Momilactones A and B: Optimization of Yields from Isolation and Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preaparation of Rice Husks and Extracts
2.2. Extraction and Isolation of MA and MB
2.3. Reagents and Analytical Instruments
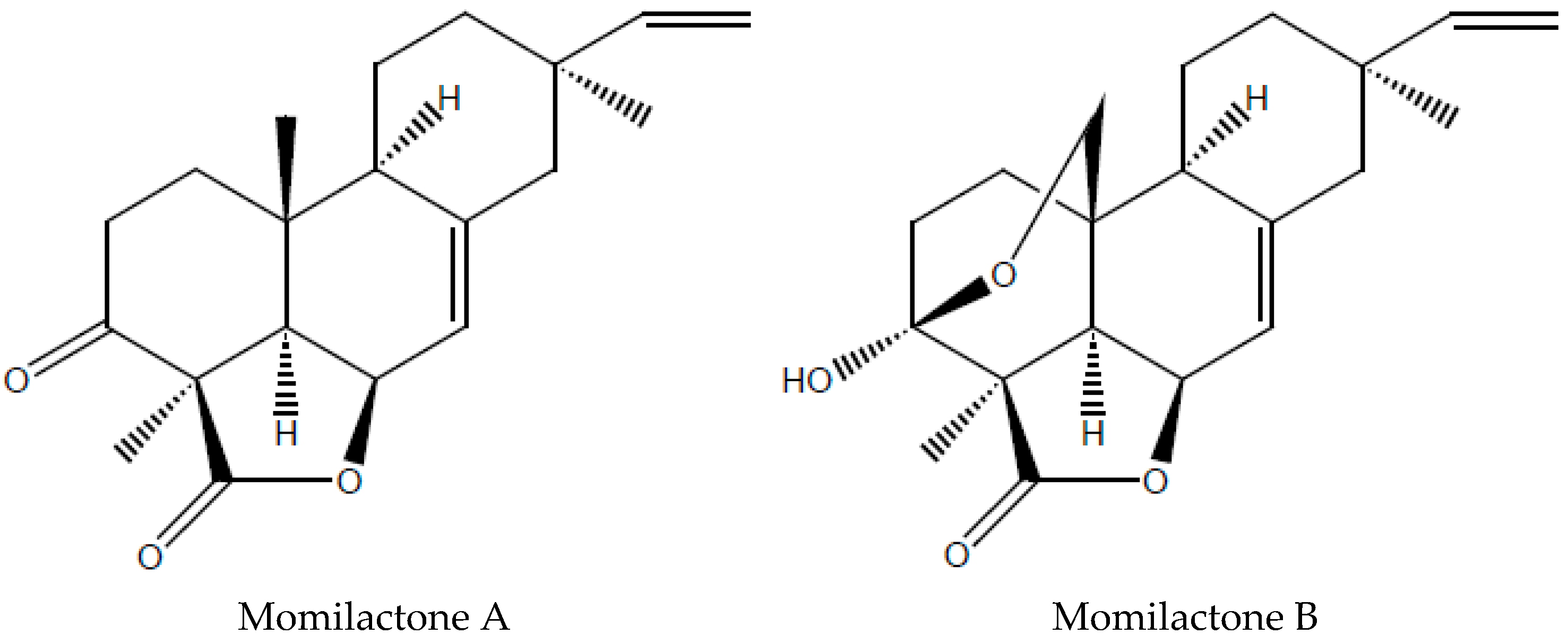
2.4. NMR Data of MA and MB
2.5. RP-HPLC and Separation Conditions
2.6. MA and MB Enrichment
2.7. Preparation of MA, MB, and Extracts for Quantification by HPLC
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanh, T.D.; Chung, M.I.; Xuan, T.D.; Tawata, S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agron. Crop Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E.; Terao, H.; Matstuo, M.; Khanh, T.D.; Murayama, S.; Hong, N.H. Alfalfa, rice by-products and their incorporation for weed control in rice. Weed Biol. Manag. 2003, 3, 137–144. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Min, C.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2015, 24, 197–206. [Google Scholar] [CrossRef]
- Lee, C.-W.; Yoneyama, K.; Takeuchi, Y.; Ryu, S.N. Quantification of momilactones A and B in rice straw. Korean J. Crop Sci. 2002, 47, 283–285. [Google Scholar]
- Kato-Noguchi, H.; Ino, T.; Kujime, H. The relation between growth inhibition and secretion level of momilactone B from rice root. J. Plant Interact. 2010, 5, 87–90. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bio-activities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- Kato-Noguchi, H.; Ino, T.; Ota, K. Secretion of momilactone A from rice roots to the rhizosphere. J. Plant Physiol. 2008, 165, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Hahn, S.J.; Ahmad, A. Evaluation of allelopathic potential and quantification of momilactone A, B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006, 54, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Toyomatsu, T.; Kagahara, T.; Odaka, K.; Koga, J.; Hasegawa, M.; Mitsuhashi, W.; Sassa, T.; Yamane, H. Diterpene phytoalexins are biosynthesized in and exudated from roots of rice seedlings. Biosci. Biotechnol. Biochem. 2008, 72, 563–567. [Google Scholar]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedon Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Kato, T.; Tsunakawa, M.; Sasaki, N.; Aizawa, H.; Fujita, K.; Kitahara, Y.; Takahashi, N. Growth and germination inhibitors in rice husk. Phytochemistry 1977, 16, 45–48. [Google Scholar] [CrossRef]
- Takahashi, N.; Kato, T.; Tsunagawa, M.; Sasaki, N.; Kitahara, Y. Mechanisms of dormancy in rice seeds. II. New growth inhibitors, momilactone-A and -B isolated from the hulls of rice seeds. Jpn. J. Breed. 1976, 26, 91–98. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Kodama, O.; Suzuki, T.; Miyakawa, J.; Akatsuka, T. Ultraviolet induced accumulation of phytolexins in rice leaves. Agric. Biol. Chem. 1988, 52, 2469–2473. [Google Scholar]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Khanh, T.D.; Chung, I.M. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilatones A and B. J. Plant Interact. 2007, 2, 245–251. [Google Scholar] [CrossRef]
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Noriji, H.; Yamane, H. Ecilitor induced activation of the methyerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the plant fungus. Mol. Plant Microbe Intract. 2010, 23, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, H.R.; Park, E.; Lee, S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007, 55, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.K.; Hatakeda, K.; Kato, T. A convenient method for analysis of momilactones. Jpn. J. Crop Sci. 1981, 50, 382–387. [Google Scholar] [CrossRef]
- Lee, C.W.; Yoneyama, K.; Takeuchi, Y.; Konnai, M.; Tamogami, S.; Kodama, O. Momilactones A and B in rice straw harvested at different growth stages. Biosci. Biotechnol. Biochem. 1999, 63, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, H.; Hayashi, K.; Nishimura, N.; Kawaide, H.; Matsuo, A.; Takaoka, D. Momilactone A and B as allelochemicals from moss Hypnum plumaeforme: First occurrence in bryophytes. Biosci. Biotechnol. Biochem. 2007, 71, 3127–3130. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Chung, I.M.; Hahn, S.J.; Ahmad, T. Confirmation of potential herbicidal agents in hull of rice Oryza sativa. J. Chem. Ecol. 2005, 31, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Peters, R.J. The role of momilactones in rice allelopathy. J. Chem. Ecol. 2013, 39, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kobayashi, K.; Shigemori, H. Allelopathy of the moss Hypnum plumaeforme by the production of momilactones A and B. Weed Res. 2009, 49, 621–627. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs. 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defense mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Guo, L.; Qiu, J.; Ye, C.; Jin, G.; Mao, L.; Zhang, H.; Yang, X.; Peng, Q.; Wang, Y.; Jia, L.; et al. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat. Commun. 2017, 8, 1031. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Liang, W.J.; Xu, X.H.; Hu, F. Release and activity of allelochemicals from allelopathic rice seedling. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Mottaleb, M.A.; Sarker, S.D. Accelerated solvent extraction for natural products isolation. Methods Mol. Biol. 2012, 864, 75–87. [Google Scholar] [PubMed]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H. Replacement of hazardous chemicals used in engineering plastics with safe and renewable-bond donor and acceptor solvent-pair mixtures. ACS Sustain. Chem. Eng. 2015, 3, 1881–1889. [Google Scholar] [CrossRef]
- Castro, R.O.; Contreras, H.A.C.; Rodriguez, L.M.; Bucio, J.L. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Germain, J.; Deslongchamps, P. Total synthesis of (±)-momilactone A. J. Org. Chem. 2002, 67, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
| Methods | Codes | EtOAc Crude Extract (g) | MA (µg/g DW) | MB (µg/g DW) |
|---|---|---|---|---|
| Controls (standards MA and MB by column chromatography) | M0 | 0.17 | 1.20 ± 0.05 j | 0.70 ± 0.03 h |
| MeOH 100% | M1 | 0.50 | 22.78 ± 0.19 ef | 42.80 ± 8.76 cdef |
| MeOH 70% | M2 | 0.46 | 38.35 ± 3.08 c | 67.81 ± 4.76 bc |
| MeOH 50% | M3 | 0.12 | nd | nd |
| MeOH 30% | M4 | 0.10 | nd | nd |
| MeOH 10% | M5 | 0.10 | nd | nd |
| Distilled water (room temperature) | M6 | 0.04 | nd | nd |
| Distilled water (100 °C) | M7 | 0.01 | nd | nd |
| Distilled water (100 °C, 30 min) + MeOH 100% | M8 | 0.67 | 18.54 ± 1.77 fg | 63.80 ± 5.33 cd |
| Distilled water (100 °C, 30 min) + EtOAc 100% | M9 | 0.50 | 21.27 ± 1.38 f | 49.63 ± 2.49 cde |
| Distilled water (100 °C, 1 h) + MeOH 100% | M10 | 0.45 | 28.83 ± 0.03 d | 3.02 ± 0.02 g |
| Distilled water (100 °C, 2 h) + MeOH 100% | M11 | 0.50 | 51.54 ± 0.95 b | 102.23 ± 5.32 ab |
| Distilled water (100 °C, 3 h) + MeOH 100% | M12 | 0.50 | 21.78 ± 0.79 ef | 45.65 ± 2.62 cdef |
| Distilled water (100 °C, 4 h) + MeOH 100% | M13 | 0.50 | 14.72 ± 0.19 gh | 30.65 ± 1.38 ef |
| Dried (100 °C, 1 h) + MeOH 100% | M14 | 0.45 | 58.76 ± 3.75 a | 104.43 ± 6.44 a |
| Dried (100 °C, 1 h) + MeOH 70% | M15 | 0.40 | 13.89 ± 0.62 gh | 29.68 ± 1.89 ef |
| Dried (100 °C, 1 h) + MeOH 50% | M16 | 0.17 | nd | nd |
| Dried (100 °C, 1 h) + MeOH 30% | M17 | 0.06 | nd | nd |
| Dried (100 °C, 1 h) + MeOH 10% | M18 | 0.20 | nd | nd |
| Dried (100 °C, 1 h) + distilled water (room temperature) | M19 | 0.16 | nd | nd |
| Dried (100 °C, 1 h) + distilled water (100 °C) | M20 | 0.62 | nd | nd |
| Dried (100 °C, 1 h), distilled water (100 °C, 1 h) + MeOH 100% | M21 | 0.31 | 15.03 ± 0.51 g | 14.04 ± 0.33 f |
| Dried (100 °C, 2 h), distilled water (100 °C, 2 h) + MeOH 100% | M22 | 0.50 | 11.76 ± 0.64 hi | 35.19 ± 2.43 def |
| Dried (100 °C, 3 h), distilled water (100 °C, 3 h) + MeOH 100% | M23 | 0.60 | 28.56 ± 0.09 d | 53.03 ± 2.45 cde |
| Dried (100 °C, 4 h), distilled water (100 °C, 4 h) + MeOH 100% | M24 | 0.40 | 6.68 ± 0.34 i | 20.26 ± 1.51 f |
| Distilled water (100 °C, 120 kPa) + MeOH 100% | M25 | 0.40 | 26.26 ± 1.44 de | 40.78 ± 2.82 cdef |
| Dried (100 °C, 120 kPa) + MeOH 100% | M26 | 0.50 | 17.90 ± 0.18 fg | 71.00 ± 6.14 bc |
| MA (µg/g DW) | MB (µg/g DW) | Materials | Instruments | Extraction Protocols | References |
|---|---|---|---|---|---|
| 15.0 | 10.0 | Rice husks | CC | Hexane:EtOAc (8:2) | [23] |
| 4.6 | 3.1 | Rice husks | CC | Hexane:EtOAc (8:2) | [15] |
| 4.5 | 3.0 | Rice straw | LC-MS-MS | EtOAc | [20] |
| 4.9 | 2.9 | Rice husks | GC-MS | EtOAc:H2O (1:1) | [8] |
| 140.0 | 95.0 | Whole rice plants | HPLC | EtOAc | [6] |
| 69.9–140.0 | 64.4–114.1 | Seedling * | GC-MS | EtOAc | [22] |
| 0.8 | 0.5 | Rice husks | CC | CHCl2 + EtOH | [10] |
| nd | 245.0 | Rice shoots | HPLC | MeOH | [28] |
| nd | 64.1 | Rice roots | HPLC | MeOH | [28] |
| 80.6 | nd | Rice seeding | HPLC | MeOH: H2O (8:2) | [30] |
| 1.0 | 0.8 | Rice husks | CC | Benzene:EtOAc (10:1) | [12] |
| 87.0 | 9.6 | UV-irradiated rice leaves | GC-MS-SIM | MeOH | [14] |
| 34.7 | 37.8 | Rice husks | GC-MS | EtOAc:H2O (1:1) | [8] |
| 58.7 | 23.4 | Hypnum plumaeforme L. | HPLC | EtOAc | [26] |
| 8.4 | 4.2 | Hypnum plumaeforme L. | TLC | EtOAc | [21] |
| 1.2 | 0.7 | Rice husks | CC | Hexane:EtOAc (8:2) | This study; For isolating standard MA and MB |
| 11.8–58.8 | 3.0–104.4 | Rice husks ** | HPLC | EtOAc; MeOH; Temperature; Pressure | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoc Minh, T.; Xuan, T.D.; Ahmad, A.; Abdelghany Elzaawely, A.; Teschke, R.; Van, T.M. Momilactones A and B: Optimization of Yields from Isolation and Purification. Separations 2018, 5, 28. https://doi.org/10.3390/separations5020028
Ngoc Minh T, Xuan TD, Ahmad A, Abdelghany Elzaawely A, Teschke R, Van TM. Momilactones A and B: Optimization of Yields from Isolation and Purification. Separations. 2018; 5(2):28. https://doi.org/10.3390/separations5020028
Chicago/Turabian StyleNgoc Minh, Truong, Tran Dang Xuan, Ateeque Ahmad, Abdelnaser Abdelghany Elzaawely, Rolf Teschke, and Truong Mai Van. 2018. "Momilactones A and B: Optimization of Yields from Isolation and Purification" Separations 5, no. 2: 28. https://doi.org/10.3390/separations5020028







