Olivine-Based Blended Compounds as Positive Electrodes for Lithium Batteries
Abstract
:1. Introduction
2. Results
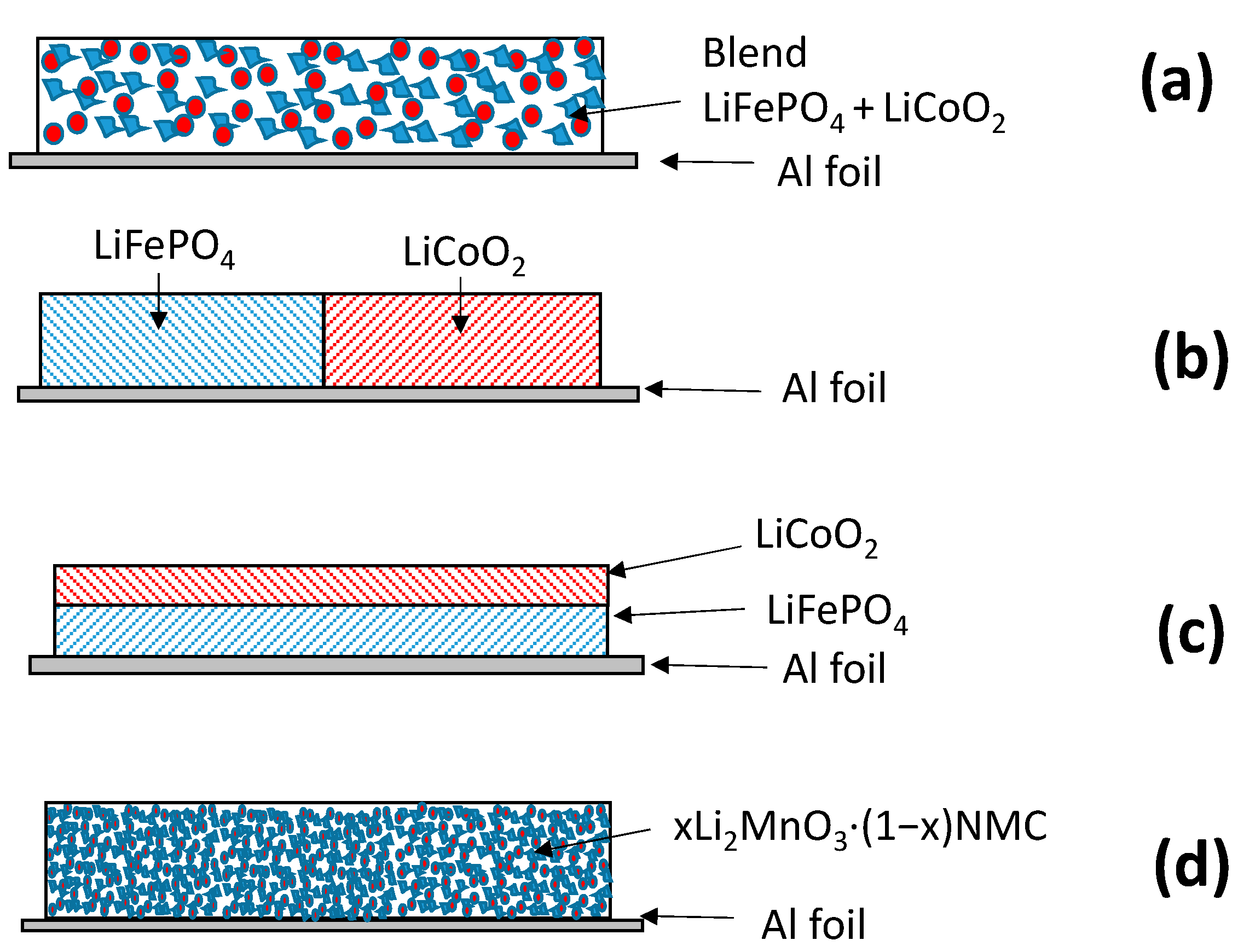
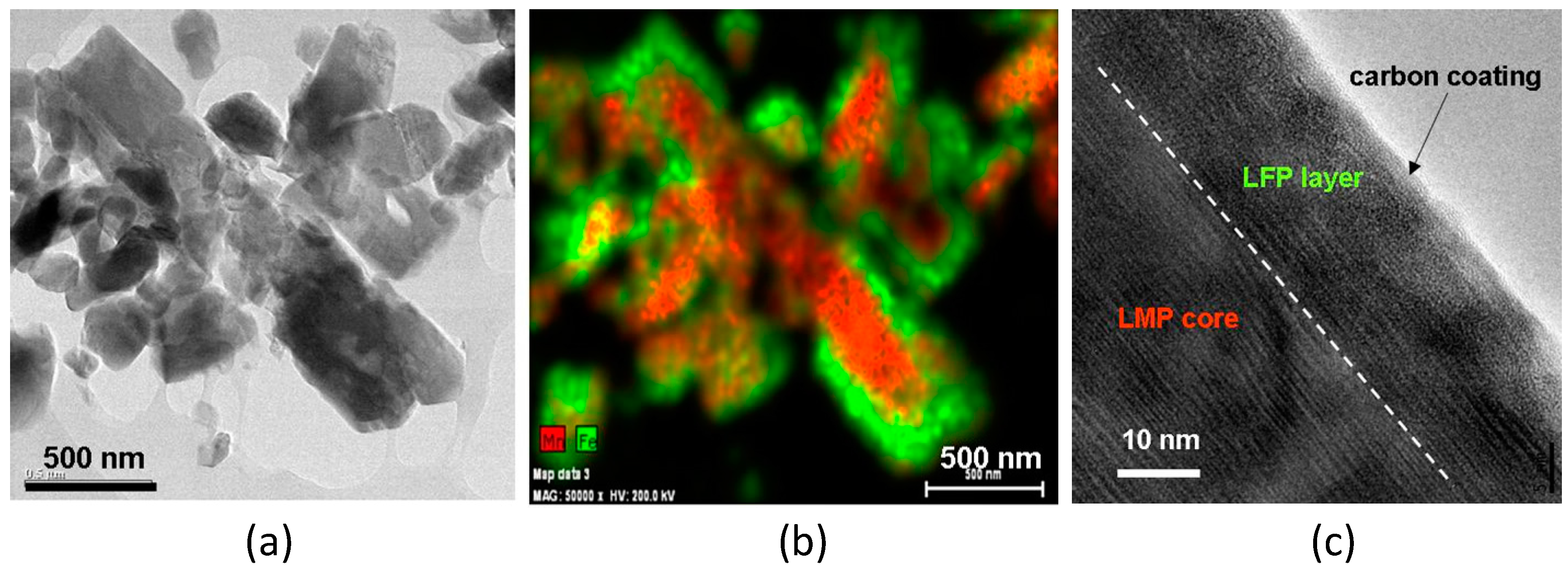
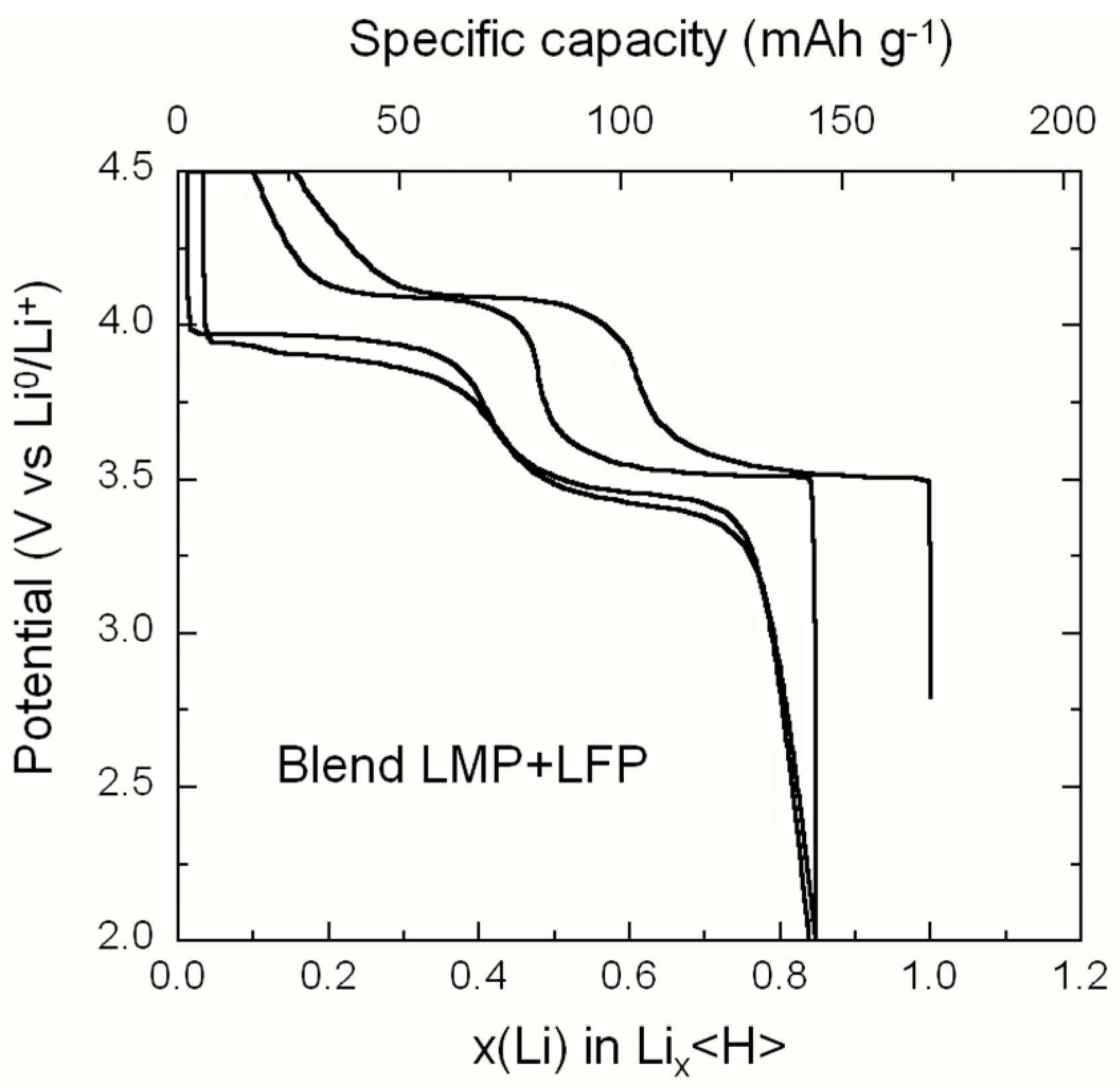
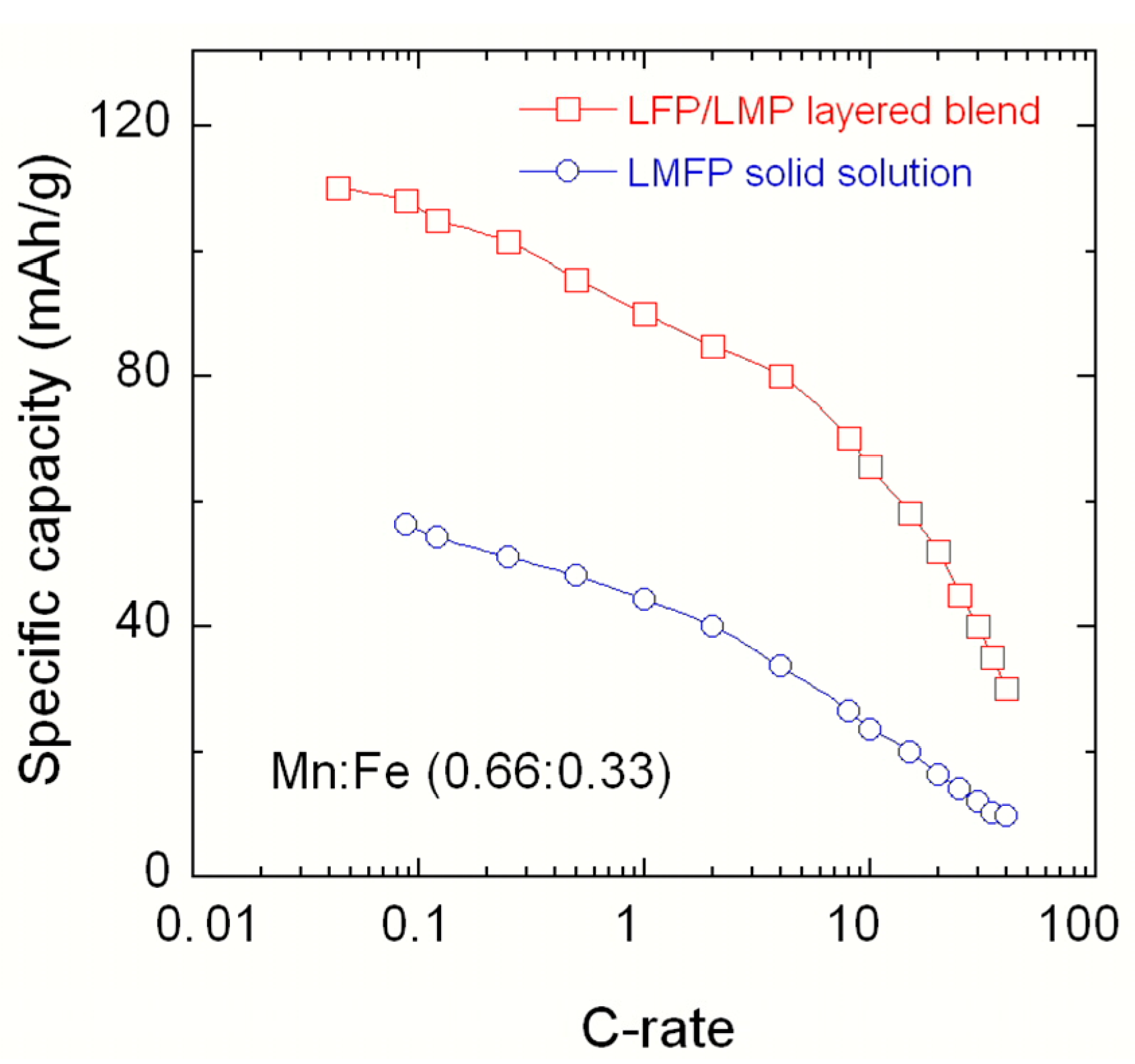
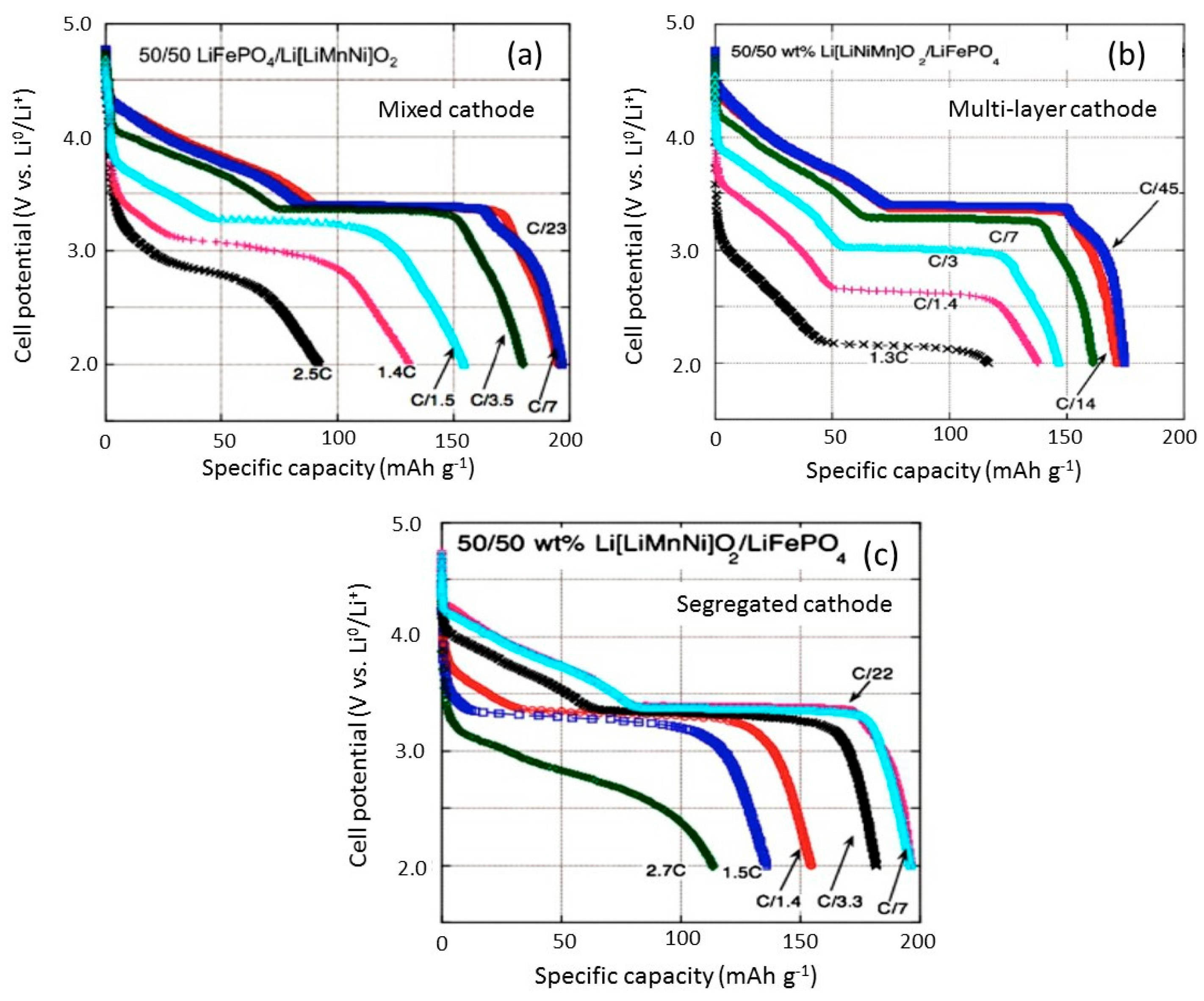
2.1. LiMnPO4–LiFePO4 (LMP–LFP) Blended Electrode
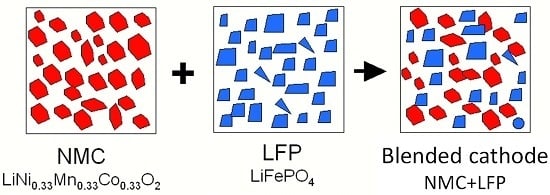
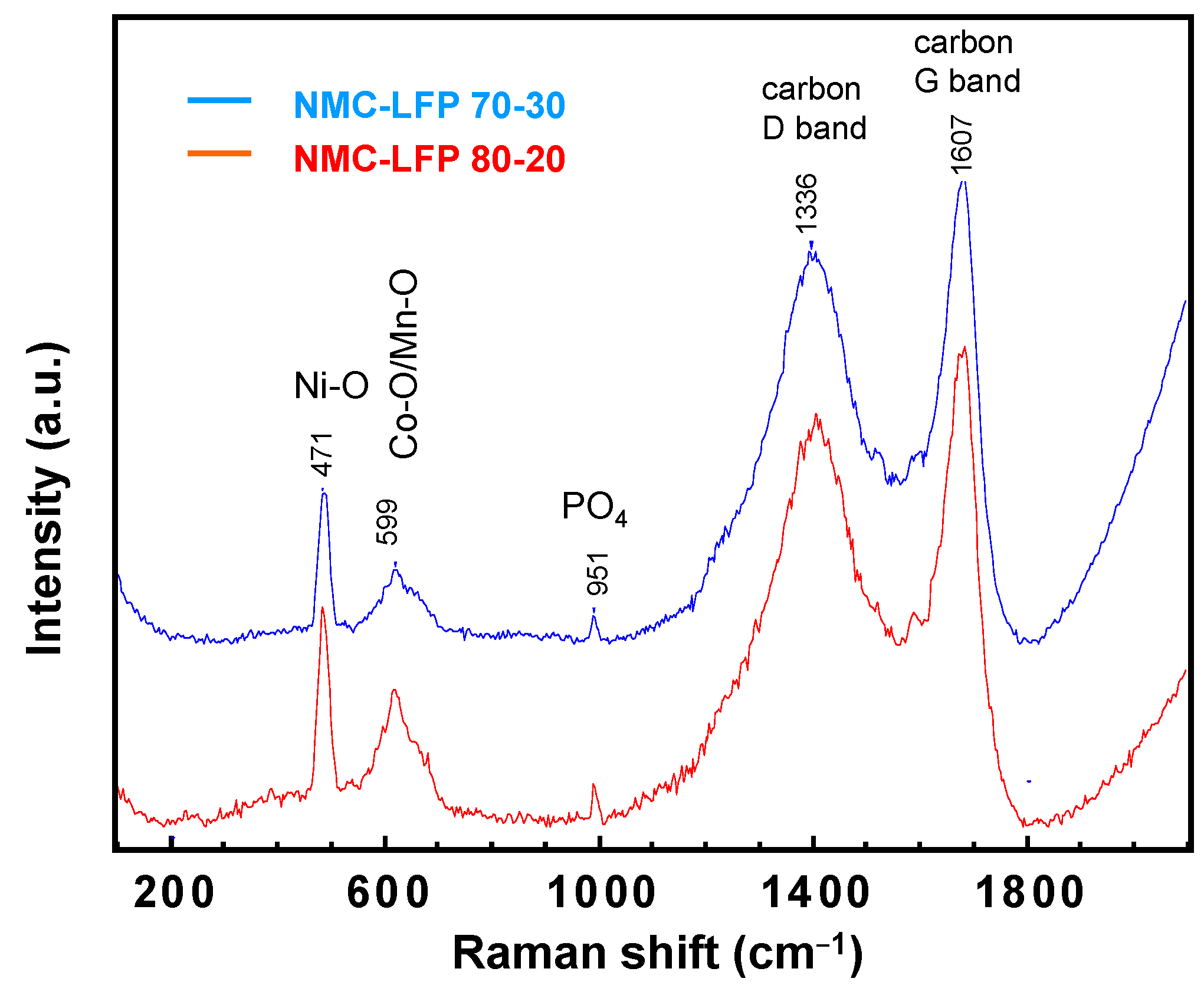
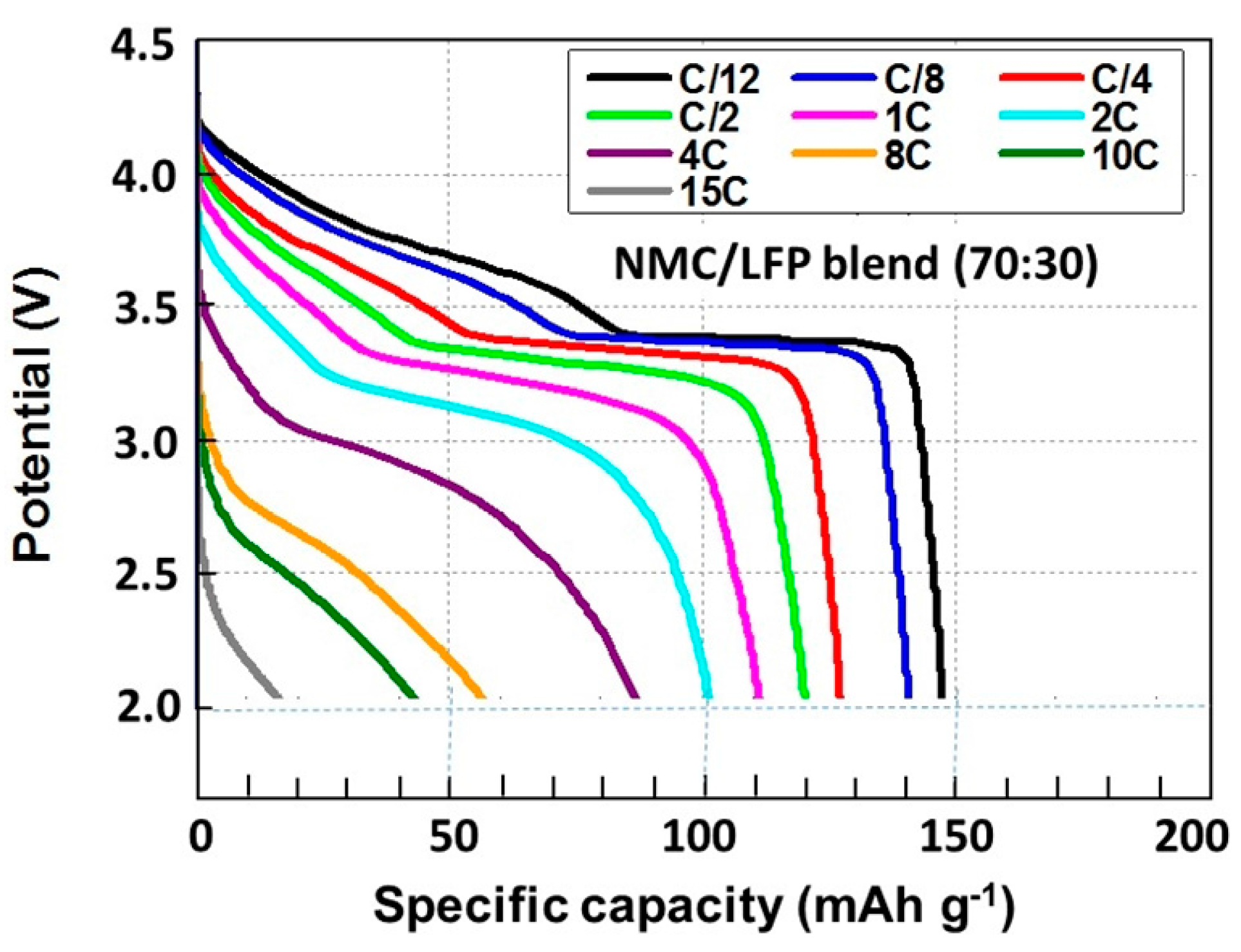
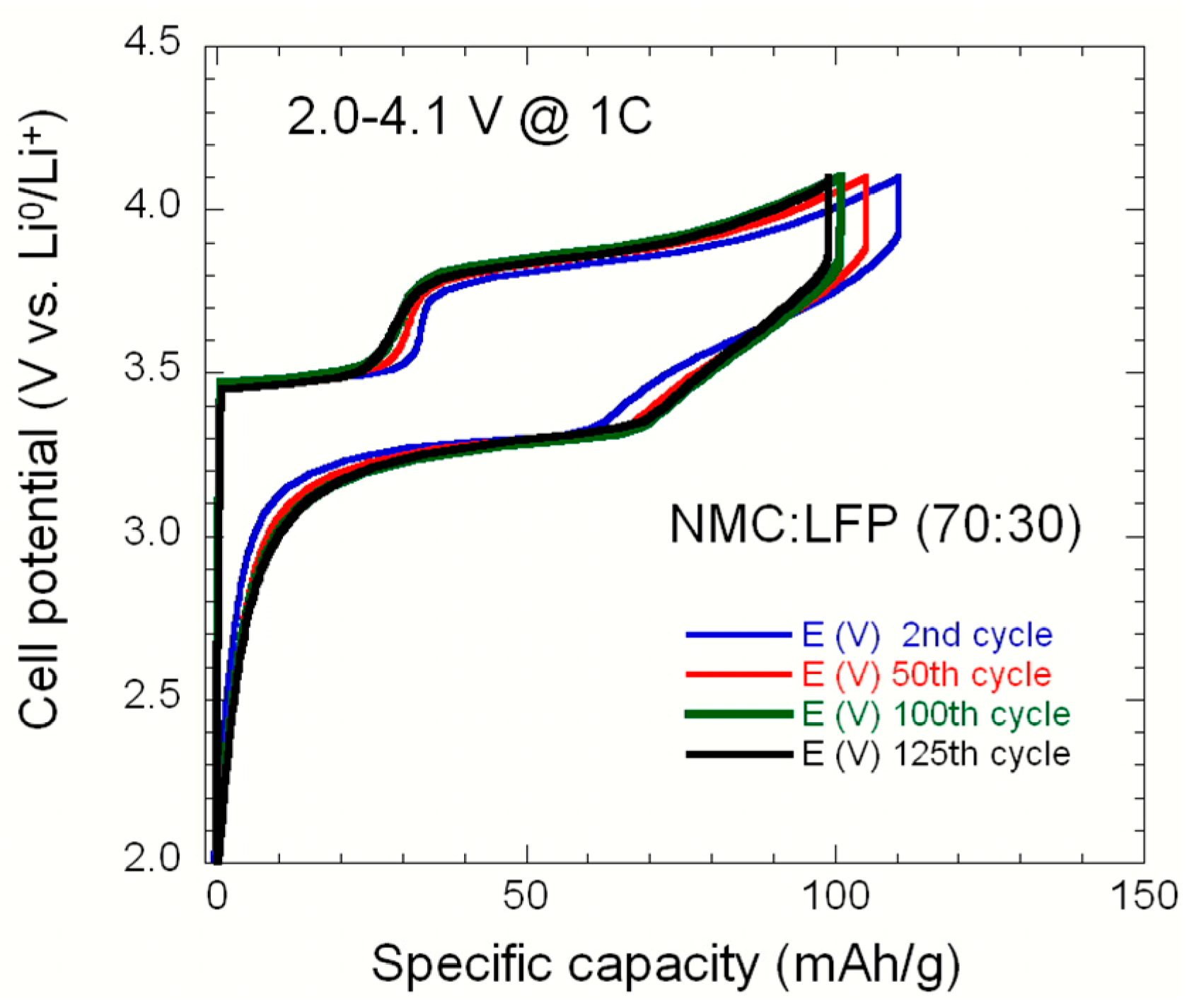
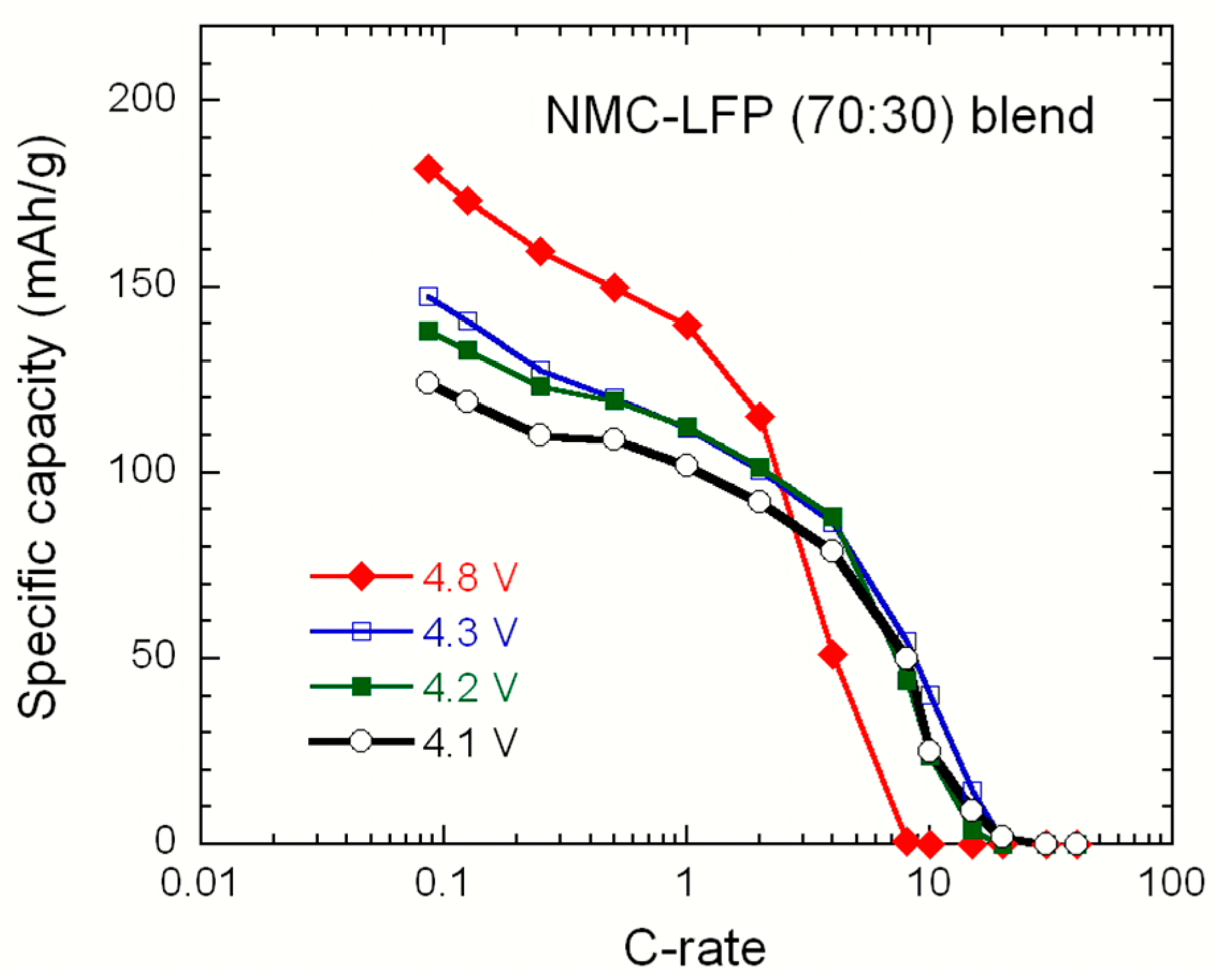
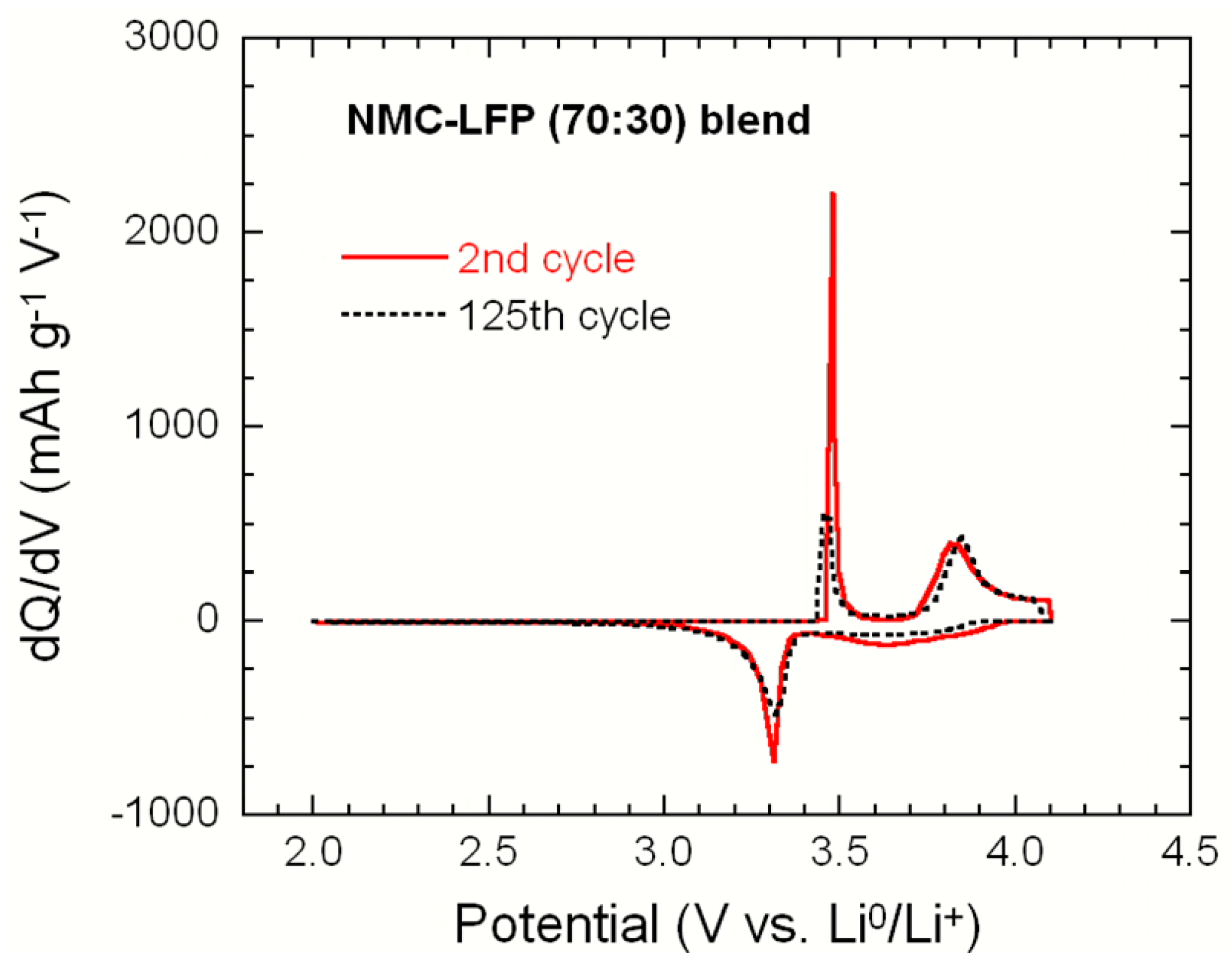
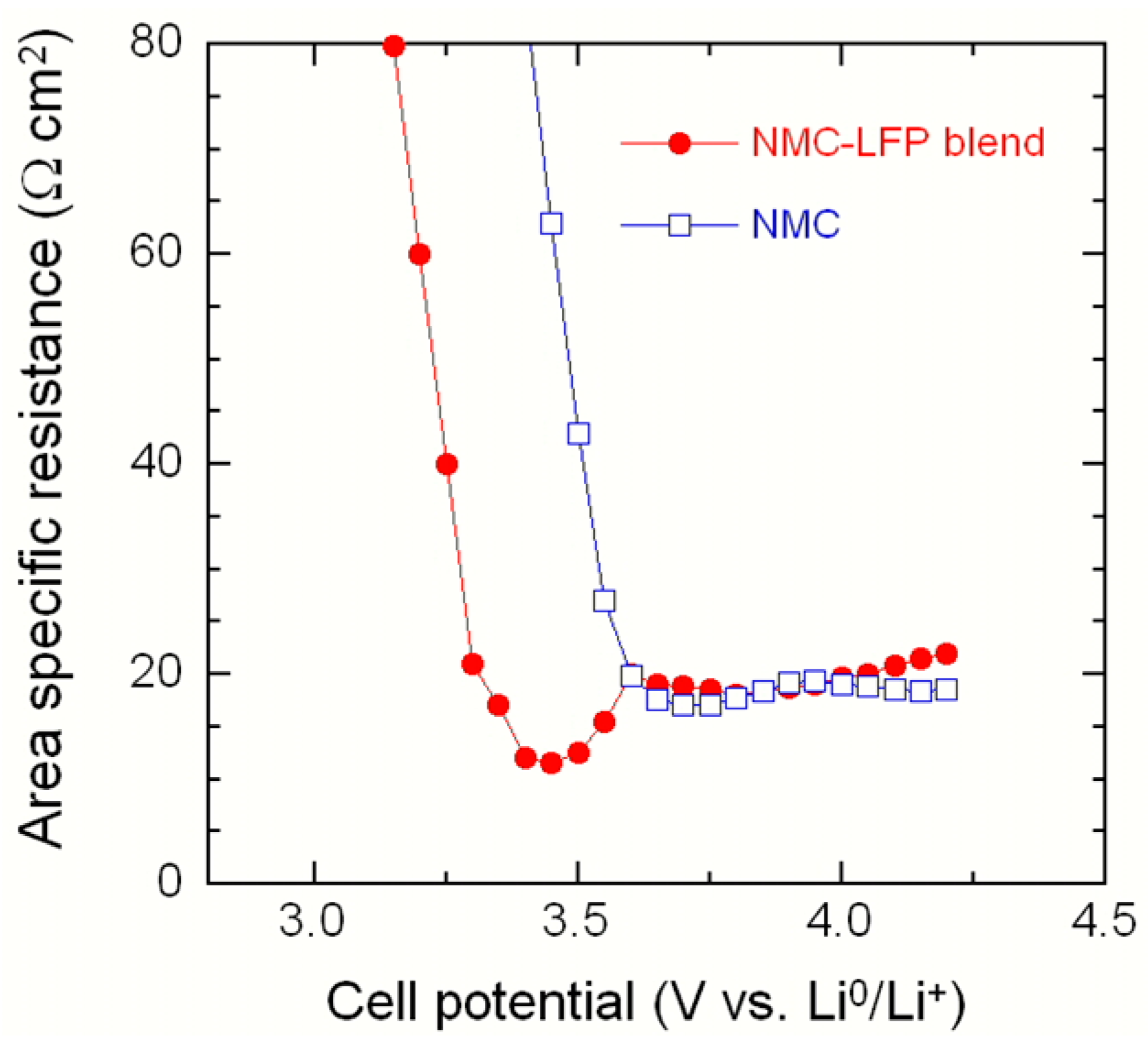
2.2. LiNi1/3Mn1/3Co1/3O2–LiFePO4 (NMC–LFP) Blended Electrode
3. Phase Evolution
4. Discussion
5. Materials and Methods
5.1. Synthesis and Characterizations
5.2. Electrochemical Tests
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chikkannanavar, S.B.; Bernardi, D.M.; Liu, L. A review of blended cathode materials for use in Li-ion batteries. J. Power Sources 2014, 248, 91–100. [Google Scholar] [CrossRef]
- Zaghib, K.; Trudeau, M.; Guerfi, A.; Trottier, J.; Mauger, A.; Julien, C.M. New advanced cathode material: LiMnPO4 encapsulated with LiFePO4. J. Power Sources 2012, 204, 177–181. [Google Scholar] [CrossRef]
- Margalit, N. Non-Aqueous Primary Battery Having a Blended Cathode Active Material. U.S. Patent 3,981,748, 21 September 1976. [Google Scholar]
- Pynenburg, R.; Barker, J. Cathode-Active Material Blends of LixMn2O4. U.S. Patent 5,429,890, 4 July 1995. [Google Scholar]
- Numata, T.; Amemiya, C.; Kumeuchi, T.; Shirakata, M.; Yonezawa, M. Advantages of blending LiNi0.8Co0.2O2 into Li1+xMn2−xO4 cathodes. J. Power Sources 2001, 97–98, 358–360. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.I.; Kim, W.S. A study on electrochemical characteristics of LiCoO2/LiNi1/3Mn1/3Co1/3O2 mixed cathode for Li secondary battery. Electrochim. Acta 2006, 52, 1457–1461. [Google Scholar] [CrossRef]
- Albertus, P.; Christensen, J.; Newman, J. Experiments on and modeling of positive electrode with multiple active materials for lithium-ion batteries. J. Electrochem. Soc. 2009, 156, A606–A618. [Google Scholar] [CrossRef]
- GM’s new battery chemistry? It’s already in Chevy Volt. Available online: http://www.popsci.com/cars/article/2011-01/gm%e2%80%99s-new-battery-chemistry-it%e2%80%99s-already-chevy-volt (accessed on 7 January 2011).
- Tran, H.Y.; Täubert, C.; Fleischhammer, M.; Axmann, P.; Küppers, L.; Wohlfahrt-Mehrens, M. LiMn2O4 spinel/LiNi0.8Co0.15Al0.05O0.2 blends as cathode materials for lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A556–A561. [Google Scholar] [CrossRef]
- Gao, J.; Manthiram, A. Eliminating the irreversible capacity loss of high capacity layered Li[Li0.2Ni0.13Mn0.54Co0.13]O2 cathode by blending with other lithium insertion hosts. J. Power Sources 2009, 191, 644–647. [Google Scholar] [CrossRef]
- Kitao, H.; Fujihara, T.; Takeda, K.; Nakanishi, N.; Nohma, T. High-temperature storage performance of Li-ion batteries using a mixture of Li–Mn spinel and Li–Ni–Co–Mn oxide as a positive electrode material. Electrochem. Solid State Lett. 2005, 8, A87–A90. [Google Scholar] [CrossRef]
- Imachi, N.; Takano, Y.; Fujimoto, H.; Kida, Y.; Fujitami, S. Layered cathode for improving safety of Li-ion batteries. J. Electrochem. Soc. 2007, 154, A412–A416. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Zaghib, K.; West, W.C.; Ratnakumar, B.V. Dual active material composite cathode structures for Li-ion batteries. J. Power Sources 2008, 177, 528–536. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Kang, S.H.; Park, S.U.; Han, S.Y. xLi2MnO3·(1−x)LiMO2 blended with LiFePO4 to achieve high energy density and pulse power capability. J. Power Sources 2011, 196, 9702–9707. [Google Scholar] [CrossRef]
- Zheng, J.C.; Li, X.; Wang, Z.X.; Niu, S.S.; Liu, D.; Wu, L.; Li, L.J.; Li, J.H.; Guo, H.J. Novel synthesis of LiFePO4–Li3V2(PO4)3 composite cathode material by aqueous precipitation and lithiation. J. Power Sources 2010, 195, 2935–2938. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, L.; Du, F.; Yang, X.; Wang, C.; Chen, G.; Wei, Y. Electrochemical performance of LiMn2O4/LiFePO4 blend cathodes for lithium ion batteries. Chem. Res. Chin. Univ. 2015, 31, 270–275. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Klein, A.; Axmann, P. Blend Performance of LiMn0.7Fe0.3PO4–LiMn1.9Al0.1O4 electrodes: Properties beyond physical mixtures? In Proceedings of the 18th Int. Meeting on Lithium Batteries, Chicago, IL, USA, 19–24 June 2016.
- Trudeau, M.L.; Laul, D.; Veillette, R.; Serventi, A.M.; Zaghib, K.; Mauger, A.; Julien, C.M. In-situ HRTEM synthesis observation of nanostructured LiFePO4. J. Power Sources 2011, 196, 7383–7394. [Google Scholar] [CrossRef]
- Julien, C. Local cationic environment in lithium nickel–cobalt oxides used as cathode materials for lithium batteries. Solid State Ion. 2000, 136–137, 887–896. [Google Scholar] [CrossRef]
- Julien, C.M.; Zaghib, K.; Mauger, A.; Massot, M.; Ait-Salah, A.; Selmane, M.; Gendron, F. Characterization of the carbon-coating onto LiFePO4 particles used in lithium batteries. J. Appl. Phys. 2006, 100, 63511. [Google Scholar] [CrossRef]
- Dubarry, M.; Svoboda, V.; Hwu, R.; Liaw, B.Y. Incremental capacity analysis and close-to-equilibrium OCV measurements to quantify capacity fade in commercial rechargeable lithium batteries. Electrochem. Solid State Lett. 2006, 9, A454–A457. [Google Scholar] [CrossRef]
- Weng, C.; Cui, Y.; Sun, J.; Peng, H. On-board state of health monitoring of lithium-ion batteries using incremental capacity analysis with support vector regression. J. Power Sources 2013, 235, 36–44. [Google Scholar] [CrossRef]
- Dubarry, M.; Liaw, B.Y.; Chen, M.S.; Chyan, S.S.; Han, K.C.; Sie, W.T.; Wu, S.H. Identifying battery aging mechanisms in large format Li ion cells. J. Power Sources 2011, 196, 3420–3425. [Google Scholar] [CrossRef]
- Shinbrot, T.; Muzio, F.J. Noise to order. Nature 2001, 410, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mullin, T. Coarsening of self-organized clusters in binary mixtures of particles. Phys. Rev. Lett. 2000, 84, 4741–4745. [Google Scholar] [CrossRef] [PubMed]
- Vediappan, K.; Guerfi, A.; Gariépy, V.; Demopoulos, G.P.; Hovington, P.; Trottier, J.; Mauger, A.; Zaghib, K.; Julien, C.M. Stirring effect in hydrothermal synthesis of C–LiFePO4. J. Power Sources 2014, 266, 99–106. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Li, Q.; Gendron, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julien, C.M.; Mauger, A.; Trottier, J.; Zaghib, K.; Hovington, P.; Groult, H. Olivine-Based Blended Compounds as Positive Electrodes for Lithium Batteries. Inorganics 2016, 4, 17. https://doi.org/10.3390/inorganics4020017
Julien CM, Mauger A, Trottier J, Zaghib K, Hovington P, Groult H. Olivine-Based Blended Compounds as Positive Electrodes for Lithium Batteries. Inorganics. 2016; 4(2):17. https://doi.org/10.3390/inorganics4020017
Chicago/Turabian StyleJulien, Christian M., Alain Mauger, Julie Trottier, Karim Zaghib, Pierre Hovington, and Henri Groult. 2016. "Olivine-Based Blended Compounds as Positive Electrodes for Lithium Batteries" Inorganics 4, no. 2: 17. https://doi.org/10.3390/inorganics4020017