; Arnold, W.H.; Epple, M. Chlorhexidine-loaded calcium phosphate nanoparticles for dental maintenance treatment: Combination of mineralising and antibacterial effects. RSC Adv. 2012, 2, 870–875. [Google Scholar] [CrossRef]
- Salama, A.; Abou-Zeid, R.E.; El-Sakhawy, M.; El-Gendy, A. Carboxymethyl cellulose/silica hybrids as templates for calcium phosphate biomimetic mineralization. Int. J. Biol. Macromol. 2015, 74, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Kovtun, A.; Prymak, O.; Meyer-Zaika, W.; Kubareva, E.; Romanova, E.A.; Oretskaya, T.S.; Heumann, R.; Epple, M. Functionalization of calcium phosphate nanoparticles by oligonucleotides and their application for gene silencing. J. Mater. Chem. 2007, 17, 721–727. [Google Scholar] [CrossRef]
- Sokolova, V.; Prymak, O.; Meyer-Zaika, W.; Cölfen, H.; Rehage, H.; Shukla, A.; Epple, M. Synthesis and characterization of DNA functionalized calcium phosphate nanoparticles. Materialwiss. Werkst. 2006, 37, 441–445. [Google Scholar] [CrossRef]
- Sokolova, V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Radtke, I.; Meyer-Zaika, W.; Heumann, R.; Epple, M. Transfection of cells with custom-made calcium phosphate nanoparticles coated with DNA. J. Mater. Chem. 2004, 14, 2213–2217. [Google Scholar] [CrossRef]
- Anonymous. Biomin bioglass toothpaste may better protect sensitive teeth and find its way into us market. Am. Ceram. Soc. Bull. 2016, 95, 22–23. [Google Scholar]
- Kim, J.Y.; Kim, K.O. The effects of sensory and nonsensory factors on consumer acceptability and purchase intention of commercial toothpaste. J. Sens. Stud. 2014, 29, 395–403. [Google Scholar] [CrossRef]
- Elschner, T.; Heinze, T. A promising cellulose-based polyzwitterion with pH-sensitive charges. Beilstein J. Org. Chem. 2014, 10, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.C.; Barrias, C.C.; Barbosa, M.A. Preparation and characterisation of calcium-phosphate porous microspheres with a uniform size for biomedical applications. J. Mater. Sci. Mater. Med. 2006, 17, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Baltrusaitis, J.; Schuttlefield, J.; Jensen, J.H.; Grassian, V.H. FTIR spectroscopy combined with quantum chemical calculations to investigate adsorbed nitrate on aluminium oxide surfaces in the presence and absence of co-adsorbed water. Phys. Chem. Chem. Phys. 2007, 9, 4970–4980. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.C.; Bartlett, M.L.; Young, R.A. Compositional analysis of apatites with Laser-Raman spectroscopy: (OH,F,Cl)Apatites. Arch. Oral Biol. 1974, 19, 995–1006. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman studies on rock-forming minerals. Part II. Minerals containing MO3, MO4, and MO6 groups. J. Chem. Soc. A 1970, 286–291. [Google Scholar] [CrossRef]
- DeAza, P.N.; Santos, C.; Pazo, A.; deAza, S.; Cusco, R.; Artus, L. Vibrational properties of calcium phosphate compounds. 1. Raman spectrum of β-tricalcium phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- DeAza, P.N.; Guitian, F.; Santos, C.; deAza, S.; Cusco, R.; Artus, L. Vibrational properties of calcium phosphate compounds. 2. Comparison between hydroxyapatite β-tricalcium phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier-transform Raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Arends, J. Raman-spectra of human dental calculus. J. Dent. Res. 1993, 72, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, Y.; Saito, N.; Arai, M. Raman spectral studies of aqueous zinc nitrate solution at high temperatures and at a high pressure of 30 Mpa. J. Phys. Chem. B 1998, 102, 3029–3035. [Google Scholar] [CrossRef]
- Ianoul, A.; Coleman, T.; Asher, S.A. UV resonance Raman spectroscopic detection of nitrate and nitrite in wastewater treatment processes. Anal. Chem. 2002, 74, 1458–1461. [Google Scholar] [CrossRef]
- Irish, D.E.; Davis, A.R. Interactions in aqueous alkali metal nitrate solutions. Can. J. Chem. 1968, 46, 943–951. [Google Scholar] [CrossRef]
- Fowler, B.O.; Markovic, M.; Brown, W.E. Octacalcium phosphate. 3. Infrared and Raman vibrational-spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; Van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaitre, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, S81–S84. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.F.; Pogson, R.E.; Millar, G.J.; Tan, K.Q.; Palmer, S.J. Raman spectroscopy of synthetic CaHPO4·2H2O– and in comparison with the cave mineral brushite. J. Raman Spectrosc. 2012, 43, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Evis, Z. Raman spectroscopy investigation of nano hydroxyapatite doped with yttrium and fluoride ions. Spectr. Lett. 2014, 47, 24–29. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Friedrich, A.; Taubert, A. Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride. J. Mater. Chem. 2008, 24, 1008. [Google Scholar] [CrossRef]
- Joshi, V.S.; Joshi, M.J. FTIR spectroscopic, thermal and growth morphological studies of calcium hydrogen phosphate dihydrate crystals. Cryst. Res. Technol. 2003, 38, 817–821. [Google Scholar] [CrossRef]
- Fujisato, K.; Habu, H.; Miyake, A.; Hori, K. Thermal decomposition of ammonium nitrate modeling of thermal dissociation in thermal analysis. Sci. Technol. Energ. Mater. 2014, 75, 28–36. [Google Scholar]
- Mulley, V.J.; Cavendis, C.D. A thermogravimetric method for analysis of mixtures of brushite and monetite. Analyst 1970, 95, 304–307. [Google Scholar] [CrossRef]
- Sugawara, A.; Yamane, S.; Akiyoshi, K. Nanogel-templated mineralization: Polymer-calcium phosphate hybrid nanomaterials. Macromol. Rapid. Commun. 2006, 27, 441–446. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F.; Boivin, D. The XPP Procedure Applied to Quantitative EDS X-ray Analysis in the SEM; San Francisco Press: San Francisco, CA, USA, 1990. [Google Scholar]
- Pouchou, J.L.; Pichoir, F. quantitative analysis of homogeneous or stratified microvolumes applying the model “pap”. In Electron Probe Quantitation; Heinrich, K.F.J., Newbury, D.E., Eds.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
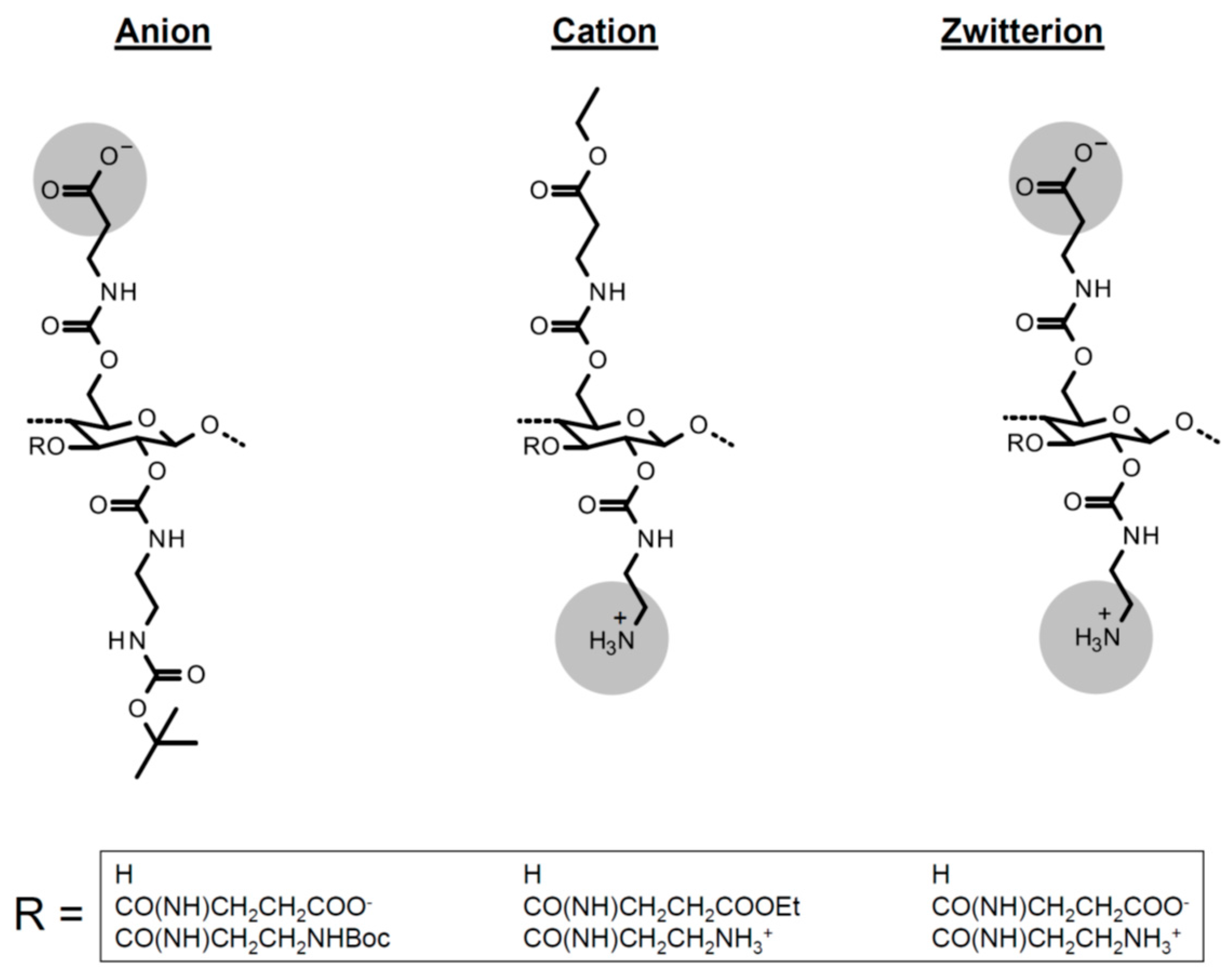
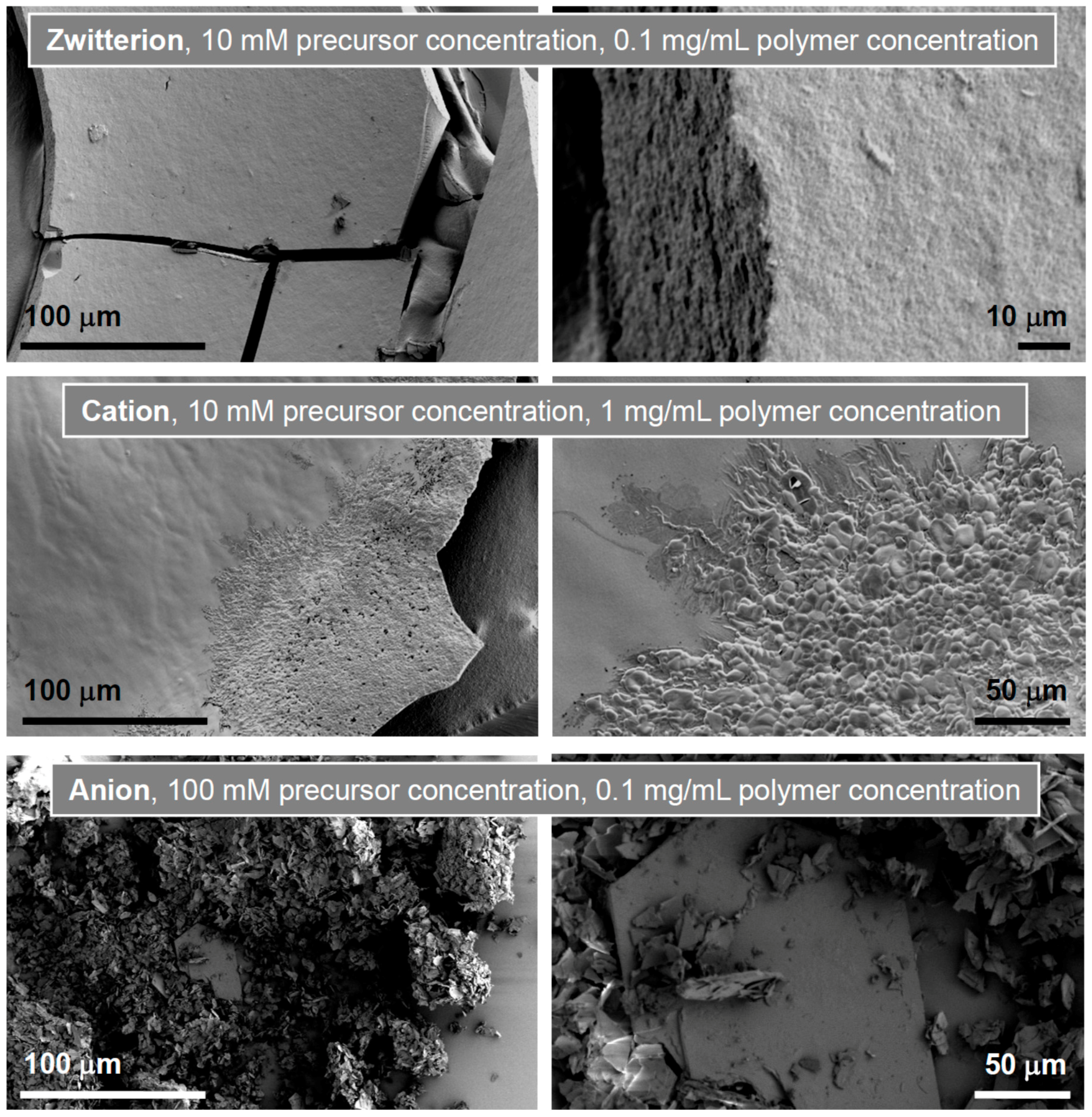
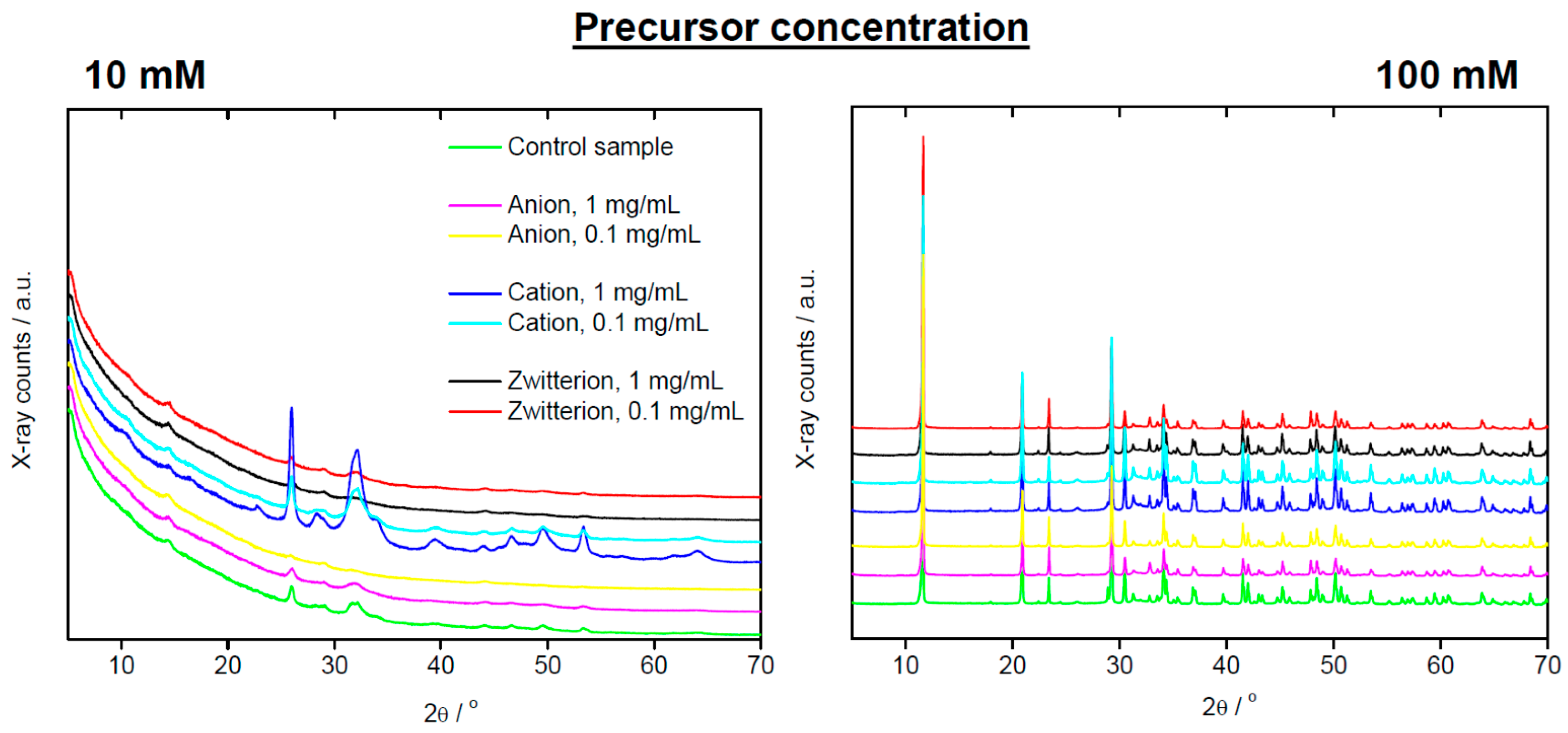
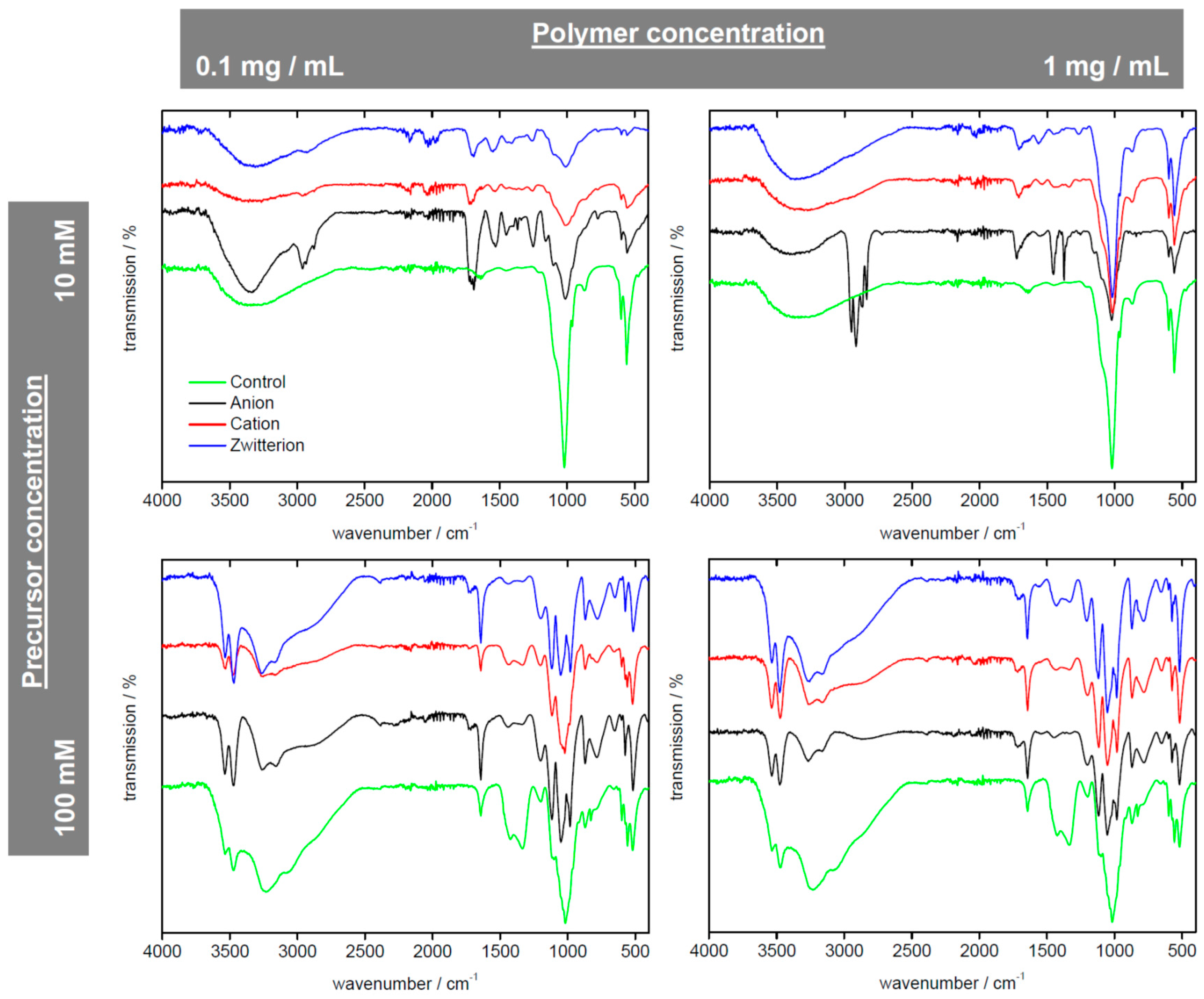
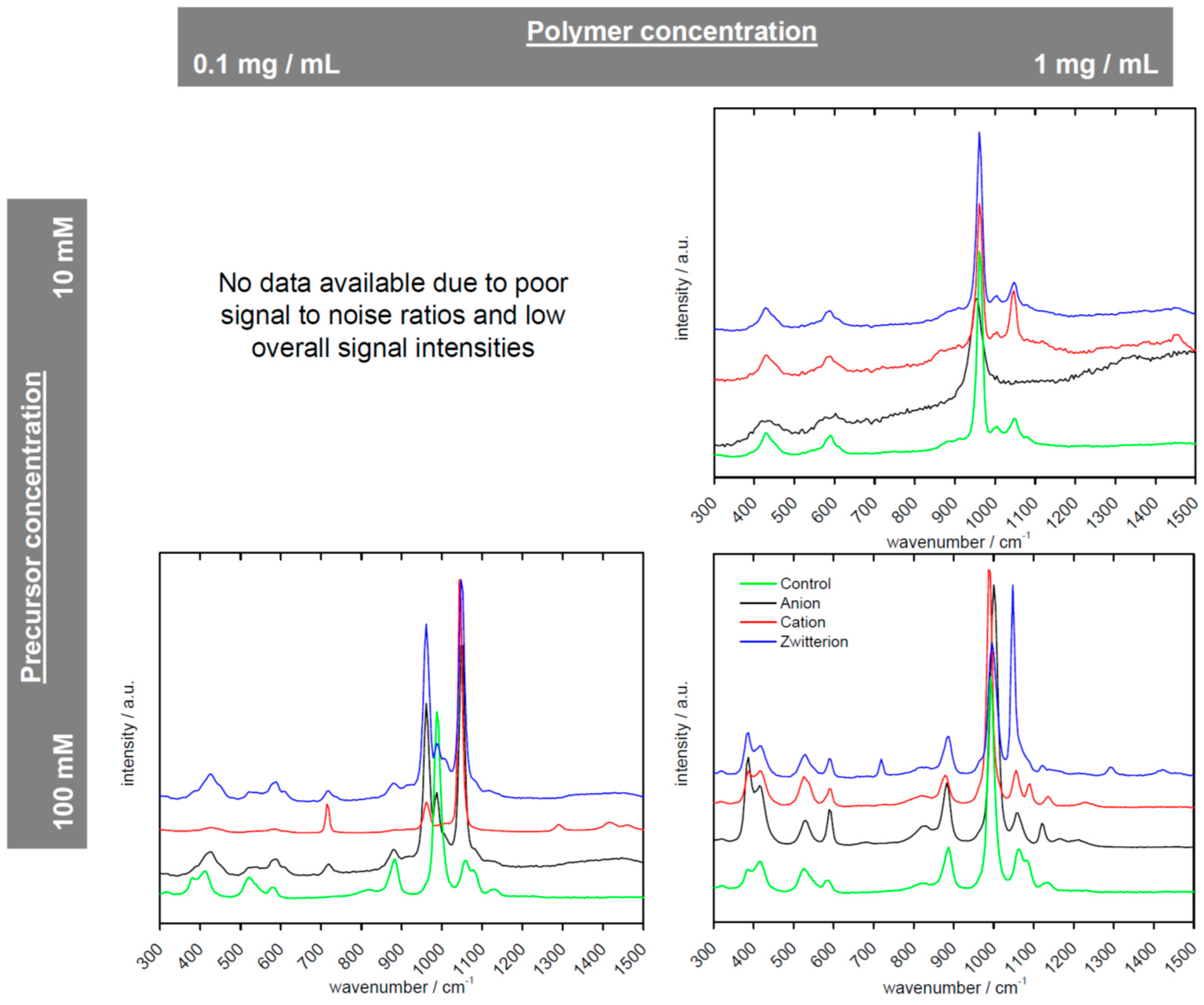
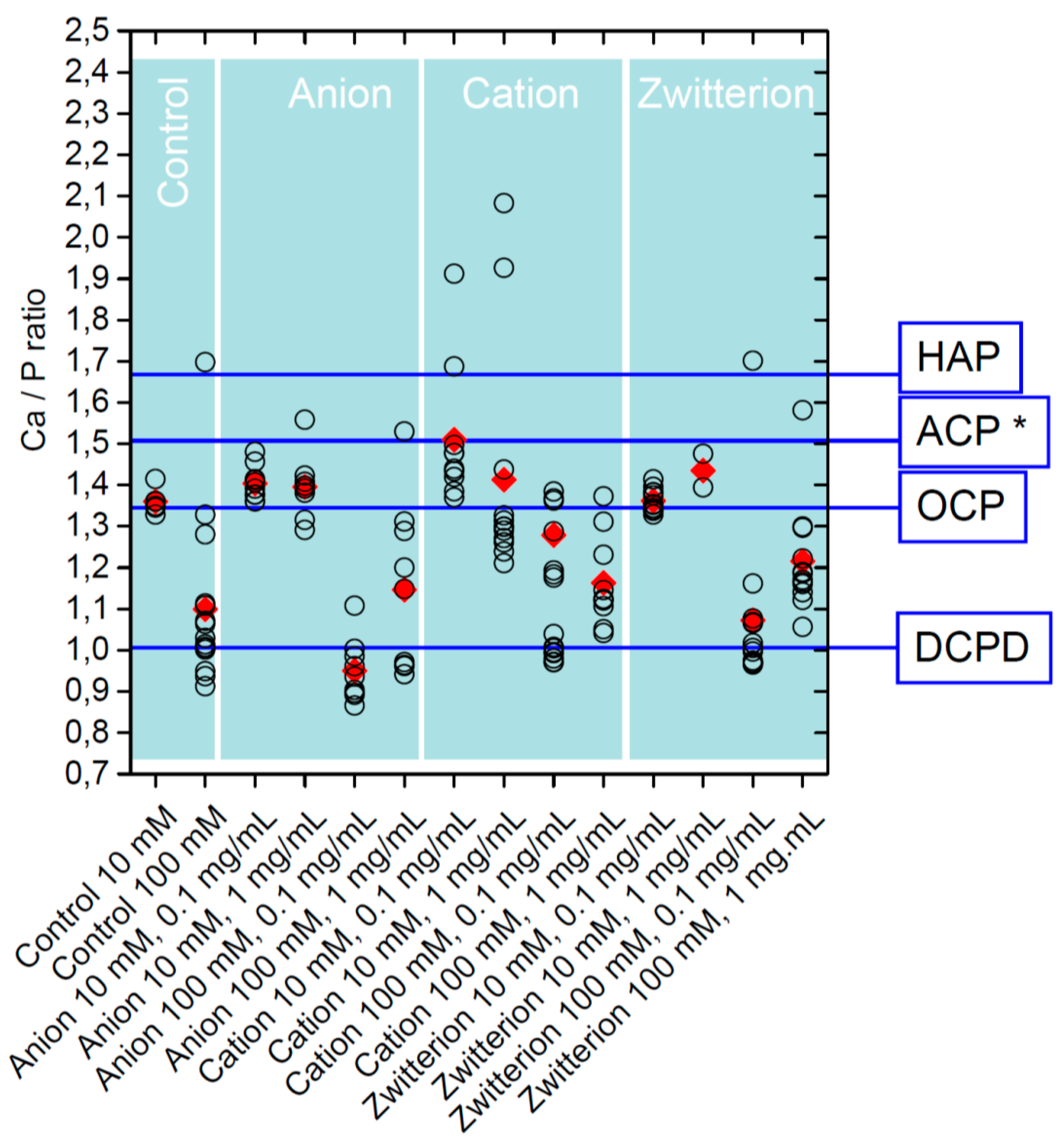
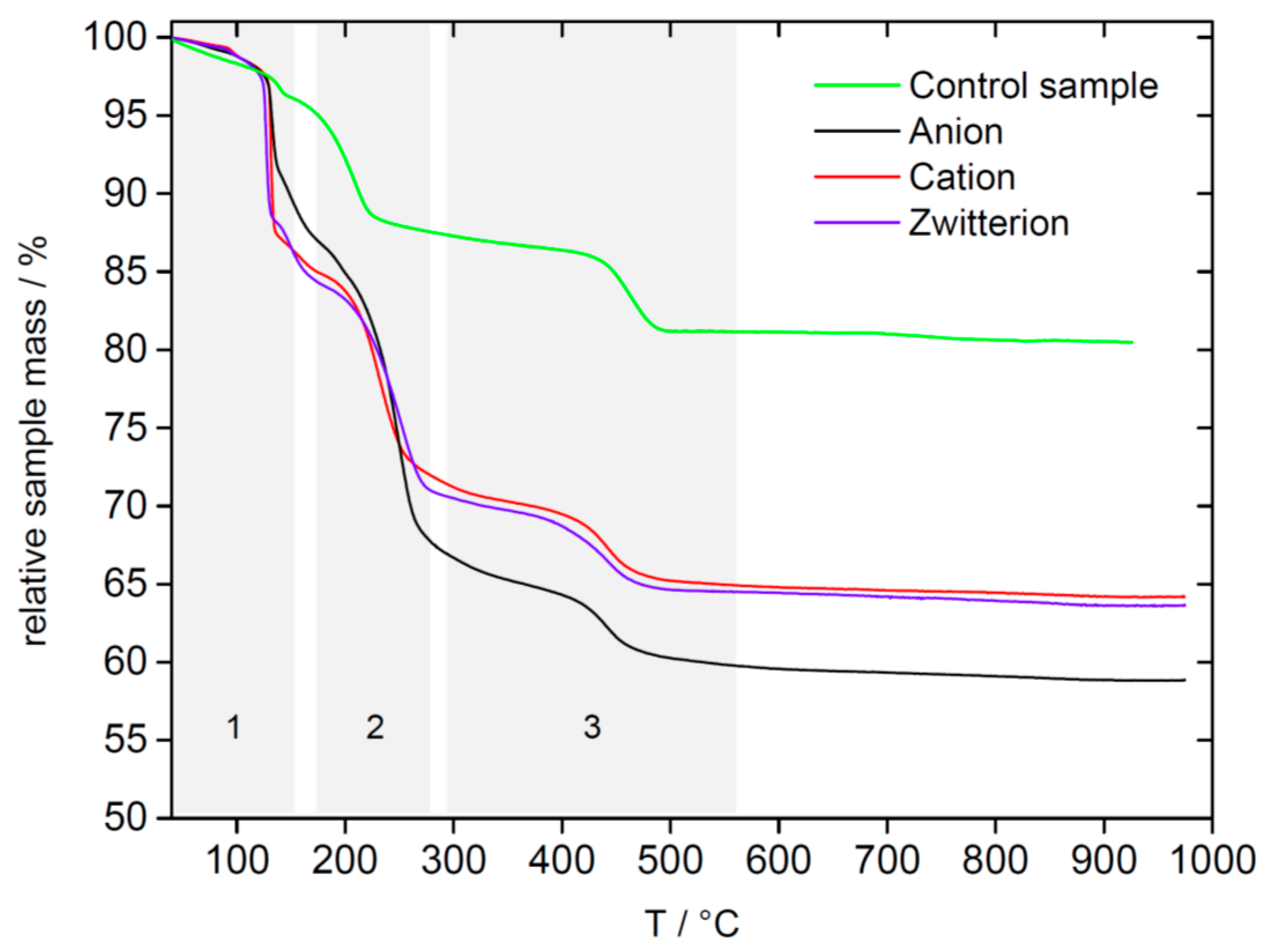
| Polymer | Precursor Concentration/mM | Polymer Concentration/mg/mL | C (%) | H (%) | N (%) |
|---|---|---|---|---|---|
| Control | 10 | 0 | n.d. | n.d. | 1.500 ± 0.530 |
| 100 | 0 | n.d. | 2.423 ± 0.11 | 4.881 ± 0.607 | |
| Anion | 10 | 1 | 10.01 ± 0.02 | 3.571 ± 0.01 | 5.094 ± 0.198 |
| 10 | 0.1 | 4.59 ± 0.08 | 1.284 ± 0.01 | 2.684 ± 0.078 | |
| 100 | 1 | 2.41 ± 0.01 | 2.522 ± 0.05 | 4.080 ± 0.15 | |
| 100 | 0.1 | n.d. | 2.511 ± 0.07 | 3.444 ± 0.09 | |
| Cation | 10 | 1 | 7.47 ± 0.16 | 1.558 ± 0.01 | 3.403 ± 0.09 |
| 10 * | 0.1 | 3.75 ± 0.30 | 1.150 ± 0.30 | 2.481 ± 0.30 | |
| 100 | 1 | 1.43 ± 0.04 | 2.448 ± 0.09 | 4.925 ± 0.20 | |
| 100 | 0.1 | n.d. | 2.307 ± 0.02 | 4.279 ± 0.06 | |
| Zwitterion | 10 | 1 | 16.94 ± 0.20 | 3.103 ± 0.05 | 4.300 ± 0.01 |
| 10 | 0.1 | 4.71 ± 0.05 | 1.164 ± 0.03 | 2.463 ± 0.07 | |
| 100 | 1 | 2.52 ± 0.01 | 2.572 ± 0.09 | 5.165 ± 0.18 | |
| 100 | 0.1 | n.d. | 2.343 ± 0.01 | 4.387 ± 0.11 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taubert, A.; Balischewski, C.; Hentrich, D.; Elschner, T.; Eidner, S.; Günter, C.; Behrens, K.; Heinze, T. Water-Soluble Cellulose Derivatives Are Sustainable Additives for Biomimetic Calcium Phosphate Mineralization. Inorganics 2016, 4, 33. https://doi.org/10.3390/inorganics4040033
Taubert A, Balischewski C, Hentrich D, Elschner T, Eidner S, Günter C, Behrens K, Heinze T. Water-Soluble Cellulose Derivatives Are Sustainable Additives for Biomimetic Calcium Phosphate Mineralization. Inorganics. 2016; 4(4):33. https://doi.org/10.3390/inorganics4040033
Chicago/Turabian StyleTaubert, Andreas, Christian Balischewski, Doreen Hentrich, Thomas Elschner, Sascha Eidner, Christina Günter, Karsten Behrens, and Thomas Heinze. 2016. "Water-Soluble Cellulose Derivatives Are Sustainable Additives for Biomimetic Calcium Phosphate Mineralization" Inorganics 4, no. 4: 33. https://doi.org/10.3390/inorganics4040033