Gilded Hope for Medicine
Abstract
:1. Introduction
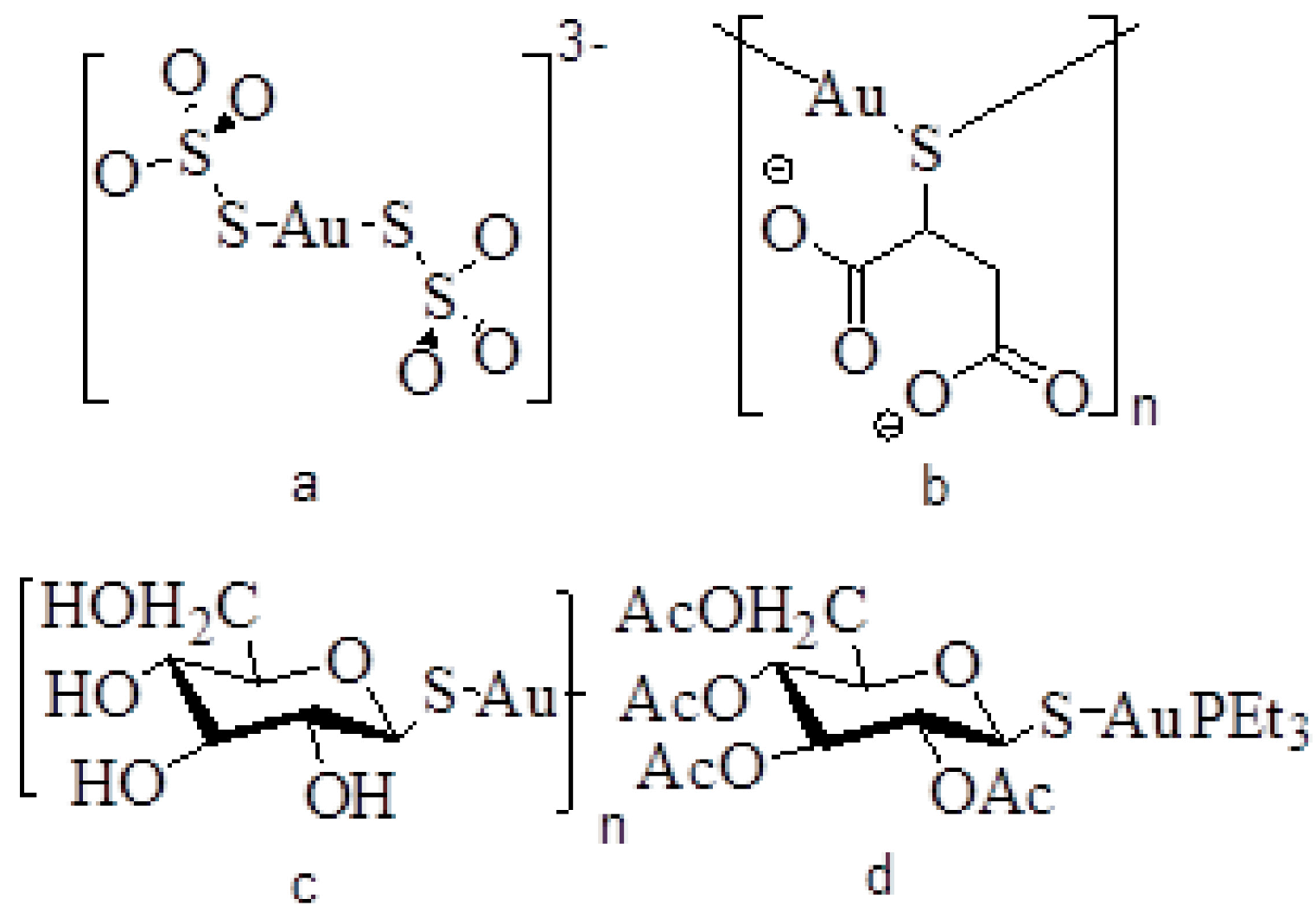
2. Rheumatoid Arthritis
3. Gold Oxidation States
4. Structure of Arthritic Gold Drugs
| Drug name | Trade name | Formula |
|---|---|---|
| Gold thiosulfate | Sanochrysin | Na3[Au(S2O3)2] 2H2O |
| Gold thiomalate | Myochrysine | Na2[AuTm] |
| Gold thioglucose | Solganol | [AuTG]n |
| Gold tetraacetylthioglucose | Auranofin | Et3PAuTATG |

5. Mechanism of Action and Fate of Gold Drugs
5.1. Fate of Auranofin in Stomach Hydrochloric Acid
5.2. Interaction of Gold Drugs with Proteins “Shuttle Thiol Mechanism”
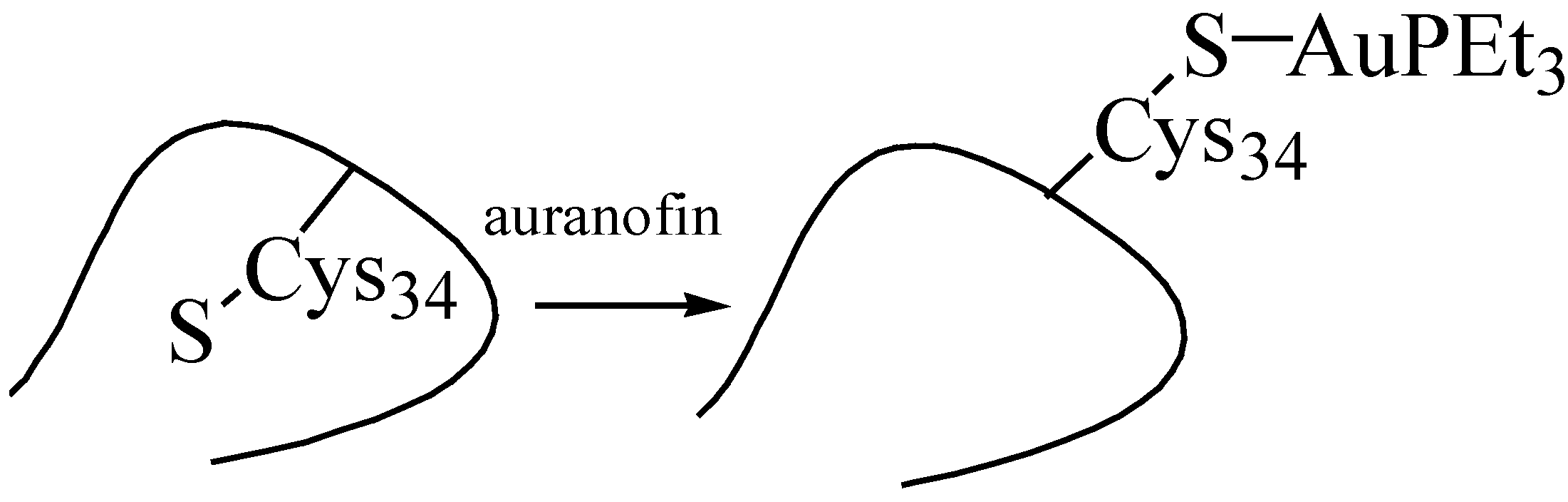
5.3. Mild Oxidation of Auranofin
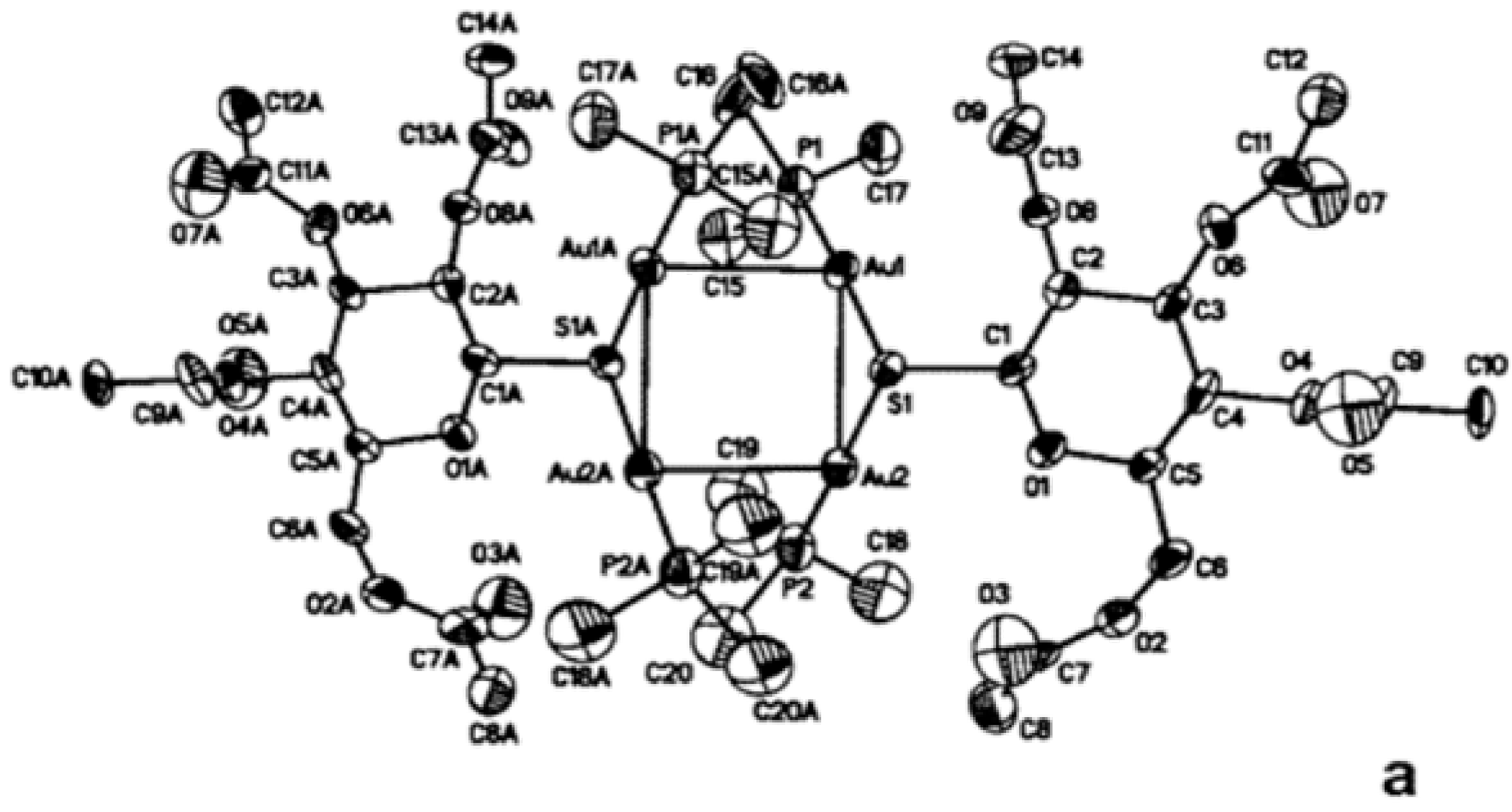
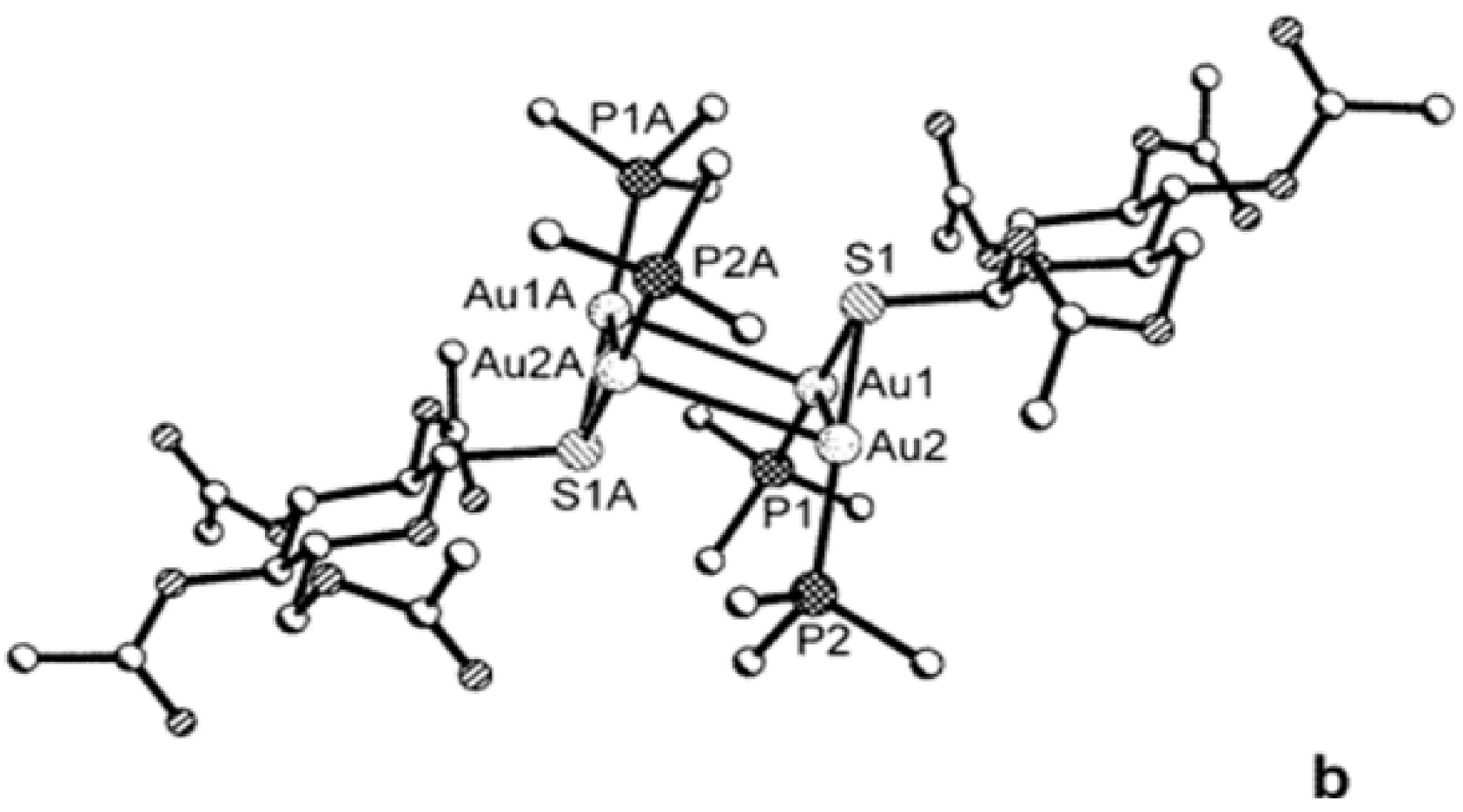
5.4. Reduction of Gold(I) to Gold(0) in Joints “Aurosomes”
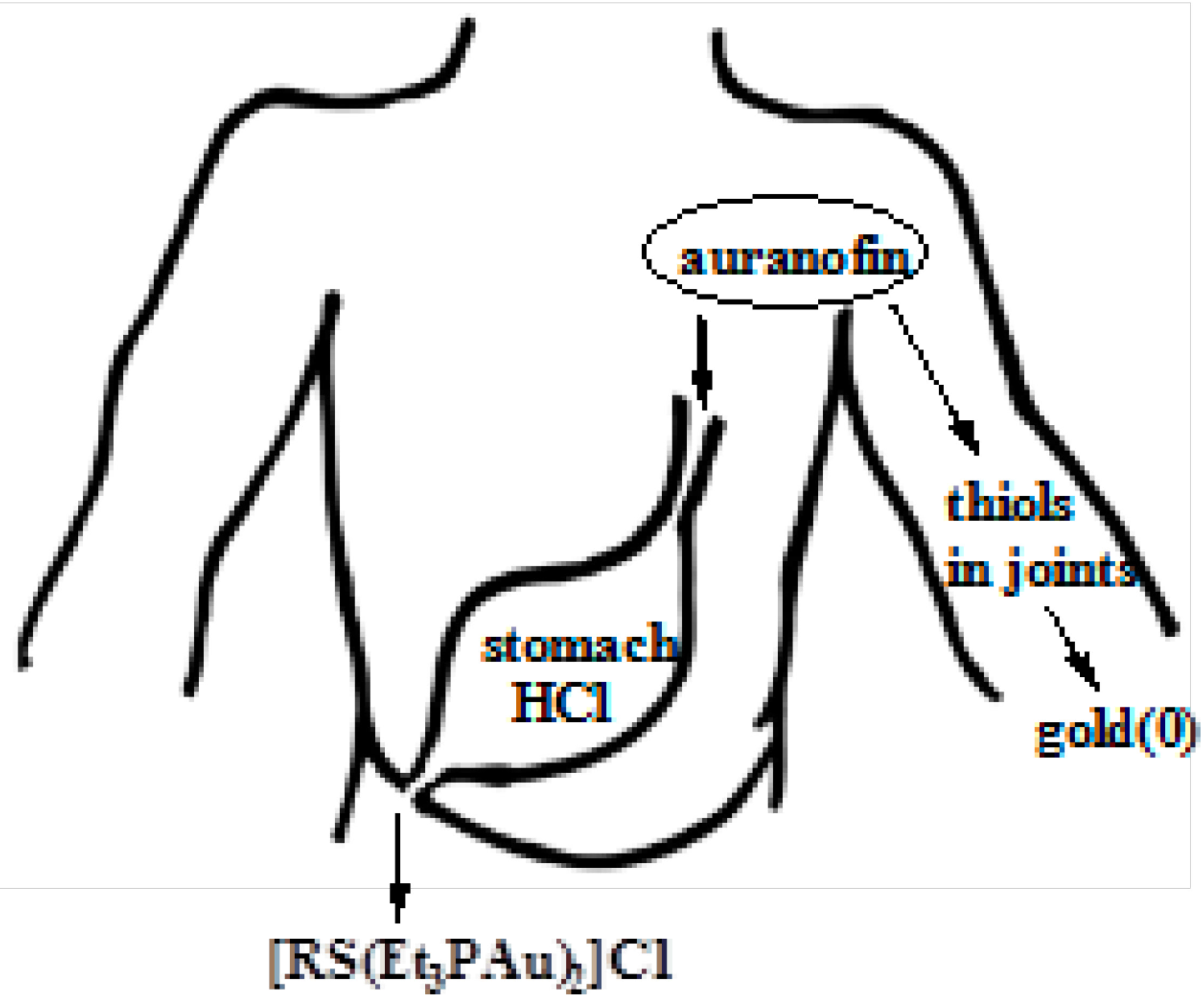
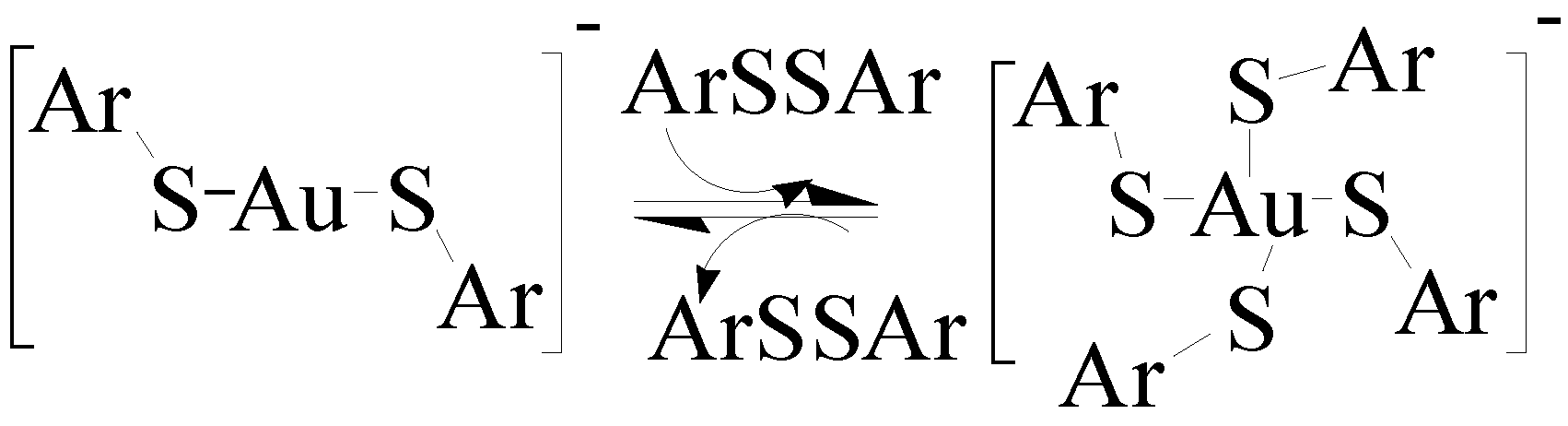
5.5. Strong Oxidation of Gold Drugs
5.6. Activation of Gold(I) Drugs by Forming Gold(I) Cyanide Metabolite

5.7. Smoking and the Clinical Response to Gold Drugs
5.8. Toxicity of Gold Rheumatoid Drugs
5.9. General Comments on the Mechanism of Action
6. Promising Gold Therapeutic Anticancer Properties

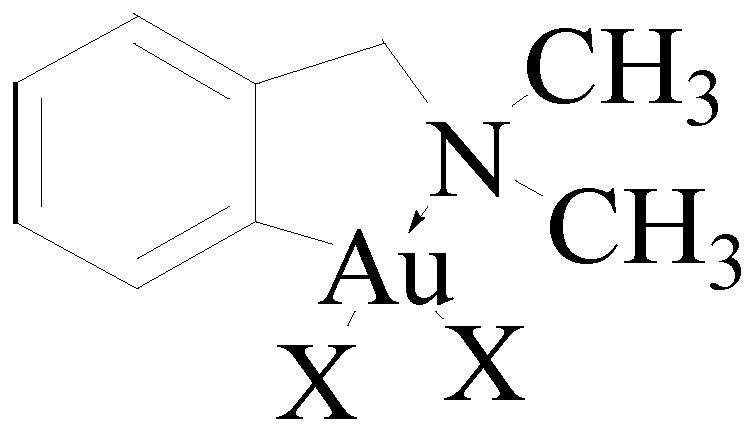
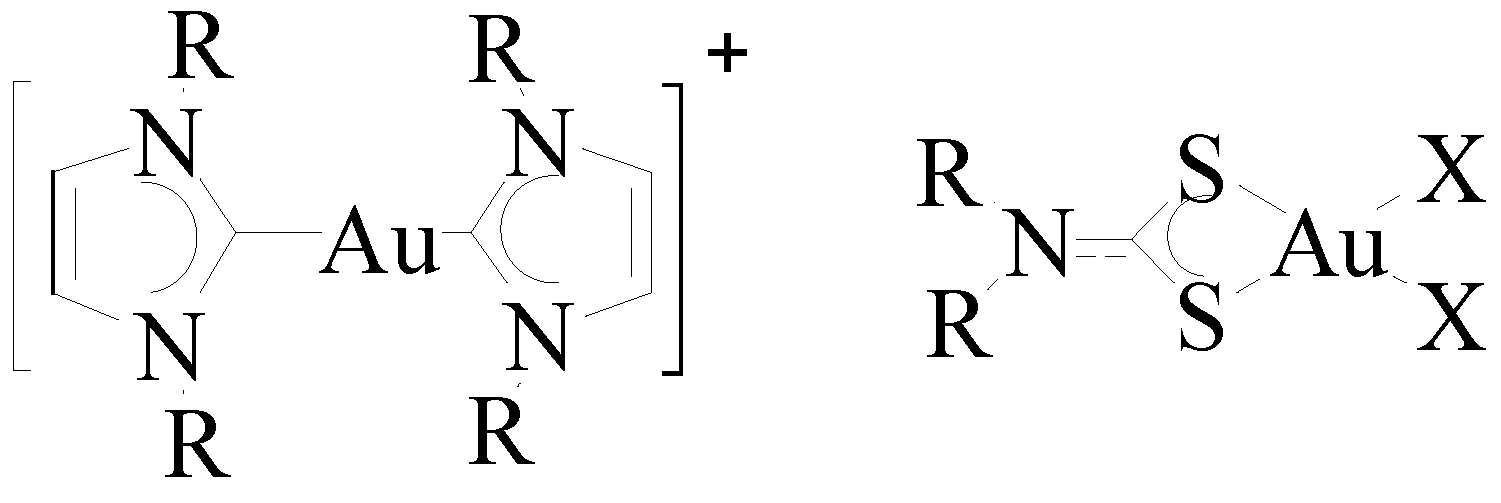
7. Anti-HIV Activity of Gold
8. Current Status of Gold Nanoparticles Therapy
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Corti, C.; Holliday, R. Gold: Science and Applications; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Schmidbaur, H. Gold Progress in Chemistry, Biochemistry, and Technology; Wiley: West Sussex, UK, 1999. [Google Scholar]
- Mohr, F. Gold Chemistry. Applications and Future Directions in Life Sciences; John Wiley & Sons: Weinheim, Germany, 2009. [Google Scholar]
- Thompson, C.J.S. Alchemy and Alchemists; Dover Publications, Inc.: Mineola, NY, USA, 2002; pp. 18, 61. [Google Scholar]
- Boyle, R. The Sceptical Chymist; Dover Publication, Inc.: Mineola, NY, USA, 2003; p. 31. [Google Scholar]
- Goodman, L.S.; Gilman, A. The Pharmacological Basis of Therapeutics, 3rd ed.; The Macmillan Co: New York, NY, USA, 1965; Volume 155, p. 957. [Google Scholar]
- Wood, H.C.; Remington, J.P.; Sadtler, S.P. United States Dispensatory, 19th ed.; J.B. Lippincott Co.: Philadelphia, PA, USA, 1907; p. 220. [Google Scholar]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. London 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Norton, S. A Brief History of Potable Gold. Mol. Interv. 2008, 8, 120–123. [Google Scholar] [CrossRef]
- Hauser, E.A. Aurum Potabile. J. Chem. Edu. 1952, 29, 456–458. [Google Scholar] [CrossRef]
- Fricker, S.P. Medical Uses of Gold Compounds: Past, Present and Future. Gold Bull. 1996, 29, 53–60. [Google Scholar] [CrossRef]
- Koch, R. An Address on Bacteriological Research. Brit. Med. J. 1890, 2, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Aurothioglucose and Auranofin. In Mosby's GenRx. The Complete Reference for Generic and Brand Drug, 12th ed.; Mosby Year Book: St. Louis, MO, USA, 2002.
- American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the Management of Rheumatoid Arthritis. Arthritis Rheum. 2002, 46, 328–346. [Google Scholar]
- Henderson, B.; Edwards, J.; Pettipher, E.R. Mechanisms and Models in Rheumatoid Arthritis. Academic Press: London, UK, 1995. [Google Scholar]
- Hitchon, C.A.; El-Gabalawy, H.S. Oxidation in Rheumatoid Arthritis. Arthritis Res Ther. 2004, 6, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. Organic Derivatives of Gold and Silver. Patai, S., Rappoport, Z., Eds.; John Wiley & Sons, Ltd.: Weinheim, Germany, 1999. [Google Scholar]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Perspectives: Chemistry: Noblesse Oblige. Science 2000, 290, 64–65. [Google Scholar] [CrossRef]
- Abdou, H.E.; Mohamed, A.A.; Fackler, J.P., Jr. Gold Chemistry: Highlights and Future Directions; Mohr, F., Ed.; Wiley & Sons: Weinheim, Germany, 2009; pp. 1–45. [Google Scholar]
- Elder, R.C.; Tepperman, K.G.; Eidsness, M.K.; Heeg, M.J.; Shaw, C.F., III; Schaeffer, N.A. Gold-Based Antiarthritic Drugs and Metabolites. Extended X-ray Adsorption Fine Structure (EXAFS) Spectroscopy and X-ray Absorption Near Edge Spectroscopy (XANES). ACS Symp. Ser. 1983, 209, 385–400. [Google Scholar]
- Elder, R.C.; Ludwig, K.; Cooper, J.N.; Eidsness, M.K. EXAFS and WAXS Structure Determination for an Antiarthritic Drug, Sodium Gold(I) Thiomalate. J. Am. Chem. Soc. 1985, 107, 5024–5026. [Google Scholar] [CrossRef]
- Bau, R. Crystal Structure of the Antiarthritic Drug Gold Thiomalate (Myochrysine): A Double-Helical Geometry in the Solid State. J. Am. Chem. Soc. 1998, 120, 9380–9381. [Google Scholar] [CrossRef]
- Ruben, H.; Zalkin, A.; Faltens, M.O.; Templeton, D.H. Crystal Structure of Sodium Gold(I) thiosulfate dihydrate, Na3Au(S2O3)2.2H2O. Inorg. Chem. 1974, 13, 1836. [Google Scholar] [CrossRef]
- Hill, D.T.; Sutton, B.M. (2,3,4,6-Tetra-O-acetyl-1-thio-β-d-glucopyranosato-S) (triethylphosphine)gold, C20H34AuO9PS. Crystal Structure Commun. 1980, 9, 679–86. [Google Scholar]
- Bryan, D.L. B.; Mikuriya, Y.; Hempel, J.C.; Mellinger, D.; Hashim, M.; Pasternack, R.F. Reactions of Auranofin ((1-thio-β-d-glucopyranose 2,3,4,6-tetraacetato-S)(triethylphosphine)gold(I)) in Aqueous Hydrochloric Acid. Inorg. Chem. 1987, 26, 4180–4185. [Google Scholar] [CrossRef]
- Christodoulou, J.; Sadler, P.J.; Tucker, A. A New Structural Transition of Serum Albumin Dependent on the State of Cys34. Detection by 1H-NMR Spectroscopy. Eur. J. Biochem. 1994, 225, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Isab, A.A.; Shaw, C.F., III; Locke, J. GC-MS and Oxygen-17 NMR Tracer Studies of Triethylphosphine Oxide Formation from Auranofin and Water-17O in the Presence of Bovine Serum Albumin: an in vitro Model for Auranofin Metabolism. Inorg. Chem. 1988, 27, 3406–3409. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abdou, H.E.; Chen, J.; Bruce, A.E.; Bruce, M.R.M. Perspectives in Inorganic and Bioinorganic Gold Sulfur Chemistry. Comments Inorg. Chem. 2002, 23, 321–334. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Bruce, M.R.M.; Bruce, A.E. Cyclic Voltammetry of Auranofin. Metal Based Drugs 1999, 6, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Chen, J.; Krause Bauer, J.A.; Hill, D.T.; Bruce, A.E.; Bruce, M.R.M. Formation of a Cationic Gold(I) Complex and Disulfide by Oxidation of the Antiarthritic Gold Drug Auranofin. Inorg. Chem. 2003, 42, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.B.; Yuan, J.; Narayanaswamy, R.; Young, M.A.; Elder, R.C.; Bruce, A.E.; Bruce, M.R.M. Solid State EXAFS and Luminescence Studies of Neutral, Dinuclear Gold(I) Complexes. Gold(I)-Gold(I) Interactions in the Solid State. Inorg. Chem. 1995, 34, 1996–2001. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, T.; Wei, G.; Mohamed, A.A.; Homrighausen, C.; Bauer, J.; Bruce, A.E.; Bruce, M.R.M. Electrochemical and Chemical Oxidation of Gold(I) Thiolate Phosphine Complexes: Formation of Gold Clusters and Disulfide. J. Am. Chem. Soc. 1999, 121, 9225–9226. [Google Scholar] [CrossRef]
- Mendez, J.H.; Perez, A.S.; Zamarreno, M.D. Electrochemical Behavior and Polarographic Determination of Auranofin. J. Pharm. Sci. 1989, 78, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.C.; Eidsness, M.K. Synchrotron X-ray Studies of Metal-Based Drugs and Metabolites. Chem. Rev. 1987, 87, 1027–1046. [Google Scholar] [CrossRef]
- Ghadially, F.N. The Aurosome. J. Rheum. 1979, 6, 45–50. [Google Scholar]
- Garrett, I.R.; Whitehouse, M.W.; Vernon-Roberts, B. Ambivalent Properties of Gold Drugs in Adjuvant Induced Polyarthritis in Rats. J. Rheumatol. 1985, 12, 1079–1082. [Google Scholar] [PubMed]
- Goebel, C.; Kubicka-Muranyi, M.; Tonn, T.; Gonzalez, J.; Gleichmann, E. Phagocytes Render Chemicals Immunogenic: Oxidation of Gold(I) to the T cell-Sensitizing Gold(III) Metabolite Generated by Mononuclear Phagocytes. Arch. Toxicol. 1995, 69, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Gleichmann, E.; Kubicka-Muranvi, M.; Kind, P.; Goldermann, R.; Goerz, G.; Merk, H.; Rau, R. Insights into the Mechanism of Gold Action Provided by Immunotoxicology: Biooxidation of Gold(I) to Gold(III) Detected by Sensitized T-cells. Rheum. Int. 1991, 11, 219–220. [Google Scholar] [CrossRef]
- Shaw, C.F. The Mammalian Biochemistry of Gold: an Inorganic Perspective of Chrysotherapy. Inorg Perspect Biol Med. 1979, 2, 287–355. [Google Scholar]
- Canumalla, A.J.; Al-Zamil, N.; Phillips, M.; Isab, A.A.; Shaw, C.F., III. Redox and Ligand Exchange Reactions of Potential Gold(I) and Gold(III)-Cyanide Metabolites under Biomimetic Conditions. J. Inorg. Biochem. 2001, 85, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.C.; Zhao, Z.; Zhang, Y.; Dorsey, J.G.; Hess, E.V.; Tepperman, K. Dicyanogold(I) Is a Common Human Metabolite of Different Gold Drugs. J. Rheumatol. 1993, 20, 268–272. [Google Scholar] [PubMed]
- Graham, G.G.; Bales, J.R.; Grootveld, M.C.; Sadler, P.J. 1H, 13C NMR, and Electronic Absorption Spectroscopic Studies of the Interaction of Cyanide with Aurothiomalate. J. Inorg. Biochem. 1985, 25, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Dale, M.M. The Activation of Gold Complexes by Cyanide Produced by Polymorphonuclear Leukocytes. II. Evidence for the Formation and Biological Activity of Aurocyanide. Biochem. Pharmacol. 1990, 39, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Kettle, A. The Activation of Gold Complexes by Cyanide Produced by Polymorphonuclear Leukocytes. III. The Formation of Aurocyanide by Myeloperoxidase. J. Biochem. Pharmacol. 1998, 56, 307–312. [Google Scholar] [CrossRef]
- Shaw, G.F.; Schraa, S.; Gleichmann, E.; Grover, Y.P.; Dunemann, L.; Jagarlamudi, A. Redox Chemistry and [Au(CN)2]− in the Formation of Gold Metabolites. Metal Based Drugs 1994, 1, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Yangyuoru, P.M.; Webb, J.W.; Shaw, C.F., III. Glutathionato-S-Gold(III) Complexes Formed as Intermediates in the Reduction of Auricyanide by Glutathione. J. Inorg. Biochem. 2008, 102, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Haavisto, T.M.; Jones, H.M.; Champion, G.D. The Effect of Cyanide on the Uptake of Gold by Red Blood Cells. Biochem. Pharmacol. 1984, 33, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Haavisto, T.M.; McNaught, P.J. The Effect of Smoking on the Distribution of Gold in Blood. J. Rheumatol. 1982, 9, 527–531. [Google Scholar] [PubMed]
- Kean, W.F.; Kean, I.R. Clinical Pharmacology of Gold. Inflammopharmacology 2008, 16, 107. [Google Scholar] [PubMed]
- Tiekink, E.R.T. Gold Compounds in Medicine: Potential Anti-Tumour Agents. Gold Bull. 2003, 36, 117. [Google Scholar] [CrossRef]
- Ward, J.R.; Williams, N.J.; Egger, J.J.; Reading, J.C.; Boyce, E.; Altz-Smith, M.; Samuelson, C.O.; Willkens, R.F.; Solsky, M.A.; Hayes, S.P.; et al. Comparison of Auranofin, Gold Sodium thiomalate, and Placebo in the Treatment of Rheumatoid Arthritis. A controlled Clinical Trial. Arthritis Rheum. 1983, 26, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fackler, J.P., Jr. Gold Thiolate Complexes with Short Intermolecular Gold-Gold Distances from Reactions of Organic Disulfides with Gold(I) Complexes. Syntheses and Crystal Structures of [AuI2(PPh3)2(m-SCH2Ph)](NO3) and AuIIICl4(m-SPh)2. Inorg. Chem. 1990, 29, 4404–4407. [Google Scholar]
- Lioe, H.; Duan, M.; O’Hair, R.A.J. Can Metal Ions be Used as Gas-Phase Disulfide Bond Cleavage Reagents? A Survey of Coinage Metal Complexes of Model Peptides Containing an Intermolecular Disulfide Bond. Rapid Commun. Mass Spectrom. 2007, 21, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.M.; Musaev, D.G.; Morokuma, K. Theoretical Studies of Oxidative Addition of E-E Bonds (E = S, Se and Te) to Pd(0) and Pt(0) Complexes. Organometallics 2005, 24, 4908–4914. [Google Scholar] [CrossRef]
- Bachman, R.E.; Bodolosky-Bettis, S.A.; Pyle, C.J.; Gray, M.A. Reversible Oxidative Addition and Reductive Elimination of Fluorinated Disulfides at Gold (I) Thiolate Complexes: A new Ligand Exchange Mechanism. J. Am. Chem. Soc. 2008, 130, 14303–14310. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Platinum Binding to DNA: Structural Controls and Consequences. J. Chem. Soc. 2001, 19, 2711–2718. [Google Scholar]
- Berners-Price, S.J.; Mirabelli, C.K.; Johnson, R.K.; Mattern, M.R.; McCabe, F.L.; Faucette, L.F.; Sung, C.-M.; Mong, S.-M.; Sadler, P.J.; Crooke, S.T. In vivo Antitumor Activity and in vitro Cytotoxic Properties of Bis[1,2-bis(diphenylphosphino)ethane]Gold(I) Chloride. Cancer Res. 1986, 46, 5486–5493. [Google Scholar] [PubMed]
- McKeage, M.J.; Berners-Price, S.J.; Galletis, P.; Bowen, R.J.; Brouwer, W.; Ding, L.; Zhuang, L.; Baguley, B. Role of Lipophilicity in Determining Cellular Uptake and Antitumour Activity of Gold Phosphine Complexes. Cancer Chemother. Pharmacol. 2000, 46, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Tiekink, E.R.T. Gold Derivatives for the Treatment of Cancer. Crit. Rev. Oncol. Hematol. 2002, 42, 225–248. [Google Scholar] [CrossRef] [PubMed]
- McKeage, M.J.; Maharaj, L.; Berners-Price, S. Mechanisms of Cytotoxicity and Antitumor Activity of Gold(I) Phosphine Complexes: the Possible Role of Mitochondria. J. Coord. Chem. Rev. 2002, 232, 127–135. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Sadler, P.J. Phosphines and Metal Phosphine Complexes: Relationship of Chemistry to Anticancer and Other Biological Activity. Struct. Bond. 1988, 70, 27–102. [Google Scholar]
- Barnard, P.J.; Berners-Price, S.J. Targeting the Mitochondrial Cell Death Pathway with Gold Compounds. Coord. Chem. Rev. 2007, 251, 1889–1902. [Google Scholar] [CrossRef]
- Hoke, G.D.; Macia, R.A.; Meunier, P.C.; Bugelski, P.J.; Mirabelli, C.K.; Rush, G.F.; Matthews, W.D. In Vivo and In Vitro Cardiotoxicity of a Gold-Containing Antineoplastic Drug Candidate in the Rabbit. Toxicol. Appl. Pharmacol. 1989, 100, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.G.; Elsome, A.M.; Fricker, S.P.; Henderson, G.R.; Theobald, B.R.C.; Parish, R.V.; Howe, B.P.; Kelland, L.R. Antitumor Properties of Some 2-[(Dimethylamino)methyl]phenylgold(III) Complexes. J. Med. Chem. 1996, 39, 5208–5214. [Google Scholar] [CrossRef] [PubMed]
- De Vos, D.; Ho, S.Y.; Tiekink, E.R.T. Cytotoxicity Profiles for a Series of Triorganophosphinegold(I) Dithiocarbamates and Triorganophosphinegold(I) Xanthates. Bioinorg. Chem. Appl. 2004, 2, 141–154. [Google Scholar] [CrossRef]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A Novel Anticancer Gold(III) Dithiocarbamate Compound Inhibits the Activity of a Purified 20S Proteasome and 26S Proteasome in Human Breast Cancer Cell Cultures and Xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Macca, C.; Trevisan, A.; Fregona, D. Gold(III) Dithiocarbamate Derivatives for the Treatment of Cancer: Solution Chemistry, DNA Binding, and Hemolytic Properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Giovagnini, L.; Marzano, C.; Bettio, F.; Grziani, R.; Pilloni, G.; Fregona, D. Gold Dithiocarbamate Derivatives as Potential Antineoplastic Agents: Design, Spectroscopic Properties, and in Vitro Antitumor Activity. Inorg. Chem. 2005, 44, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.J.B.; Vasam, C.S. Review of Gold(I) N-heterocyclic Carbenes. Can. J. Chem. 2005, 83, 812–825. [Google Scholar] [CrossRef]
- Barnard, P.J.; Bakers, M.V.; Berners-Price, S.J.; Day, D.A. Mitochondrial Permeability Transition Induced by Dinuclear Gold(I)-Carbene Complexes: Potential New Antimitochondrial Antitumour Agents. J. Inorg. Biochem. 2004, 98, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.V.; Barnard, P.J.; Berners-Price, S.J.; Brayshaw, S.K.; Hickey, J.L.; Skelton, B.W.; White, A.H. Synthesis and Structural Characterization of Linear Au(I) N-heterocyclic Carbene Complexes: New Analogues of the Au(I) Phosphine Drug Auranofin. J. Organomet. Chem. 2005, 690, 5625–5635. [Google Scholar] [CrossRef]
- Okada, T.; Patterson, B.K.; Ye, S.; Gurney, M.E. Aurothiolates Inhibit HIV-1 Infectivity by Gold(I) Ligand Exchange with a Component of the Virion Surface. Virology 1993, 192, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Traber, K.E.; Okamoto, H.; Kurono, C.; Baba, M.; Saliou, C.; Soji, T.; Parker, L.; Okamoto, T. Anti-rheumatic Compound Aurothioglucose Inhibits Tumor Necrosis Factor-α-Induced HIV-1 Replication in Latently Infected OM10.1 and Ach2 Cells. Int. Immunol. 1999, 11, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.L.; Masci, J.R. Treatment of HIV Associated Psoriatic Arthritis with Oral Gold. J. Rheumatol. 1996, 23, 1818–1820. [Google Scholar] [PubMed]
- Tepperman, K.; Zhang, Y.; Roy, P.W.; Floyd, R.; Zhao, Z.; Dorsey, J.G.; Elder, R.C. Transport of the Dicyanogold(I) Anion. Metal-Based Drugs 1994, 1, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hess, E.V.; Pryhuber, K.G.; Dorsey, J.G.; Tepperman, K.; Elder, R.C. Gold Binding Sites in Red Blood Cells. Inorg. Chim. Acta 1995, 229, 271–280. [Google Scholar] [CrossRef]
- Fonteha, P.; Meyer, D. Novel Gold(I) Phosphine Compounds Inhibit HIV-1 Enzymes. Metallomics 2009, 1, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.J.; Shanov, V.N.; Yun, Y. Nanomedicine Design of Particles, Sensors, Motors, Implants, Robots, and Devices; Artech House, Incorporated: Norwood, MA, USA, 2009. [Google Scholar]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential Oxidative Stress of Gold Nanoparticles by Induced-NO Releasing in Serum. J. Am. Chem. Soc. 2009, 131, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Whitehouse, M.W.; Tiekink, E.R. T.; Bushell, G.R. Colloidal Metallic Gold is not Bio-inert. Inflammopharmacology 2008, 16, 133–137. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naggar, M.E.; Shehadi, I.; Abdou, H.E.; Mohamed, A.A. Gilded Hope for Medicine. Inorganics 2015, 3, 139-154. https://doi.org/10.3390/inorganics3020139
Naggar ME, Shehadi I, Abdou HE, Mohamed AA. Gilded Hope for Medicine. Inorganics. 2015; 3(2):139-154. https://doi.org/10.3390/inorganics3020139
Chicago/Turabian StyleNaggar, Mohamed El, Ihsan Shehadi, Hanan E. Abdou, and Ahmed A. Mohamed. 2015. "Gilded Hope for Medicine" Inorganics 3, no. 2: 139-154. https://doi.org/10.3390/inorganics3020139





