1. Introduction
The concept of “green solvents” pertains to the wider area of “green chemistry”, for which several principles have been established. These include waste prevention, safety—no toxic or hazardous materials should be used or produced—maximization of energy efficiency, and minimization of the potential for accidents—explosion, fire, and pollution possibilities must be kept in mind. In view of these principles, the chemical community is proceeding in recent years towards sustainable industrial processes. Water is the “greenest solvent” imaginable: it is readily available at the required purity, it is cheap, it is readily recycled, non-toxic, non-flammable, and environmentally friendly. In its supercritical form, water is also considered as a “green solvent” [
1]. Supercritical ethanol has also been mentioned as a “green solvent” and even supercritical methanol (although much less often). Therefore, the use of such fluids, also in their subcritical forms, is nowadays considered to be at the forefront of efforts to apply neoteric solvents to chemical processes. On the other hand, supercritical water in the presence of an oxidant is used for total oxidation of hazardous substances [
1], and this aspect of its use should be kept in mind when its use for the extraction of useful products is considered.
Extraction by subcritical and supercritical water, methanol, and ethanol and their mixtures has been applied to many materials for the recovery of useful products from them. The treatment of coals and oil shales with these solvents, after possible desulfurization and denitrification, may yield liquid fuels of better environmental impact. Many plant and some animal biomass materials are sources of bio-active products, such as anti-oxidants, biofuels, and bio-oils, when subjected to extraction with these solvents at the named conditions. Some of the biomass is conventionally extracted by soxhlet devices, but accelerated solvent extraction (ASE) is faster and economically superior; the term ASE means the use of pressurized liquid extraction (PLE) or supercritical fluid extraction (SFE). However, SFE is generally understood as extraction with supercritical carbon dioxide (scCO2), possibly modified by co-solvents, but extraction with this particular method is outside the scope of this review, which is limited to neat supercritical water, methanol, ethanol, or their mixtures.
In order to be able to understand and follow the many publications that deal with the extraction by subcritical and supercritical water, methanol, ethanol and their mixtures and apply them in the future it is necessary to know their physical and chemical properties. These are summarized in
Section 2 of this review and in several tables. Water, methanol, and ethanol at ambient conditions have highly hydrogen-bonded structures, which are destroyed to an appreciable extent as the temperature is increased. This phenomenon reduces their dielectric permittivities and makes them less polar and tight, and facilitates the dissolution of non-polar substances in them. However, the increased temperatures, at liquid-like densities, do not detract from the dipolarity and hydrogen bond donation and acceptance abilities of these solvents so that they also dissolve and solvate polar solutes.
An extensive body of publications deals with the application of subcritical and supercritical water, methanol, and ethanol and their mixtures to the production of liquid and gaseous fuels from coals and oil sands. These are reviewed in
Section 3, with the understanding that the reports dealt with are illustrative rather than comprehensive regarding this topic. Extraction with the title solvents of biomass of various origins has also been extensively dealt with in the literature. Pressurized liquid extraction with hot-but-subcritical water, methanol, and ethanol and their mixtures is dealt with in
Section 4. The extraction of phenolic bio-active compounds, useful as antioxidants, as well as other kinds of useful products, is reviewed in this section, again with the restriction that the reports are merely illustrative. Extraction with the supercritical solvents of cellulosic and lignin biomass is reviewed in
Section 5, where their application to algae and other plant materials is also dealt with. Finally, in
Section 6, some methods, such as ultrasound- and microwave-assisted extraction combined with the pressurized liquid extraction are shown, leading to optimization of the extraction processes.
2. Properties of Subcritical and Supercritical Water, Methanol, and Ethanol and Their Mixtures
The critical properties: critical temperature,
Tc, critical pressure,
Pc, critical density,
ρc, and critical molar volume,
Vc =
M/
ρc (
M is the molar mass) of the three pure fluids water, methanol, and ethanol are presented in
Table 1. The critical compressibility factor,
Zc =
PcVc/
RTc, is also shown there, being nearly the same for these three fluids.
The properties of hot, subcritical water, methanol, and ethanol along the saturation line are shown in
Table 2,
Table 3 and
Table 4, respectively [
1,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. These properties include the vapor pressures
p, the molar enthalpies of vaporization Δ
VH, the density
ρ, the isobaric expansibility
αP, the isothermal compressibility
κT, the internal pressure
Pint =
TαP/
κT −
p, the isobaric molar heat capacity
Cp, and the static permittivity
ε. In the case of water (
Table 2) data on the surface tension, the dynamic viscosity
η, and the specific electrical conductivity
κ could also be found and are included. The surface tension,
σ, of methanol and ethanol is available only for the liquids below their normal boiling points, diminishing linearly with the temperature. Up to 333.15 K the values are
σ/mN m
−1 = 19.41 − 0.0774 (
T/K) for methanol and 19.06 − 0.0832 (
T/K) for ethanol [
14].
For water and ethanol at elevated temperatures the solvatochromic indices, indicating the polarity/polarizability (Kamlet-Taft
π*), hydrogen bond donation ability (Kamlet-Taft
α) and hydrogen bond acceptance ability (Kamlet-Taft
β) are shown in
Table 2 and
Table 4. No such data were found for methanol.
It should be noted that the polarities and permittivities of the solvents considered here diminish with increasing temperatures, and do so drastically as the critical point is approached. Supercritical water, for instance, is capable of dissolving hydrocarbons such as hexane. Such behavior is connected with the destruction of the three-dimensional hydrogen bond structure of water and the chain-like and ring hydrogen bonded structures of the alkanols [
1,
10,
13].
The density of supercritical water is shown in
Table 5 at several pressures (
Pr =
P/Pc) and temperatures and the densities of supercritical methanol and ethanol at similar reduced pressures and temperatures are shown in
Table 6.
The critical data (temperatures, pressures, densities, and molar volumes) of mixtures of water with the co-solvents methanol and ethanol are shown in
Table 7 as functions of their mole fractions
x. The densities,
ρ/kg m
−3 of supercritical aqueous mixtures of methanol and ethanol at specified mole fractions of the alkanol, temperatures, and pressures are shown in
Table 8. The values were interpolated from detailed
T-P-ρ-x tables of data and are deemed accurate to ±2%.
3. Supercritical Extraction Applied to Fuels
One of the aspects of the application of supercritical fluids to the extraction of coals is their desulfurization, involving the removal of both inorganic and organic sulfur compounds. As an example, Ohio coals contain 3.3 wt % total sulfur, of which some 60% are organic. When extracted with supercritical methanol, aqueous methanol, or water (at 560–712 K and 10.5–28.6 MPa) some 20% to 40% of the sulfur is removed. Water is most effective for the removal of pyritic sulfur, whereas methanol is most effective for the removal of organic sulfur [
18]. Illinois coals may contain less sulfur, 1.4 wt %, of which up to 60% could be removed by supercritical methanol at 673 K [
19]. Both supercritical aqueous methanol and aqueous ethanol were applied to Midwestern bituminous coals, where again the removal of the inorganic (pyritic and sulfate) forms of sulfur was more efficient than that of the organic sulfur [
20]. Chinese coals are richer in sulfur, and a high-ranking coal with 4.0 wt % sulfur was treated with supercritical ethanol (which may contain a small amount of water) at 673 K and 12 MPa. Some 30% of the sulfur could be removed, most effectively with 95% ethanol [
21]. Supercritical methanol at 673 to 723 K removed about 45% of the organic sulfur from Canadian coal [
22]. Desulfurization of low-ranking coal from Thailand appears to require the addition of potassium hydroxide to the supercritical ethanol. Up to 48% of the sulfur could be removed with 5 g dm
−3 of KOH at 623 K and 8.3 MPa of supercritical ethanol [
23]. Desulfurization of coal with supercritical methanol or ethanol leads to partial transformation of the pyrite to pyrrhotite and to eventual coke formation. For a given level of sulfur removal, methanol produces a lower coke yield than ethanol [
24]. In analogy with desulfurization may be considered the removal of oxygen from sub-bituminous coals on supercritical water extraction. When the temperature was increased from 633 to 673 K the oxygen removal increased from 53% to 71% [
25].
Another aspect of the application of supercritical fluids to coal is the winning of liquid fuels from it. The conversion of South African coals to liquid and gaseous products by supercritical fluid extraction was studied with 18 fluids, among which were water, methanol, and ethanol. At 723 K and 20 MPa extraction conditions these supercritical fluids resulted in 18.4%, 18.9%, and 25.5% conversion, rather less than obtained with some other supercritical fluids (tetralin,
m-cresol, aniline, and cyclohexanone) at the same conditions [
26]. Illinois No. 6 bituminous coal was subjected to supercritical fluid extraction at 673 K with water (at 21.2–24.7 MPa), methanol (at 8.6 MPa), and ethanol (at 11.9 MPa), among other solvents, but in this study the conversion yield with the water (34% and 39%) was larger than with methanol (8.4%) and ethanol (17.5%) [
27]. Supercritical ethanol at 673 K and 20.5 MPa yielded 35.7% conversion to liquid products, but only 78.6% ethanol recovery, which was increased to 84.3 recovery on lowering the pressure at 673 K to 12.7 MPa, but at the expense of a much lower conversion to liquid products, 22.2% [
28]. Brazilian coals were extracted by supercritical methanol and ethanol (also propanols, butanols, and pentanols) with recovery of >40% of liquid and gaseous products [
29]. High-volatile bituminous coal from the Saar region in Germany was subjected to supercritical extraction with methanol and ethanol and the dependence of the extraction yields on the conditions employed was studied [
30]. Both pure ethanol and its aqueous mixtures (also isopropanol) were applied as supercritical fluids at 598–698 K and ≤12.5 MPa to high-ash Brazilian coal, with increased temperatures and pressures increasing the conversion yield [
31].
Supercritical water was found to act as both an extracting solvent as well as a reactant, converting coal to liquid and gaseous products [
32]. Supercritical water at 633–673 K applied to sub-bituminous coals resulted in 36% to 44% total conversion [
25]. Supercritical water at 673–698 K and 14–24.5 MPa was applied to Western Canadian lignite and high-volatile bituminous coals, with 50% and 35% maximal conversion (on a dry, ash-free basis) to liquid and gaseous products [
33]. Supercritical, but also subcritical, water was applied to lignite coal for the extraction of liquid products, applied to Turkish [
34] and Dayan lignite coals [
35]. Their yield increased with increasing pressure and temperatures of 673 to 723 K were effective, but with increasing temperatures mainly gas and light oil production was enhanced. A similar study with sub- and supercritical water was conducted on hard coals at 573 to 673 K. Admixture in a 1:1 mass ratio of methanol and more so ethanol to the water increased the yield of liquid products significantly (at 623 K) [
36]. Sub- and supercritical water were also applied to some Chinese coals, the maximal extraction rate occurred near 670 K and the gas (CO, H
2, CH
4) and extract yields increased with the pressure [
37]. Higher temperatures 673 to 1033 K, and pressures, 30 MPa, were used for the supercritical extraction of brown coal from Russia, with conversion of 48% to 63% to liquid and gaseous products [
38]. Russian brown coal was treated with supercritical water at 673 to 873 K for the production of combustible volatile products in a continuously supplied reactor [
39,
40]. Bitumen has been subjected to sub- and supercritical water extraction for the production of gaseous fuel, mainly hydrogen and carbon monoxide at supercritical conditions [
41]. The catalytic gasification of bituminous Chinese coal by supercritical water in the presence of potassium carbonate catalyst was studied in [
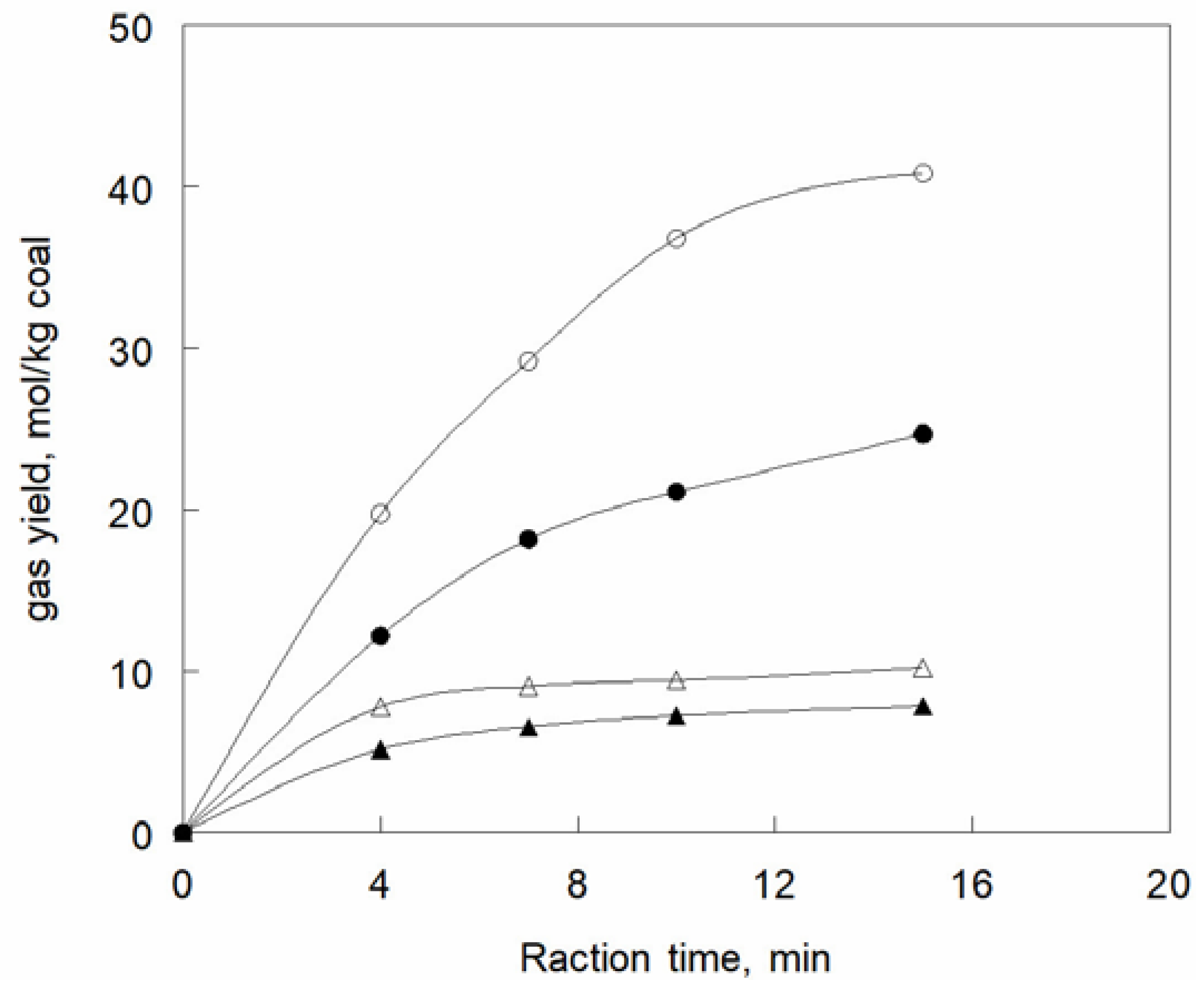
42] and the rates of production of hydrogen and methane are shown in
Figure 1. The methane yield reaches a plateau at an early stage, but that of hydrogen increases with time and is more sensitive to the temperature. The co-liquefaction of coal and
Dunaliella tertiolecta algae for the production of liquid fuel by 60 vol % ethanol in water mixtures at 633 K (supercritical for the mixture) was studied in [
43].
Several studies dealt with some aspects of the application of the supercritical fluids considered here to coals that were not the winning of liquid fuels per se. The interaction of two model compounds, quinoline and isoquinoline with supercritical water was examined. It was found that these compounds were more reactive in the presence of supercritical water than when undergoing inert pyrolysis. Some other compounds (benzonitrile, aniline, tetralin, dihydroanthracene, and ethylbenzene) were studied less intensively with regard to their interaction with supercritical water, and alkyl side chains on aromatics were found to be somewhat reactive and that carbon atoms reacted with the water forming hydrogen [
44]. Alkylation of C atoms, but also of O, S, and N atoms, was found spectroscopically to occur on extraction of Spanish lignite coal with supercritical methanol and ethanol at 523 to 623 K and up to 15.2 MPa [
45]. Coal char is constituted mainly by polycyclic aromatic hydrocarbons (PAH), of which anthracene is the model compound. Its conversion to hydrogen by supercritical water at 873 to 1023 K was studied in [
46].
Another kind of material treated by supercritical fluids in order to obtain liquid (eventually also gaseous) fuels is oil shales. Extraction of Green River oil shale with supercritical water, methanol, and their mixtures at 473 K showed that part of the methanol was consumed by yielding esterification products, but it produced liquid organic materials beyond these [
47]. Use of a 50 vol % mixture of water and methanol yielded 90% of the organic material in the shale on extraction at 673 K for 34 min [
48]. Supercritical water was applied to Chinese Maoming oil shales at 673 K with 45% oil recovery when heated at this temperature for 1 h [
49]. Extraction of Chinese (Huadian) oil shales with water for 30 min at 633 K (subcritical) to 723 K showed a maximal yield of 81.2% at 673 K, of which one half was oil and the rest asphaltene and pre-asphaltene [
50]. Supercritical water was applied at a lower temperature, 648 K, to extract oil from Turkish oil shale, with 75% yield after 1 h treatment [
51]. Turkish (Beypazari) oil shales were extracted with water at 623 (subcritical) to 698 K, with the asphaltene fraction diminishing but the polar fraction of the produced oil increasing in this temperature range [
52]. Supercritical water applied to Moroccan oil shales at 653 K to 673 K yielded oils with increasing yields and diminishing fractions of asphaltenes [
53]. Jordanian (El-Lajjun) oil shales were subjected to retorting and supercritical extraction with CO
2 and with water. Supercritical water at 712 K and 24.9 MPa produced the highest oil yield, nearly 47% [
54]. Russian oil shales (Bazhenov formation) were treated with water at temperatures from 573 K (subcritical) to 753 K (supercritical) and liquid products were recovered at 673 K and 30 MPa, whereas at the highest temperature secondary hydrocarbon formation was observed [
55]. Supercritical water extraction was applied to Russian (Krassava) and Bulgarian (Syssola) oil shales at 623 K, and admixtures of methanol and ethanol at 50 wt % to the water enhanced the oil recovery from 18% to 37% and 44% for the Russian shale, but less (from 13% to 18% and 35%) for the Bulgarian shale [
36]. Spanish oil shales (from Puertollano) were treated by supercritical methanol at 553 to 623 K and the kerogen produced was mainly constituted by aliphatic hydrocarbon, contrary to the nature of the kerogen produced by supercritical toluene, which was mostly aromatic [
37].
All these reports are summarized in
Table 9.
4. Pressurized Subcritical Liquid Extraction from Bio-Materials
Pressurized liquid extraction (PLE) applies subcritical hot solvents at elevated pressures for the efficient extraction of useful products from bio-materials. This technique has also been called accelerated solvent extraction (ASE) [
56]. Bio-active products are apt to decompose at very high temperatures, hence supercritical extraction may not be applicable to them. The application of near- and supercritical water to biorefineries has been summarized in a book [
57]. Pressurized liquid extraction is preferable to extraction with supercritical water, methanol, ethanol and their mixtures when materials are to be recovered that are sensitive to the higher temperatures needed for the supercritical conditions.
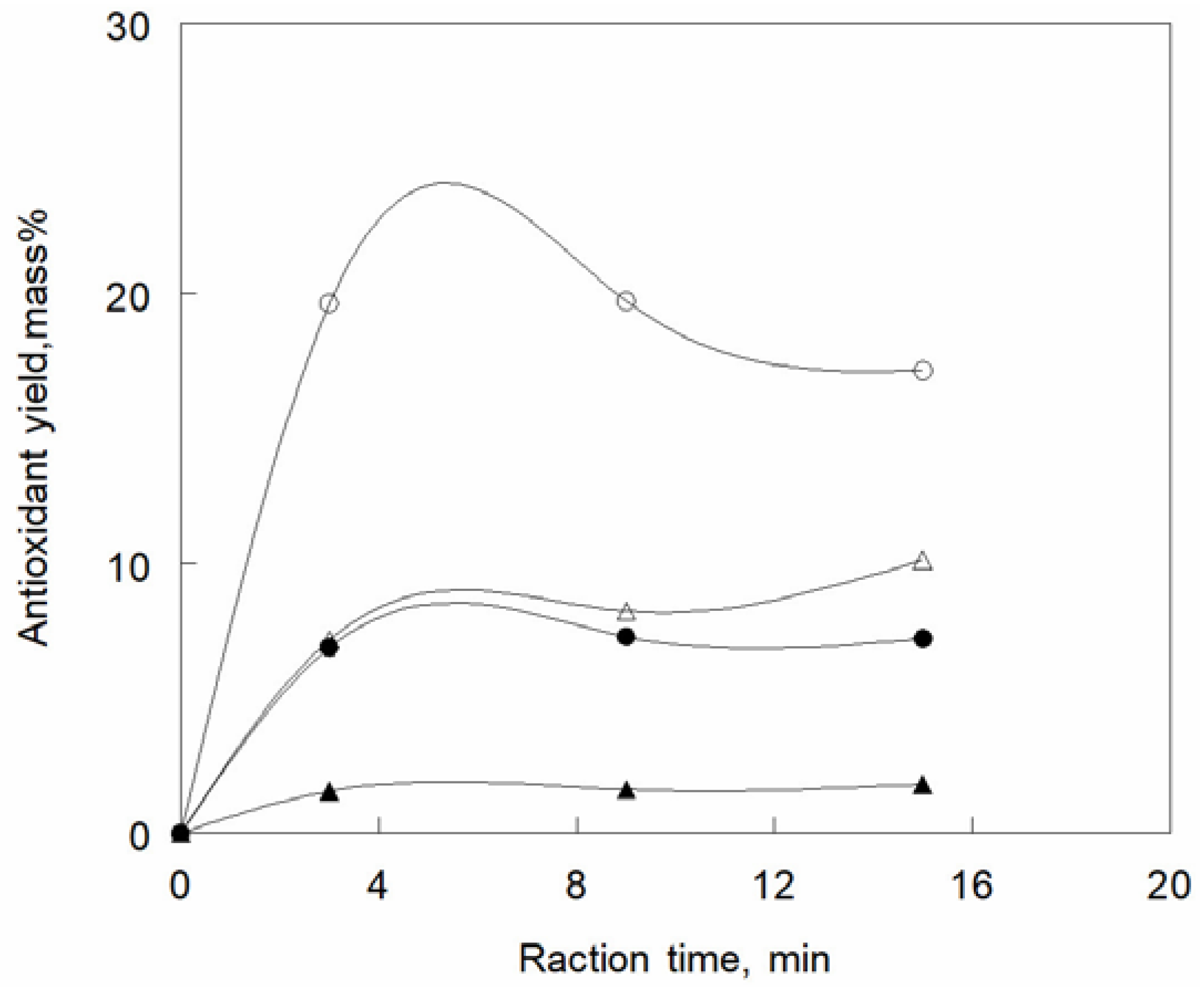
A class of products that has been obtained by the PLE technique is anti-oxidants, such as carotenoids and polyphenols, and a fairly large set of studies has been devoted to this topic in recent years. Microalgae are a source of carotenoid antioxidants. Pressurized water and ethanol at 383 and 443 K were applied to
Spirulina platensis microalgae to extract antioxidants, the activity of which was immediately ascertained [
58], ethanol being the optimal solvent [
59]. The rate of the extraction is shown in
Figure 2 [
60]. Pressurized water and ethanol were applied at 313, 373 and 433 K to
Dunaliella salina microalgae to extract antioxidants, the best results being obtained with ethanol at 433 K [
61]. Pressurized ethanol has again been applied to a microalga,
Haematococcus fluvialis, at temperatures of 323, 373, 423, and 473 K, and although an increase in the temperature enhances the yield, this was deleterious to the antioxidant activity [
62].
Pressurized water, methanol and ethanol were applied at 373 to 473 K for the extraction of the antioxidants catechin and epicatechin from tea leaves and grape seeds. Methanol was most efficient and temperatures <403 K led to ≥95% yields [
63]. Polyphenolic antioxidants were obtained by the application of pressurized water and ethanol to olive leaves. Water at 473 K and ethanol at 423 K were most effective [
64]. A pressurized 50 mass% ethanol + water mixture at 473 K produced the best yield of phenolic antioxidants from black bamboo leaves [
65]. Pressurized 80 mass% ethanol in water at 433 K was effective for the extraction of antioxidants from bitter gourd fruits (
Momordica charantia). Pressurized water and ethanol were used to extract phenolic antioxidants from spent coffee grounds and a temperature pf 468 K was found to be most useful [
66].
Red grape skins appear to be a good source for phenolic and flavonoid antioxidants. Pressurized methanol at 423 K was effective for the extraction of phenolic antioxidants from grape skins, but flavonoids tended to degrade after prolonged treatment [
67]. On the other hand, pressurized water at 383 to 433 K was effective for the extraction of anthocyanin antioxidants from red grape skins, with the yield increasing with the temperature in this range, but the anti-oxidative effect decreased [
68]. Temperatures of up to 393 K were most effective for pressurized water extraction from grape skins, and this solvent was more efficient than pressurized methanol or ethanol [
69]. A 50 mass% ethanol + water mixture, pressurized to 9 MPa at 393 K, was the most efficient for the extraction of phenolic antioxidants from red grape pomace, compare with neat water or ethanol in these conditions [
70]. Pressurized ethanol at 5 to 15 MPa and 313 to 393 K was effective for the extraction of anthocyanin antioxidants from Brazilian jabuticaba skins [
71].
Further studies of the pressurized liquid extraction of phenolic compounds from bio-materials, without stressing their antioxidant properties have also been reported. PLE was applied to methanol, ethanol, and their mixtures with water for the extraction of phenolic compounds from parsley (
Petroselinum crispum) flakes, and 50 vol % ethanol+water appeared to be the most effective [
72]. A 60 vol % methanol+water mixture at 363 K was best for PLE of phenolic acids from black cohosh (
Cimicifuga racemose) [
73]. Water and 70 mass% ethanol in water mixtures were used for PLE at 323 to 463 K of spinach to yield flavonoids, the mixture being more effective than neat water [
74].
Phenolic bio-active compounds were extracted from virgin olive oil filter cake by pressurized mixtures of water and ethanol, the best yield was with 50 vol % mixtures at 393 K [
75]. Pressurized water and ethanol were also used to extract phenolic compounds from olive leaves, and neat ethanol at 463 K proved to be optimal on-the-whole, but a 43:53 mixture at this temperature increased the yield of oleorupein [
76]. Pressurized water and ethanol mixtures were also used for extraction from defatted sesame seeds, in this case a 63.5% ethanol mixture at 493 K led to maximal yields of flavones and other phenolic compounds with radical scavenging properties [
77]. Brazilian pepper fruits were submitted to PLE with slightly acidified 54.2% aqueous ethanol at 348 K to yield the maximal amount of phenolic compounds [
78]. Phenolic compounds and anthocyanines were extracted from juçara (
Euterpe edulis Mrt.) residues using PLE with water, ethanol, their mixtures, and their acidified liquids. The highest yield of phenolic compounds was obtained with the acidified mixtures at 353 K [
79].
Non-phenolic compounds were also obtained from natural materials by pressurized liquid extraction (PLE). Ketones of polycyclic aromatic hydrocarbons were obtained from contaminated soil by PLE with ethanol at 373 K and 15 MPa. Precipitates formed after cooling the extract, so that dichloromethane was added to prevent this [
80]. Polar and non-polar lipids were obtained by PLE from corn and oats with several solvents including ethanol. Only for the polar compounds were extractions with ethanol at 373 K more effective than with the other solvents (hexane, methylene chloride, and isopropanol) [
81]. Capsaicinoids were extracted from peppers with pressurized methanol and ethanol containing up to 20% water at 323 to 473 K. The presence of the water had relatively little effect on the yields of the different capsaicinoids, whereas increasing temperatures increased the yields [
82]. Charantin was extracted from
Momordica charantia fruits by PLE with several solvents, including water and ethanol at 323 to 423 K and 10 MPa. Ethanol (also acetone) was found to be more effective than dichloromethane and water for this extraction [
83]. A mixture of lactose and lactulose was separated (to obtain the lactulose component) by PLE with mixtures of water and ethanol at 313 to 403 K and 10 MPa. Optimal extractions were obtained at the lowest temperature with 70% ethanol [
84]. Pressurized hot water at 373 and 393 K was used for the extraction of thermally labile bioactive compounds (gastrodin and vanillyl alcohol) from
Gastrodia elata Blume, with results similar to those with reflux, but at a somewhat shorter time [
85]. Pressurized extraction with at 403 K with 86% ethanol with water was found to be optimal for recovery of spicatoside A from
Liriope platyphylia [
86]. The active constituents from ginger,
Zingiber cassumunar Roxh rhizome, were recovered by PLE with methanol, ethanol, or water at 373 K for the alkanols and at 413 K for the water [
87]. Another kind of ginger,
Zingiber officinale Roscoe, was submitted to PLE with bio-ethanol or 70% ethanol in water at 373 K and 10 MPa with high efficiency [
88]. Fucoxanthin was extracted most effectively from brown algae,
Eisenia bicyclis (Kjellman), by pressurized 90% aqueous ethanol at 383 K [
89]. A green microalga,
Chlorella ellipsoidea, was subjected to PLE with ethanol at 389 K for the recovery zeaxanthin, this being more efficient than PLE with hexane or isopropanol [
90]. Constituents, such as 1,8-cineol and
α-terpinenyl, were effectively recovered from cardamom seeds by PLE with 75% aqueous ethanol at 363 K [
91]. Olive pomace was the raw material for the recovery of sugars and lignin, the former of which being eventually converted to bio-ethanol, by PLE with water at 453 to 493 K [
92].
Pressurized 88% aqueous ethanol at 513 K was more effective than at lower temperatures, down to 453 K, and more than pressurized water at 453 K for the PLE of carbohydrates from barley hulls, but phenolic compounds were best recovered by pressurized 59% aqueous
N-methyl-2-hydroxyethyl-ammonium acetate at 433 K and 15 MPa [
93]. Proteins and amino acids were extracted from porcine placenta by pressurized water at 443 K and 1MPa. The addition of ethanol to the extracting fluid was deleterious [
94].
All these reports are summarized in
Table 10.
5. Supercritical Fluid Extraction from Bio-Materials
An extensive amount of work has been reported regarding the extraction with supercritical water, methanol, and ethanol, but the term “supercritical fluid extraction” generally pertains to extraction with supercritical carbon dioxide, possibly with modifiers, such as methanol and ethanol. This latter field of study is outside the scope of the present paper.
Cellulosic materials, including lignin, have been subjected to extraction with supercritical water, methanol, and ethanol. Japanese sugi wood (
Cryptomeria japonica) was treated with supercritical water and various products could be identified in the water-soluble, methanol-soluble, and methanol-insoluble fractions [
95]. Japanese beech (
Fagus crenata Blume) was treated with supercritical methanol containing some water, and 10 vol % water at 623 K was found to be optimal for obtaining products from the lignocelluloses [
96]. Japanese beech as well as oil palm trunks were treated with supercritical water at 653 K and 100 MPa to obtain low molecular size products [
97]. German beech wood was treated with supercritical ethanol, optimally at 589 K, to produce bio-oil [
98]. Holm oak was treated by water at 453 to 623 K to hydrolyze hemicellulose, and then supercritical water at 669 K at 24.5 MPa was used for extraction of low molecular mass products, e.g., lactic acid [
99].
Non-wood cellulosic and lignin materials have also been subjected to supercritical fluid extraction, for example bagasse of sugar cane stalks, treated by supercritical ethanol at 603 K to yield bio-oil [
100]. Supercritical water can deal directly with wet biomass (olive husk, cotton cocoon shell, and tea waste), without need for drying, to produce hydrogen and methane beside carbon oxides. The yields increase with temperature in the range 650 to 700 K and with pressure in the range 23 to 38 MPa [
101]. Two recent reviews [
102,
103] dealt with the hydrolysis, fractionation, and extraction of biomass containing cellulose, hemicellulose, and lignin by sub- and supercritical water at 623 and 673 K. The yields of phenolics, for example, increased with increasing density (pressure) and temperature of the water. Lignin has been effectively converted to biofuel by treatment with supercritical methanol catalyzed by the layered (hydrotalcite-like) copper-doped magnesium-aluminium porous metal oxide [
104].
Algae are a source of useful products when treated with supercritical fluids, e.g., supercritical methanol at 528 K that yields biodiesel from wet algae [
105]. Direct transesterification of algal biomass under supercritical methanol at 533 K and microwave irradiation was compared [
106]. Supercritical ethanol yields ethyl esters by transesterification when wet algae are treated by it at 533 K and microwave irradiation [
107]. Supercritical water at 24 MPa and 936 K was used for the gasification of
Nannochloropsis gaditana microalgae, producing mainly hydrogen, methane, and carbon dioxide [
108]. The same microalgae as wet biomass were subjected to hydrothermal liquefaction with subcritical water at 623 K and subsequent extraction with supercritical waterat 673 K in the presence of hydrogen gas to produce water-soluble biocrudes [
109].
Other plant materials were also treated by supercritical water, methanol, or ethanol in order to recover valuable products. Supercritical water at 650 to 750 K and 23 to 48 MPa was used to produce hydrogen-rich gas from almond, hazelnut, walnut, sunflower shells and cotton cocoon as wet biomass [
110]. Bullrush plants,
Typha latifolia, were subjected to supercritical methanol and ethanol (also acetone and 2-butanol) for supercritical fluid extraction and liquefaction at 518 to 558 K [
111]. Stinging nettle was subjected to supercritical ethanol extraction at 608 K, yielding various esters, phenols, indoles and nitrogen-containing compounds [
112]. Another kind of thistle stalks,
Onopordum heteracanthum, was subjected to liquefaction to produce bio-oils by means of supercritical methanol or ethanol (also acetone) at 523 to 563 K [
113]. Red grape stems were used for the extraction of antioxidants by supercritical denatured ethanol up to 573 K [
114]. Supercritical water at 923 K and 25 MPa was used for the gasification of glucose to produce hydrogen [
115], consisting mainly of fatty acid esters [
116]. Sea mango oil (
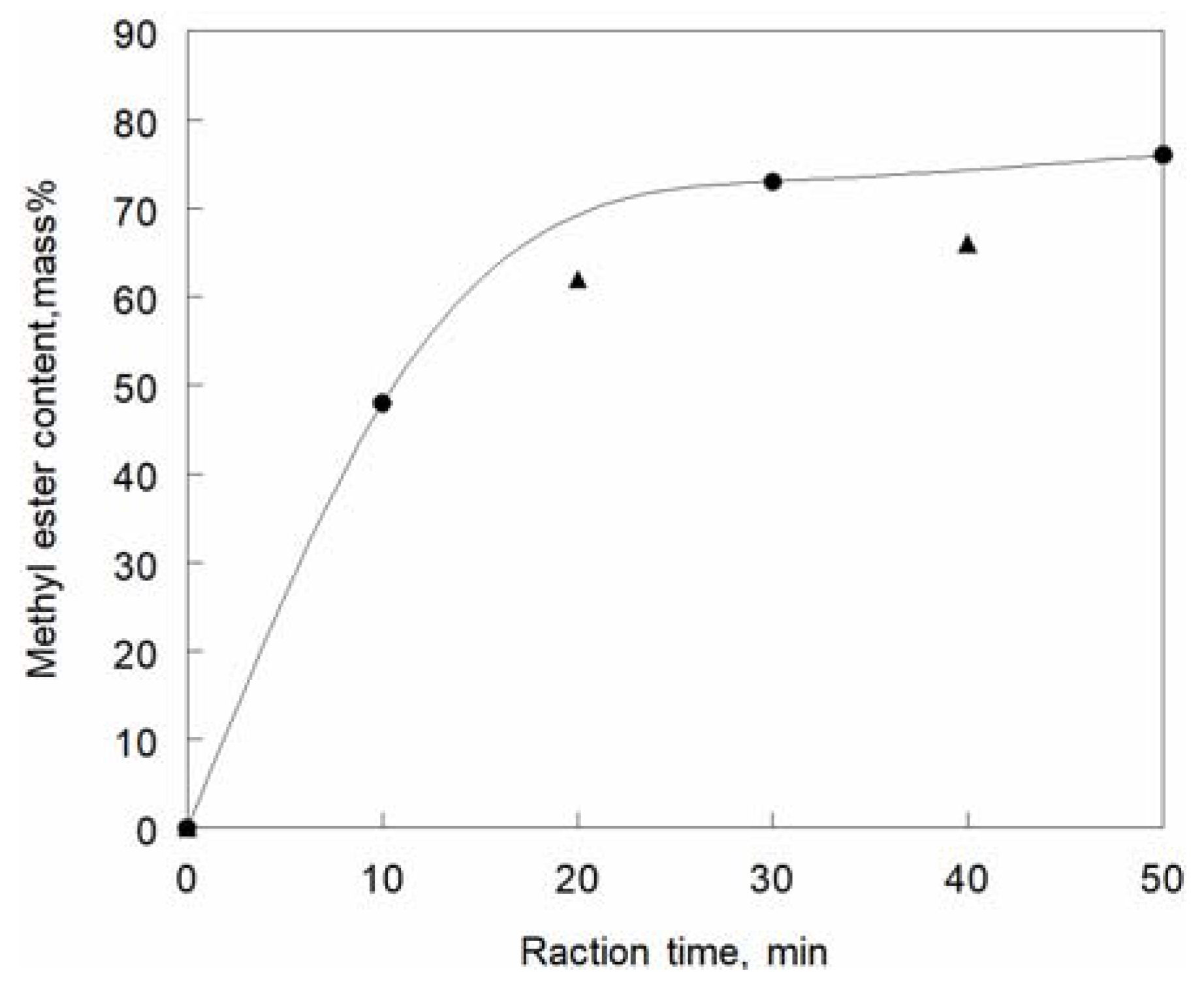
Cerberaodollam) was subjected to transesterification by supercritical methanol, and the rate of the formation of the methyl esters of the fatty acid contents is shown in
Figure 3 [
117].
All these reports are summarized in
Table 11.
6. Methods Used with Sub- and Supercritical Fluid Extraction
Several methods are being employed to enhance the yields and qualities of the products of solvent extraction processes, among which are solid micro-extraction and ultrasound- and microwave-assisted extraction. However, pressurized liquid extraction used with subcritical water, methanol, ethanol, and their mixtures as well as supercritical fluid extraction with these fluids are sufficiently powerful, so that these enhancement methods have only seldom been needed. Nevertheless, there are situations, for instance with temperature-sensitive products, for which lowering the extraction temperature and enhancement of the process with either one of these methods has proved useful.
Microwave assistance has been employed for the PLE with water of thermally labile bioactive compounds from
Gastrodia elata Blume, shortening their residence time at the high temperature, 373 to 393 K [
85]. Transesterification of algal biomass with supercritical methanol [
106] and ethanol [
107] at 533 K benefited from microwave irradiation. Phenolic compounds were extracted from
Equisetum arvense L. herb with pressurized hot methanol and aqueous methanol, and ultrasound-assisted extraction with 80% methanol proved to be the optimal procedure [
118]. The recovery of anthocyanins from various plant materials (blueberries, blackberries, grumixama residues) by means of PLE at 353 K with water and aqueous ethanol alone and combined with ultrasound assistance was examined. The use of pressurized 70% ethanol combined with ultrasound assistance provided the largest total phenolics yield, but ultrasound-assisted extraction with this solvent at ambient conditions yielded the most anthocyanin product [
119]. Ultrasound assistance combined with PLE with 80% aqueous methanol at 403 K and 13 MPa was used for the pretreatment of four varieties of algae for the recovery of phenolics from them [
120].
In some cases, the efficiency of extraction by pressurized liquids or supercritical fluids is enhanced by the use of catalysts. Supercritical methanol and ethanol (also acetone) at 523 to 563 K, used for the liquefaction of
Onopordum heteracanthum stalks for the production of bio-oils, was enhanced by the use of potassium hydroxide or zinc chloride as catalysts [
106]. Catalytic amounts of potassium hydroxide were also needed for the desulfurization of low rank coal with supercritical ethanol at 623 K and 8.3 MPa of [
23].
The optimization of the extraction processes with pressurized subcritical and with supercritical water, methanol, and their mixtures has been studied in many cases, using a central composite design [
121] or response surface methodology [
76,
112,
122]. In all these methods, the optimal temperature, choice of solvent (or its composition, in the case of aqueous mixtures), and the duration of the extraction process were sought. The best results of these methods are described in the previous sections.