Detection and Characterization of Ignitable Liquid Residues in Forensic Fire Debris Samples by Comprehensive Two-Dimensional Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
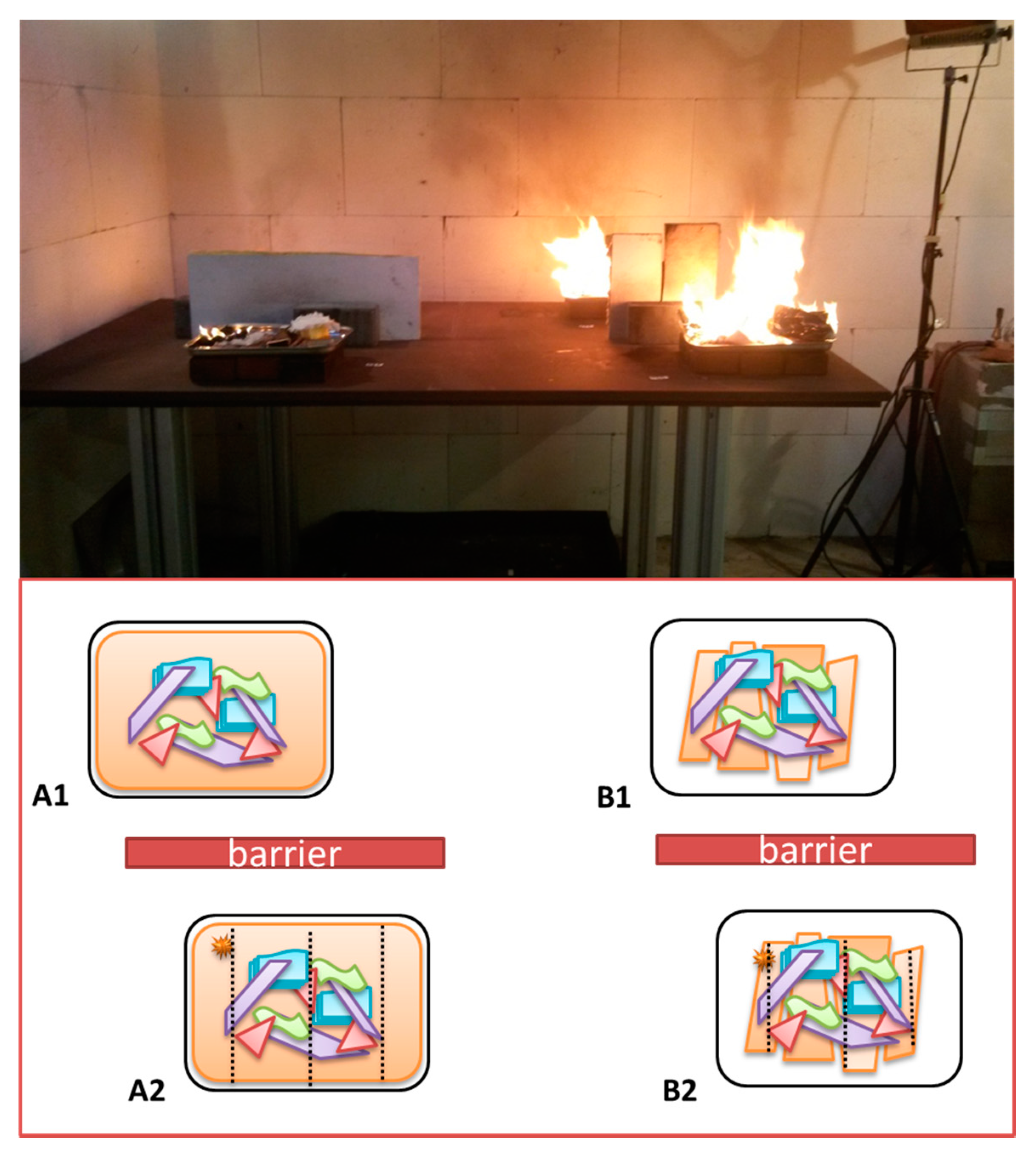
2.2. Preparation of Laboratory-Scale Fire Debris Samples
2.3. Protocol for Burn Experiments
2.4. Preparation of Headspace Extracts
2.5. Instrumental Analysis
2.5.1. GC-FID
2.5.2. GC×GC-FID
2.5.3. GC×GC-TOFMS
3. Results and Discussion
3.1. GC×GC-FID Method Development
3.2. Characterization of ILs
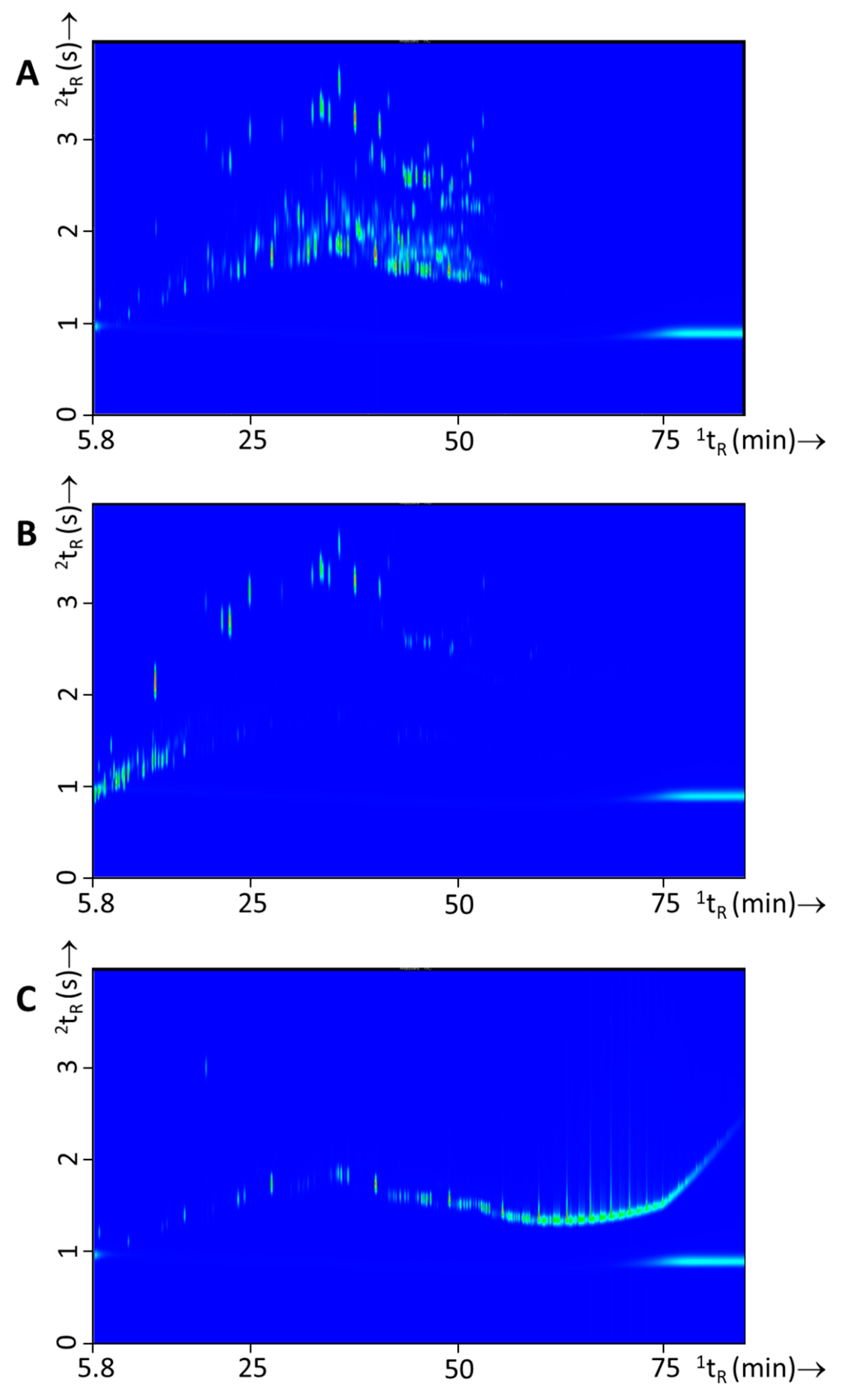
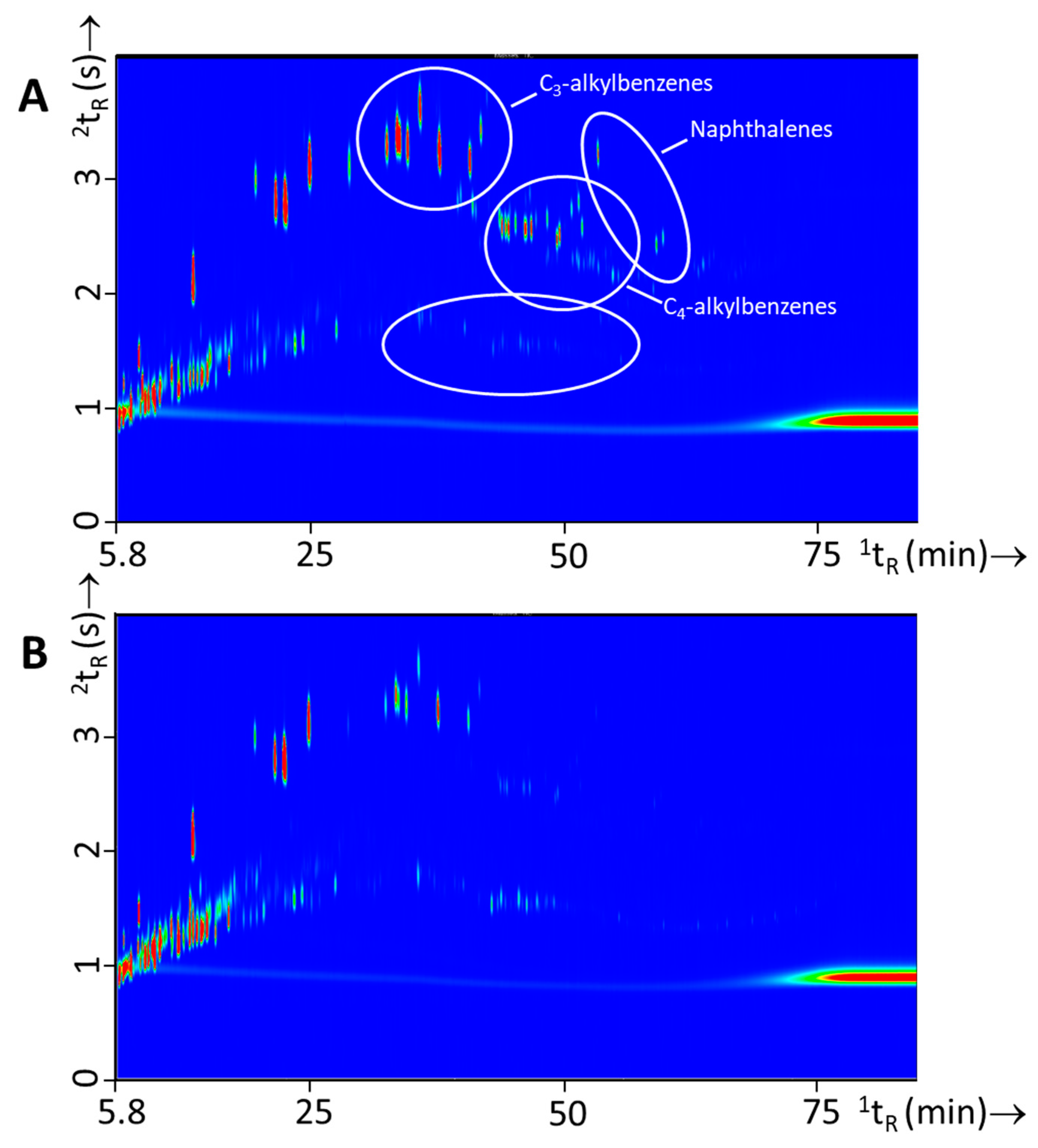
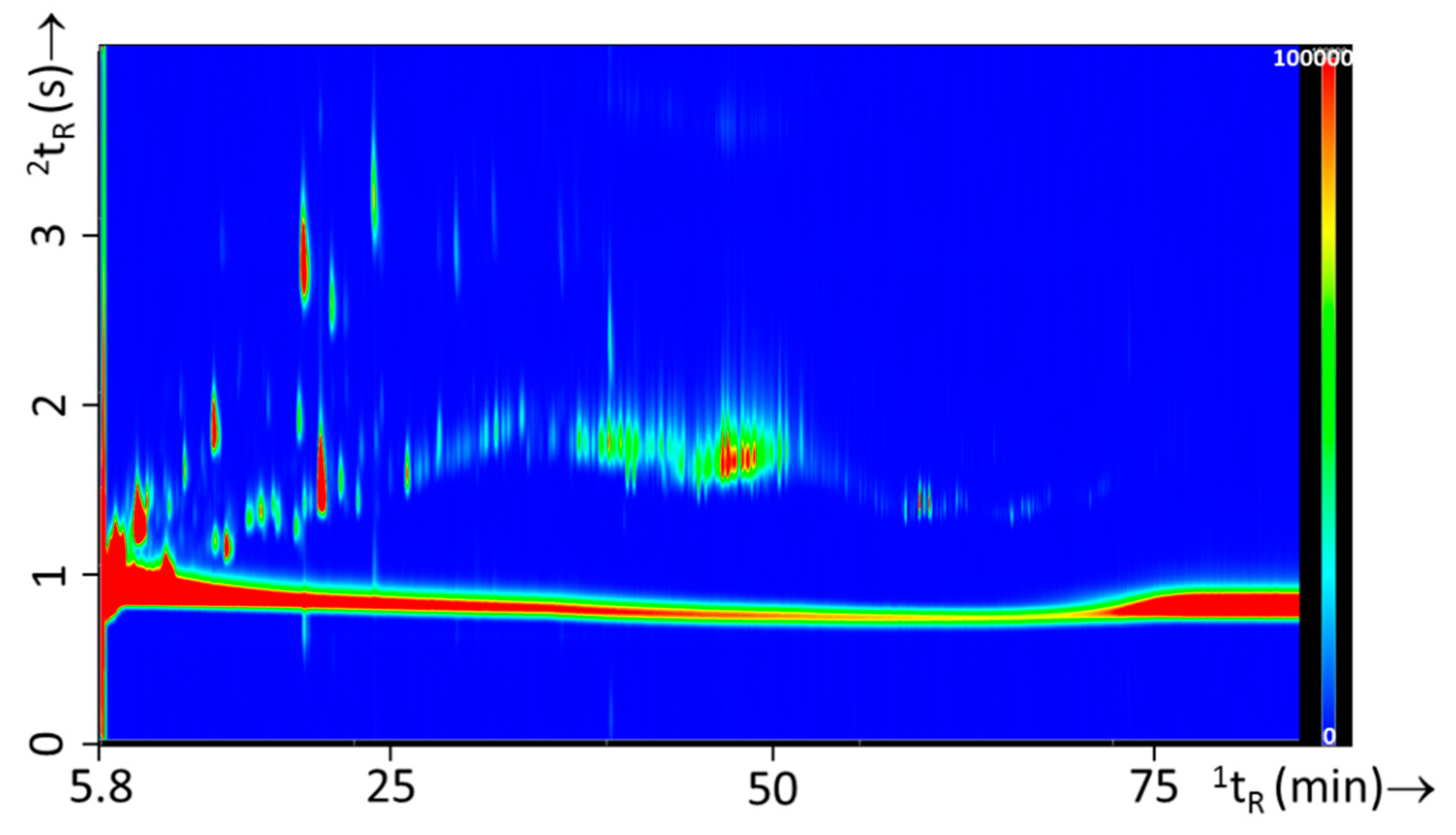
3.3. Fire Experiments without IL: Headspace and Pyrolysis Compounds from Single Substrates and Substrate Mixtures
3.4. Fire Experiments with IL: Ignitable Liquid Detection and Classification in Fire Debris
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stauffer, E.; Dolan, J.A.; Newman, R. Fire Debris Analysis; Academic Press, Elsevier: London, UK, 2008; ISBN 9780126639711. [Google Scholar]
- ASTM E1618-14. Standard Test Method for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography-Mass Spectrometry; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Sandercock, P.M.L. Fire investigation and ignitable liquid residue analysis—A review: 2001–2007. Forensic Sci. Int. 2008, 176, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Pert, A.D.; Baron, M.G.; Birkett, J.W. Review of Analytical Techniques for Arson Residues. J. Forensic Sci. 2006, 51, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alberca, C.; Ortega-Ojeda, F.E.; García-Ruiz, C. Analytical tools for the analysis of fire debris. A review: 2008–2015. Anal. Chim. Acta 2016, 928, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sampat, A.A.S.; Lopatka, M.; Vivó-Truyols, G.; Schoenmakers, P.J.; van Asten, A.C. Towards chemical profiling of ignitable liquids with comprehensive two-dimensional gas chromatography: Exploring forensic application to neat white spirits. Forensic Sci. Int. 2016, 267, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.M.L.; Du Pasquier, E. Chemical fingerprinting of unevaporated automotive gasoline samples. Forensic Sci. Int. 2003, 134, 1–10. [Google Scholar] [CrossRef]
- Frysinger, G.S.; Gaines, R.B. Forensic analysis of ignitable liquids in fire debris by comprehensive two-dimensional gas chromatography. J. Forensic Sci. 2002, 47, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Peschier, L.J.C.; Grutters, M.M.P.; Hendrikse, J.N. Using Alkylate Components for Classifying Gasoline in Fire Debris Samples. J. Forensic Sci. 2018, 63, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Baerncopf, J.; Hutches, K. A review of modern challenges in fire debris analysis. Forensic Sci. Int. 2014, 244, e12–e20. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J. ENFSI collaborative testing programme for ignitable liquid analysis: A review. Forensic Sci. Int. 2007, 167, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.R.; McGuffin, V.L.; Waddell, S.R. Effect of evaporation and matrix interferences on the association of simulated ignitable liquid residues to the corresponding liquid standard. Forensic Sci. Int. 2012, 222, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hendrikse, J.; Grutters, M.; Schäfer, F. Identifying Ignitable Liquids in Fire Debris; Academic Press, Elsevier: London, UK, 2016; ISBN 9780128043165. [Google Scholar]
- Fernandes, M.S.; Lau, C.M.; Wong, W.C. The effect of volatile residues in burnt household items on the detection of fire accelerants. Sci. Justice 2002, 42, 7–15. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liang, D.; Shen, H. An analysis of background interference on fire debris. Procedia Eng. 2013, 52, 664–670. [Google Scholar] [CrossRef]
- Borusiewicz, R.; Zieba-Palus, J.; Zadora, G. The influence of the type of accelerant, type of burned material, time of burning and availability of air on the possibility of detection of accelerants traces. Forensic Sci. Int. 2006, 160, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.R.; Furton, K.G. Characterization of background and pyrolysis products that may interfere with the forensic analysis of fire debris. J. Anal. Appl. Pyrolysis 2004, 71, 51–67. [Google Scholar] [CrossRef]
- Americal Society for Testing Materials. ASTM E 1412-00 Standard practice for separation of ignitable liquid residues from fire debris samples by passive headspace concentration with activated charcoal. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2001; pp. 431–433. [Google Scholar]
- Lennard, C.J.; Tristan Rochaix, V.; Margot, P.; Huber, K. A GC–MS database of target compound chromatograms for the identification of arson accelerants. Sci. Justice 1995, 35, 19–30. [Google Scholar] [CrossRef]
- Schwartz, Z.; An, Y.; Konstantynova, K.I.; Jackson, G.P. Analysis of household ignitable liquids and their post-combustion weathered residues using compound-specific gas chromatography-combustion-isotope ratio mass spectrometry. Forensic Sci. Int. 2013, 233, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yoh, J.J. Fire debris analysis for forensic fire investigation using laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 134, 75–80. [Google Scholar] [CrossRef]
- Lopatka, M.; Sampat, A.A.; Jonkers, S.; Adutwum, L.A.; Mol, H.G.J.; van der Weg, G.; Harynuk, J.J.; Schoenmakers, P.J.; van Asten, A.; Sjerps, M.J.; et al. Local Ion Signatures (LIS) for the examination of comprehensive two-dimensional gas chromatography applied to fire debris analysis. Forensic Chem. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Gruber, B.; Weggler, B.A.; Jaramillo, R.; Murrell, K.A.; Piotrowski, P.K.; Dorman, F.L. Comprehensive two-dimensional gas chromatography in forensic science: A critical review of recent trends. TrAC Trends Anal. Chem. 2018, 105, 292–301. [Google Scholar] [CrossRef]
- Sampat, A.; Lopatka, M.; Sjerps, M.; Vivo-Truyols, G.; Schoenmakers, P.; van Asten, A. Forensic potential of comprehensive two-dimensional gas chromatography. TrAC Trends Anal. Chem. 2016, 80, 345–363. [Google Scholar] [CrossRef]
- Taylor, C.M. An Arson Investigation by using Comprehensive Two-dimensional Gas Chromatography-Quadrupole Mass Spectrometry. J. Forensic Res. 2012, 3. [Google Scholar] [CrossRef]
- Jonkers, S. Local Ion Signatures (LIS) for Forensic Comparison of GCxGC-MS Data; University of Amsterdam: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nizio, K.; Cochran, J.; Forbes, S. Achieving a Near-Theoretical Maximum in Peak Capacity Gain for the Forensic Analysis of Ignitable Liquids Using GC×GC-TOFMS. Separations 2016, 3, 26. [Google Scholar] [CrossRef]
- Organtini, K.L.; Myers, A.L.; Jobst, K.J.; Cochran, J.; Ross, B.; McCarry, B.; Reiner, E.J.; Dorman, F.L. Comprehensive characterization of the halogenated dibenzo-p-dioxin and dibenzofuran contents of residential fire debris using comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry. J. Chromatogr. A 2014, 1369, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Centraal Bureau voor Statistiek. Brandweerstatistiek 2013–2017; CBS: Den Haag, The Netherlands, 2013–2017. [Google Scholar]
- Mondello, L. Fundamental Principles of Comprehensive 2D GC. In GC×GC Handbook; Shimadzu Corporation: Kyoto, Japan, 2012; pp. 1–29. [Google Scholar]
- Akmeemana, A.; Williams, M.R.; Sigman, M.E. Major chemical compounds in the Ignitable Liquids Reference Collection and Substrate databases. Forensic Chem. 2017, 5, 91–108. [Google Scholar] [CrossRef]
- Sandercock, P.M.L.; Du Pasquier, E. Chemical fingerprinting of gasoline: 2. Comparison of unevaporated and evaporated automotive gasoline samples. Forensic Sci. Int. 2004, 140, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sinkov, N.A.; Harynuk, J.J. Cluster resolution: A metric for automated, objective and optimized feature selection in chemometric modeling. Talanta 2011, 83, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Adutwum, L.A.; Abel, R.J.; Harynuk, J. Total Ion Spectra versus Segmented Total Ion Spectra as Preprocessing Tools for Gas Chromatography-Mass Spectrometry Data. J. Forensic Sci. 2017, 63, 1059–1068. [Google Scholar] [CrossRef] [PubMed]





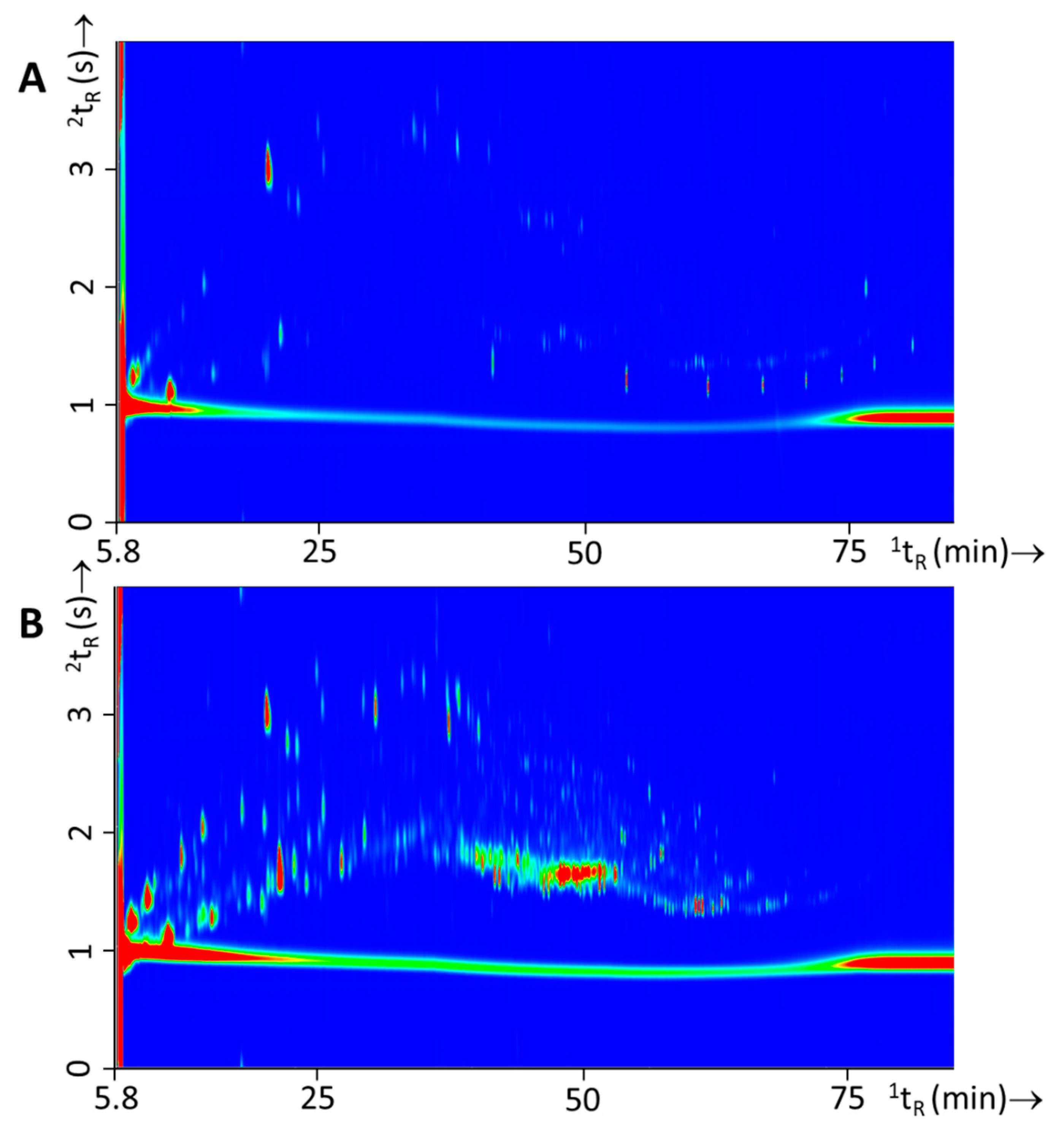

| Single Substrate Material Burns | Substrate Mixture Material Burns | Substrate Mixture Material Burns with IL | ||||
|---|---|---|---|---|---|---|
| Exp # | Substrate | Exp # | Primary Substrate | Secondary Substrates | Exp # | Substrate + IL |
| 1 | 1 | 20 | 1 | 10 12 16 18 | 65 | Same as #020 + IL 1 Bottle 1 |
| 2 | 2 | 21 | 2 | 7 10 17 19 | 66 | Same as #021 + IL 1 Bottle 2 |
| 3 | 3 | 22 | 3 | 12 7 15 17 | 67 | Same as #022 + IL 1 Bottle 3 |
| 4 | 4 | 23 | 4 | 10 13 16 18 | 68 | Same as #023 + IL 1 Bottle 4 |
| 5 | 5 | 24 | 5 | 3 6 17 19 | 69 | Same as #024 + IL 1 Bottle 5 |
| 6 | 6 | 25 | 7 | 2 4 6 10 | 70 | Same as #025 + IL 1 Bottle 6 |
| 7 | 7 | 26 | 8 | 9(1) 13 15 18 | 71 | Same as #026 + IL 1 Bottle 7 |
| 8 | 8 | 27 | 9(1) | 4 16 12 18 | 72 | Same as #027 + IL 1 Bottle 8 |
| 9 | 9(1) | 28 | 10 | 5 6 1 17 | 73 | Same as #028 + IL 1 Bottle 9 |
| 009_2 | 9(2) 1 | 29 | 11 | 19 7 9(1) 18 | 74 | Same as #029 + IL 1 Bottle 10 |
| 10 | 10 | 30 | 12 | 5 6 16 18 | 75 | Same as #030 + IL 1 Bottle 11 |
| 11 | 11 | 31 | 13 | 2 5 12 14 | 76 | Same as #031 + IL 1 Bottle 12 |
| 12 | 12 | 32 | 14 | 4 7 9(1) 11 | 77 | Same as #032 + IL 1 Bottle 13 |
| 13 | 13 | 33 | 17 | 1 2 13 9(1) | 78 | Same as #033 + IL 1 Bottle 14 |
| 14 | 14 | 34 | 19 | 7 10 13 15 | 79 | Same as #034 + IL 1 Bottle 15 |
| 15 | 15 | 35 | 1 | 8 11 12 19 | 80 | Same as #035 + IL 2 Batch 1 |
| 16 | 16 | 36 | 2 | 16 14 15 11 | 81 | Same as #036 + IL 2 Batch 2 |
| 17 | 17 | 37 | 3 | 6 7 14 19 | 82 | Same as #037 + IL 2 Batch 3 |
| 18 | 18 | 38 | 4 | 12 18 14 19 | 83 | Same as #038 + IL 2 Batch 4 |
| 19 | 19 | 39 | 5 | 2 3 6 16 | 84 | Same as #039 + IL 2 Batch 5 |
| 40 | 7 | 3 12 17 18 | 85 | Same as #040 + IL 2 Batch 6 | ||
| 41 | 8 | 5 15 14 17 | 86 | Same as #041 + IL 2 Batch 7 | ||
| 42 | 9(1) | 4 9(1) 11 15 | 87 | Same as #042 + IL 2 Batch 8 | ||
| 43 | 10 | 1 18 9(1) 13 | 88 | Same as #043 + IL 2 Batch 9 | ||
| 44 | 11 | 3 16 10 13 | 89 | Same as #044 + IL 2 Batch 10 | ||
| 45 | 12 | 7 11 13 14 | 90 | Same as #045 + IL 2 Batch 11 | ||
| 46 | 13 | 9(1) 12 14 19 | 91 | Same as #046 + IL 2 Batch 12 | ||
| 47 | 14 | 7 3 11 19 | 92 | Same as #047 + IL 2 Batch 13 | ||
| 48 | 17 | 5 9(1) 13 18 | 93 | Same as #048 + IL 2 Batch 14 | ||
| 49 | 19 | 5 8 15 17 | 94 | Same as #049 + IL 2 Batch 15 | ||
| 50 | 1 | 6 10 14 16 | 95 | Same as #050 + IL 3 Bottle 1 | ||
| 51 | 2 | 13 5 18 15 | 96 | Same as #051 + IL 3 Bottle 2 | ||
| 52 | 3 | 14 10 15 17 | 97 | Same as #052 + IL 3 Bottle 3 | ||
| 53 | 4 | 11 12 13 14 | 98 | Same as #053 + IL 3 Bottle 4 | ||
| 54 | 5 | 10 14 16 18 | 99 | Same as #054 + IL 3 Bottle 5 | ||
| 55 | 7 | 10 11 16 19 | 100 | Same as #055 + IL 3 Bottle 6 | ||
| 56 | 8 | 1 5 13 14 | 101 | Same as #056 + IL 3 Bottle 7 | ||
| 57 | 9(2) | 2 19 13 16 | 102 | Same as #057 + IL 3 Bottle 8 | ||
| 58 | 10 | 3 5 14 16 | 103 | Same as #058 + IL 3 Bottle 9 | ||
| 59 | 11 | 8 12 16 17 | 104 | Same as #059 + IL 3 Bottle 10 | ||
| 60 | 12 | 6 14 15 5 | 105 | Same as #060 + IL 3 Bottle 11 | ||
| 61 | 13 | 2 14 15 16 | 106 | Same as #061 + IL 3 Bottle 12 | ||
| 62 | 14 | 1 3 19 16 | 107 | Same as #062 + IL 3 Bottle 13 | ||
| 63 | 17 | 5 9(1) 10 6 | 108 | Same as #063 + IL 3 Bottle 14 | ||
| 64 | 19 | 17 3 12 16 | 109 | Same as #064 + IL 3 Bottle 15 | ||
| Fire Debris | ILs | |||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | n1 | 1Dtr (s) | 2Dtr (s) | Area | n | 1Dtr (s) | 2Dtr (s) | Area |
| Chlorobenzene | 43 | 0.09% | 1.74% | 23.14% | 37 | 0.08% | 0.14% | 16.42% |
| Compound | Substrates | Frequency of Occurrences [31] |
|---|---|---|
| methyl methacrylate | Mattress, computer fragments, electronics | - |
| pentanal | Floor skirting, pine floor strips, laminate flooring, living-room rug, plasterboard, particle board, wooden chair, newspaper | - |
| 1,4-dioxine | Vinyl flooring | - |
| methyl isobutyl ketone | Floor skirting, pine floor strips, oak floor strips, laminate Flooring, carpet material, carpet underlay, living-room rug, Particle board, sofa, wooden chair, mattress, magazines, Newspaper, clothing | - |
| butyl ester acetic ester | Floor skirting, carpet underlay, sofa, wooden chair | - |
| cyclopentanone | Carpet underlay, plasterboard, magazines, newspaper, Electronics | 9 × 10−3 |
| 2n-butylcrolein | Laminate flooring, particle board | - |
| styrene | Laminate flooring, carpet material, living-room rug, vinyl flooring, curtains, plasterboard, sofa, mattress, magazines, newspaper, computer fragments, clothing, electronics | 2.4 × 10−1 |
| 4-methyl-2-heptanone | Carpet material, carpet underlay, living-room rug | - |
| 4,6-dimethyl-2-heptanone | Carpet underlay | - |
| n-butyl methacrylate | Electronics | - |
| a-methylstyrene | Computer fragments, electronics | 2 × 10−2 |
| 2-ethyl-1-hexanol | Electronics | 5 × 10−2 |
| butylbenzene | Electronics | - |
| a-pinene | Floor skirting, pine floor strips, laminate flooring, plasterboard, particle board, sofa | - |
| camphene | Floor skirting, pine floor strips, particle board | - |
| b-pinene | Pine floor strips, particle boards | - |
| 2-pentylfuran | Pine floor strips, plasterboard, sofa, magazines, newspaper | 4 × 10−2 |
| nonanal | Curtains(2), plasterboard, sofa, magazines, newspaper, Clothing, electronics | 9 × 10−3 |
| decanal | Plasterboard, sofa, mattress, clothing | - |
| undecanal | Plasterboard | - |
| dodecanal | Plasterboard | - |
| tridecanal | Plasterboard | - |
| nonanoic acid | Mattress, clothing, electronics | - |
| dibutyl phthalate | Mattress, clothing, electronics | 9 × 10−3 |
| 1-chloro-decane | Plasterboard | - |
| 1-chloro-undacane | Plasterboard | - |
| butyl acrylate | Carpet underlay | - |
| 2-hexanone | Floor skirting | - |
| pentanitrile | Floor skirting | - |
| 2-propylfuran | Floor skirting | - |
| benzaldehyde | Particle board | 2.3 × 10−1 |
| camphor | Particle board | - |
| fenchone | Particle board | - |
| a-terpineol | Particle board | - |
| Compound | Substrates | White Spirit | Gasoline | Lamp Oil | Frequency of Occurrences [31] | |
|---|---|---|---|---|---|---|
| IL | Substrate | |||||
| tetrahydrofuran | Curtains (1) | t | t | - | - | - |
| benzene | Floor skirting, carpet material, carpet underlay, living-room rug, vinyl flooring, curtains, plasterboard, computer fragments, clothing, electronics | t | x | - | - | - |
| 2-pentanone | Floor skirting, pine floor strips, carpet material, carpet underlay, living-room rug, plaster board, magazines, newspaper | t | x | - | - | - |
| heptane | Floor skirting, oak floor strips, carpet material, curtains (1), particle board, sofa, wooden chair, mattress, magazines | x | x | x | - | - |
| 2,4-dimethylfuran | Carpet underlay | x | - | - | - | - |
| toluene | Floor skirting, pine floor strips, laminate flooring, carpet material, carpet underlay, living-room rug, vinyl flooring, curtains (2), plaster board, particle board, sofa, wooden chair, mattress, magazines, newspaper, computer fragments, clothing, electronics | x | x | - | 1.81 × 10−1 | 2.3 × 10−1 |
| isobutyl acetate | Floor skirting, oak floor strips | x | - | - | - | - |
| 4-ethenyl cyclohexane | Carpet material, living-room rug, magazines, computer fragments, electronics | x | - | - | - | - |
| hexanal | Floor skirting, pine floor strips, oak floor strips, laminate flooring, living-room rug, vinyl flooring, plasterboard, particle board, sofa, wooden chair, magazines, newspaper | x | - | - | - | - |
| 2,4-dimethyl-heptene | Floor skirting, oak floor strips, laminate flooring, carpet material, carpet underlay, living-room rug, vinyl flooring, curtains, plasterboard, particle board, sofa, mattress, magazines, newspaper, computer fragments, clothing, electronics | x | - | - | - | - |
| ethylbenzene | Floor skirting, pine floor strips, oak floor strips, laminate flooring, carpet material, living-room rug, vinyl flooring, curtains (2), plasterboard, sofa, wooden chair, magazines, newspaper, computer fragments, electronics | x | x | - | 5.7 × 10−2 | 8 × 10−2 |
| p-xylene | Floor skirting, pine floor strips, oak floor strips, laminate flooring, carpet material, living-room rug, vinyl flooring, curtains (2), plasterboard, sofa, wooden chair, mattress, magazines, newspaper, electronics | x | x | - | 1.4 × 10−1 | 4 × 10−2 |
| o-xylene | Vinyl flooring, curtains (2), electronics | x | x | - | 6.2 × 10−2 | 2 × 10−2 |
| 2-heptanone | Floor skirting, pine floor strips, laminate flooring, plaster board, particle board, sofa, wooden chair, magazines, newspaper | x | - | - | 8 × 10−3 | 9 × 10−3 |
| heptanal | Floor skirting, pine floor strips, laminate flooring, plasterboard, particle board, sofa, magazines, newspaper | t | t | - | - | - |
| isopropylbenzene | Computer fragments, electronics | x | x | - | - | - |
| 2-butoxy-ethanol | Sofa | x | x | - | 4.9 × 10−2 | 5 × 10−2 |
| propylbenzene | Carpet material, magazines, electronics | x | x | - | - | - |
| 3-chloromethyl-heptane | Electronics | x | - | - | - | - |
| 1,2,4-trimethylbenzene | Floor skirting, pine floor strips, oak floor strips, carpet underlay | x | x | - | 1.84 × 10−1 | 9 × 10−3 |
| furfural | Pine floor strips, oak floor strips, laminate flooring, plasterboard, sofa, magazines, newspaper, Clothing | x | - | - | - | - |
| o/p-cymene | Particle board | x | t | - | - | - |
| limonene | Pine floor strips, plasterboard, particle board, sofa, mattress, clothing | x | - | - | 5.7 × 10−2 | 6 × 10−2 |
| dietyl phthalate | Mattress, clothing, electronics | t | t | t | - | - |
| diisobutyl phthalate | Sofa, mattress, clothing, electronics | t | t | t | - | - |
| n-octane | Particle board, wooden chair | x | x | x | - | - |
| n-nonane | Floor skirting, pine floor strips, carpet underlay, particle board, magazines, clothing, electronics | x | x | x | 2.49 × 10−1 | 9 × 10−3 |
| n-decane | Floor skirting, pine floor strips, oak floor strips, laminate flooring, vinyl flooring, curtains (1), plasterboard, sofa, mattress, magazines, newspaper, clothing, electronics | x | t | x | 3 × 10−2 | 2 × 10−2 |
| n-undecane | Floor skirting, pine floor strips, oak floor strips, laminate flooring, vinyl flooring, curtains, plasterboard, sofa, mattress, magazines, newspaper, clothing, electronics | x | t | x | 3.2 × 10−1 | 3 × 10−2 |
| n-dodecane | Floor skirting, oak floor strips, laminate flooring, vinyl flooring, curtains, plasterboard, sofa, mattress, magazines, newspaper, clothing, electronics | x | t | x | 2.36 × 10−1 | 2 × 10−2 |
| n-tridecane | Floor skirting, oak floor strips, laminate flooring, vinyl flooring, curtains, plasterboard, sofa, mattress, magazines, newspaper, clothing, electronics | x | t | x | 1.59 × 10−1 | 2 × 10−2 |
| n-tetradecane | Floor skirting, oak floor strips, laminate flooring, vinyl flooring, curtains, plasterboard, sofa, mattress, magazines, newspaper, clothing, electronics | x | t | x | 1.4 × 10−1 | 9 × 10−3 |
| Compounds | White Spirit | Gasoline | Lamp Oil |
|---|---|---|---|
| Alkanes | |||
| n-Alkanes | |||
| Pentadecane | - | m | x/m |
| Hexadecane | - | m | x/m |
| Heptadecane | - | - | x/m |
| Octadecane | - | - | x/m |
| nonadecane | - | - | x/m |
| icosane | - | - | x/m |
| Cyclo-alkanes | |||
| methylcyclohexane | x | x | x/m |
| ethylcyclohexane | - | x | x/m |
| butylcyclohexane | x | - | - |
| pentylcyclohexane | x | - | - |
| Aromatics | |||
| C3-alkylbenzenes | |||
| 3-ethyltoluene | x | x | - |
| 4-ethyltoluene | x | x | - |
| 1,3,5-trimethylbenzene | x | x | - |
| 2-ethyltoluene | x | x | - |
| 1,2,3-trimethylbenzene | x | x | - |
| C4-alkylbenzene1 | |||
| 1-methylpropylbenzene | x | m | - |
| 1,3-diethylbenzene | x | x/m | - |
| 1,4-diethylbenzene | x | x/m | - |
| 1-methyl-3-propylbenzene | x | x/m | - |
| 1-methyl-4-propylbenzene | x | x/m | - |
| 2-ethyl-1,4-dimethylbenzene | x | x/m | - |
| 1,2,3,4-tetramethylbenzene | x | x/m | - |
| 1,2,4,5-tetramethylbenzene | x | x/m | - |
| Naphthalenes | |||
| naphthalene | x | x | - |
| 1,3-dimethylnaphthalene | - | x | - |
| 2,3-dimethylnaphthalene | - | x | - |
| 1-methylnaphthalene | m | x/m | - |
| 2-methylnaphthalene | m | x/m | - |
| 1,2,3,4-tetrahydronaphthalene | x | - | - |
| Indane | |||
| indane | x | x | - |
| This Study | Analysis | Lopatka et al. | Analysis | ||||
|---|---|---|---|---|---|---|---|
| ILR (−) | ILR (+) | ILR (−) | ILR (+) | ||||
| TRUE | ILR (−) | 93% | 7% | TRUE | ILR (−) | 99% | 1% |
| ILR (+) | 11% | 89% | ILR (+) | 39% | 61% | ||
| Analysis | ||||||
|---|---|---|---|---|---|---|
| White Spirit | Gasoline | Lamp Oil | ILR (−) | Not Identified | ||
| TRUE | White Spirit | 100% | 0% | 0% | 0% | 0% |
| Gasoline | 0% | 79% | 0% | 0% | 21% | |
| Lamp oil | 0% | 15% | 77% | 0% | 8% | |
| ILR (−) | 0% | 0% | 5% | 93% | 2% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampat, A.A.S.; Van Daelen, B.; Lopatka, M.; Mol, H.; Van der Weg, G.; Vivó-Truyols, G.; Sjerps, M.; Schoenmakers, P.J.; Van Asten, A.C. Detection and Characterization of Ignitable Liquid Residues in Forensic Fire Debris Samples by Comprehensive Two-Dimensional Gas Chromatography. Separations 2018, 5, 43. https://doi.org/10.3390/separations5030043
Sampat AAS, Van Daelen B, Lopatka M, Mol H, Van der Weg G, Vivó-Truyols G, Sjerps M, Schoenmakers PJ, Van Asten AC. Detection and Characterization of Ignitable Liquid Residues in Forensic Fire Debris Samples by Comprehensive Two-Dimensional Gas Chromatography. Separations. 2018; 5(3):43. https://doi.org/10.3390/separations5030043
Chicago/Turabian StyleSampat, Andjoe A. S., Brenda Van Daelen, Martin Lopatka, Hans Mol, Guido Van der Weg, Gabriel Vivó-Truyols, Marjan Sjerps, Peter J. Schoenmakers, and Arian C. Van Asten. 2018. "Detection and Characterization of Ignitable Liquid Residues in Forensic Fire Debris Samples by Comprehensive Two-Dimensional Gas Chromatography" Separations 5, no. 3: 43. https://doi.org/10.3390/separations5030043





