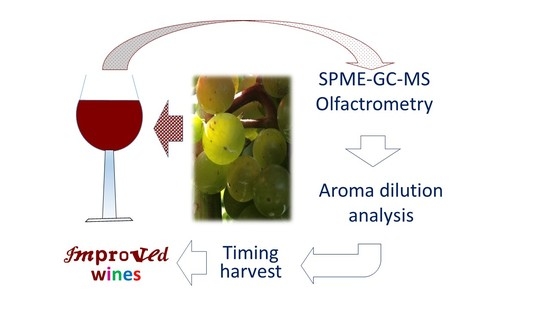
Determination of Selected Aromas in Marquette and Frontenac Wine Using Headspace-SPME Coupled with GC-MS and Simultaneous Olfactometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Standards, and Matrix Blank
2.2. Aroma Dilution Analysis
2.3. Automated GC-MS-Olfactometry System
3. Results
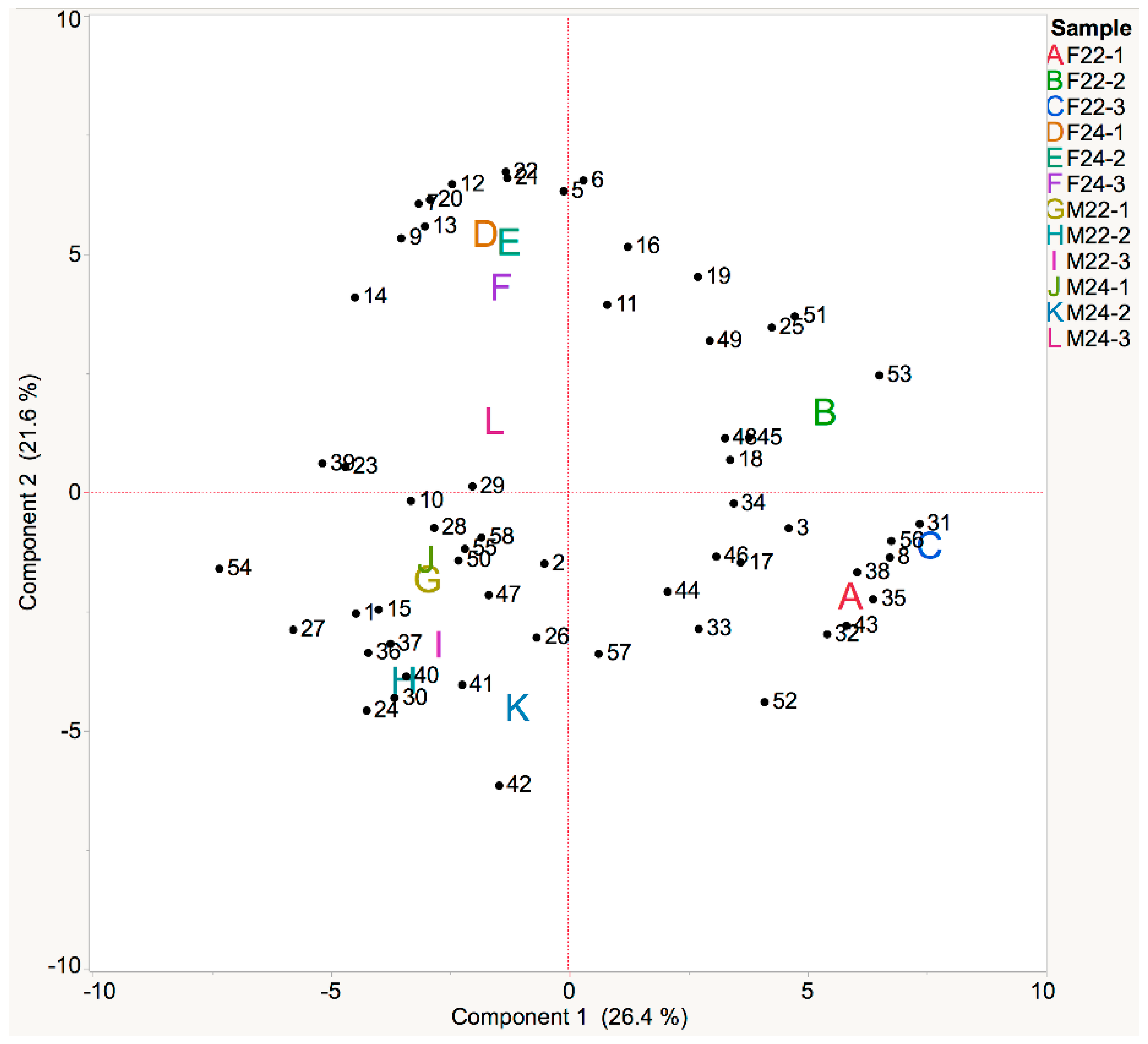
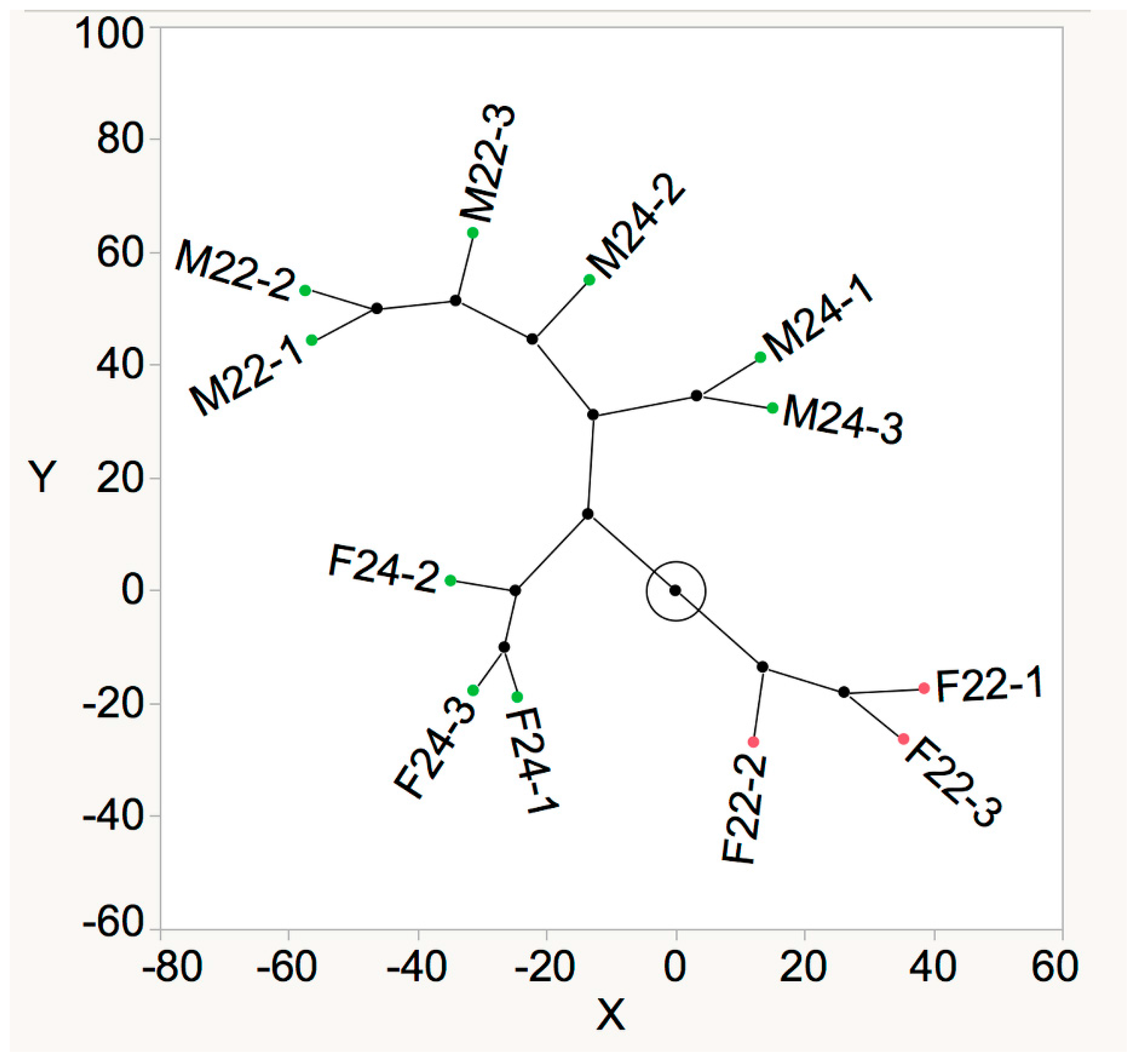
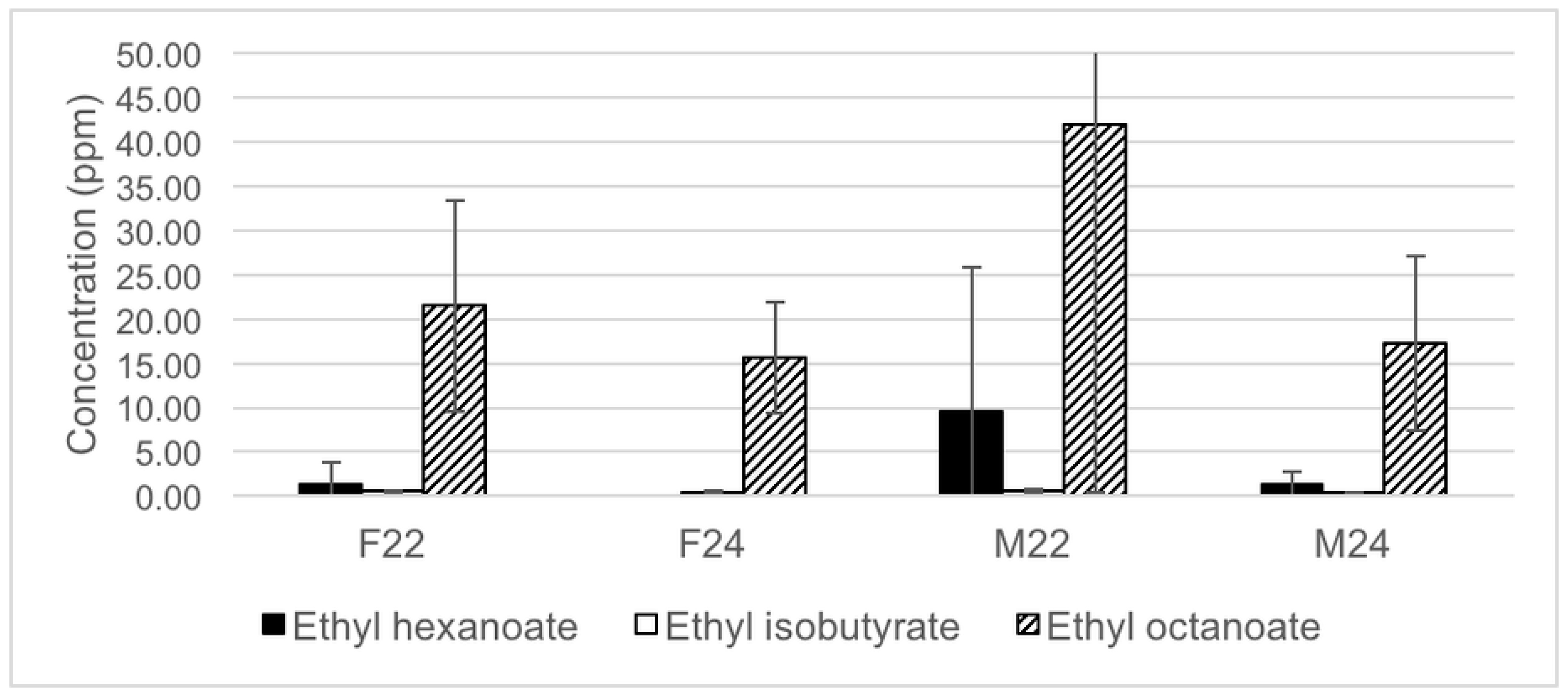
3.1. Semi-Quantitative Analysis of Volatiles in Wine
3.2. Aroma Dilution Analysis
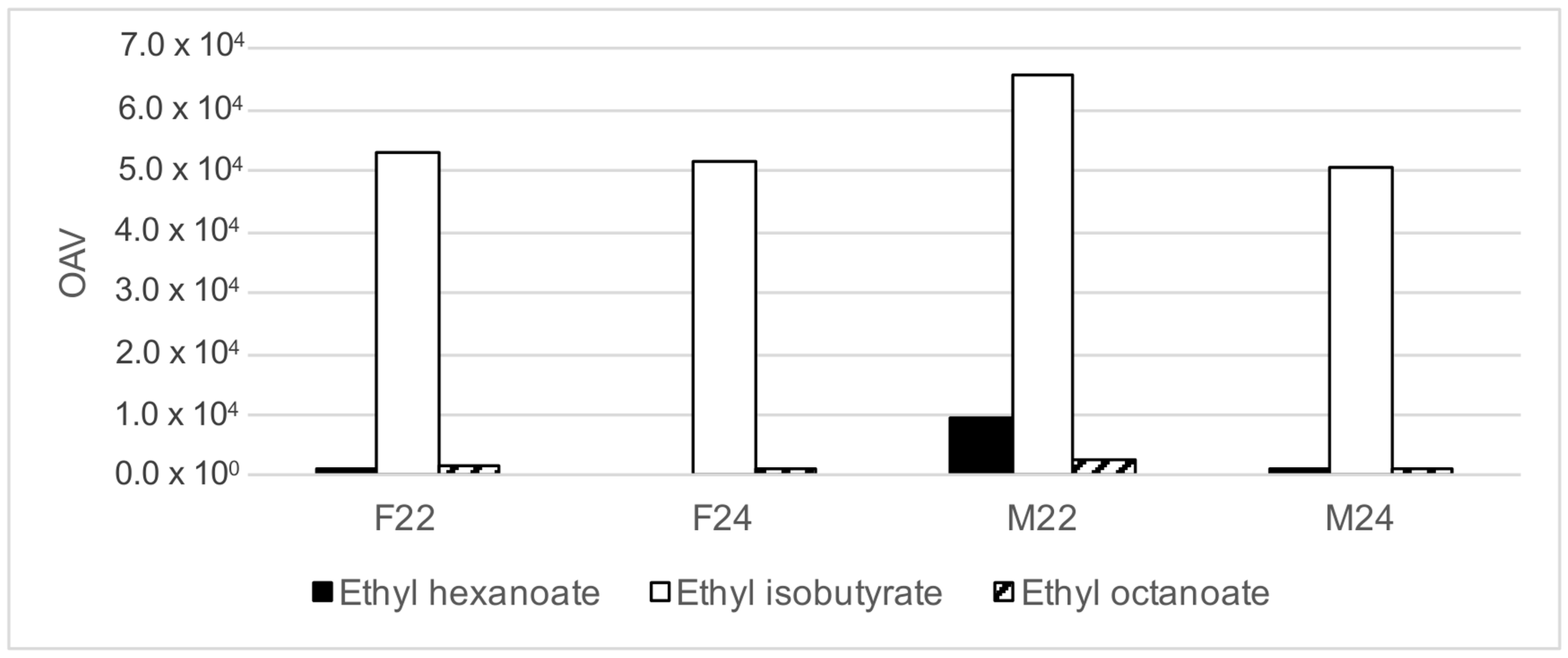
3.3. Calculation of OAV
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jackson, R.S. Wine Science Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Sanchez-Palomo, E.; Diaz-Maroto, M.C.; Gonzalez-Vinas, M.A.; Soriano-Perez, A.; Perez-Coello, M.S. Aroma profile of wines from Albillo and Muscat grape varieties at different stages of ripening. Food Control 2005, 18, 398–403. [Google Scholar] [CrossRef]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Miguez, M.J.; Gomez-Miguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of colour and aroma in white wines vinifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Bescansa, L.; Masa, A.; Oliveira, J. Changes in free and bound fractions of aroma compounds of four Vitis Vinifera cultivars at the last ripening stages. Phytochemistry 2012, 74, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, F.; Qian, M. Quantification of selected aroma-active compounds in Pinot noir wines from different grape maturities. J. Agric. Food Chem. 2006, 54, 8567–8573. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Aroma potential in early- and late- maturity Pinot noir grapes evaluated by aroma extract dilution analysis. J. Agric. Food Chem. 2016, 64, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Jeong, S.; Hur, Y.; Koh, S.; Choi, I. Changes in volatile compounds in vitis labrusca ‘Doonuri’ grapes during stages of fruit development and in wine. Hortic. Environ. Biotechnol. 2015, 56, 137–144. [Google Scholar] [CrossRef]
- Pedneault, K.; Dorais, M.; Angers, P. Flavor of cold-hardy grapes: Impact of berry maturity and environmental conditions. J. Agric. Food Chem. 2013, 64, 10418–10438. [Google Scholar] [CrossRef] [PubMed]
- Slegers, A.; Angers, P.; Ouillet, E.; Truchon, T.; Pedneault, K. Volatile compounds from grape skin, juice and wine from five interspecific hybrid grape cultivars grown in Quebec (Canada) for wine production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef] [PubMed]
- Guillaumie, S.; Ilg, A.; Rety, S.; Brette, M.; Trossat-Magnin, C.; Decroocq, S.; Leon, C.; Keime, C.; Ye, T.; Baltenweck-Guyot, R.; et al. Genetic analysis of the biosynthesis of 2-methoxy-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant Physiol. 2013, 162, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Radeka, S.; Grozaj, N.; Staver, M.; Persuric, D. Changes in physio-chemical and volatile aroma compound composition of Gewerztraminer wine as a result of late and ice harvest. Food Chem. 2016, 196, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Gametta, J.; Bastian, S.; Cozzolino, D.; Jeffery, D. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.T.; Heymann, H.; Ellersieck, M. Sensory and chemical analysis of Missouri Seyval blank wines. Am. J. Enol. Vitic. 1990, 41, 116–120. [Google Scholar]
- Canuti, V.; Conversano, M.; Calzi, M.L.; Heymann, H.; Mathews, M.A.; Eberle, S.E. Headspace solid-phase microextraction-gas chromatography-mass spectrometry for profiling the free volatile compounds in Cabernet Sauvignon grapes and wines. J. Chromatogr. A 2009, 1216, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Rocha, S.; Delgadillo, I.; Coimbra, M.A. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Onuki, S.; Koziel, J.A.; Jenks, W.S.; Cai, L.; Rice, S.; van Leeuwen, J.H. Optimization of extraction parameters for quantification of fermentation volatile by-products in industrial ethanol with solid-phase microextraction and gas chromatography. J. Inst. Brew. 2016, 122, 102–109. [Google Scholar] [CrossRef]
- Cai, L.; Rice, S.; Koziel, J.A.; Jenks, W.S.; van Leeuwen, J.H. Further purification of food-grade alcohol to make a congener-free product. J. Inst. Brew. 2016, 122, 84–92. [Google Scholar] [CrossRef]
- Robinson, A.L.; Ebeler, S.E.; Heymann, H.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J. Agric. Food Chem. 2009, 11, 10313–10322. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid phase microextraction in perspective. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Chemical Industry Press: Beijing, China, 2009; pp. 2–12. ISBN 978-7-122-04701-4. [Google Scholar]
- Cai, L.; Rice, S.; Koziel, J.A.; Dharmadhikari, M. Development of an automated method for aroma analysis of red wines from cold-hardy grapes using simultaneous solid-phase microextraction–multidimensional gas chromatography-mass spectrometry-olfactometry. Separations 2017, 4, 24. [Google Scholar] [CrossRef]
- Pavez, C.; Steinhaus, M.; Casaubon, G.; Schieberle, P.; Agosin, E. Identification, quantitation and sensory evaluation of methyl 2- and methyl 3- methylbutanoate in varietal red wines. Aust. J. Grape Wine Res. 2015, 21, 189–193. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortin, N.; Escudero, A.; Lopez, R.; Cacho, J. Chemical characterization of the aroma of Grenache rose wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.P.; Mestres, M.; Sala, C.; Busto, O.; Guasch, J. Solid-phase microextraction and gas chromatography olfactometry analysis of successively diluted samples. A new approach of the aroma extract dilution analysis applied to the characterization of wine aroma. J. Agric. Food Chem. 2003, 51, 7861–7865. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Ennis, D.M. Sensory thresholds: Concepts and methods. J. Sens. Stud. 1998, 13, 133–148. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A. Characterizing the smell of marijuana by odor impact of volatile compounds: An application of simultaneous chemical and sensory analysis. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Koziel, J.A. Odor impact of volatiles emitted from marijuana, cocaine, heroin and their surrogate scents. Data Br. 2015, 5, 653–706. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Koziel, J.A. The relationship between chemical concentration and odor activity value explains the inconsistency in making a comprehensive surrogate scent training tool representative of illicit drugs. Forensic Sci. Int. 2015, 257, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Koziel, J.A.; Dharmadhikari, M.; Fennell, A. Evaluation of tannins and anthocyanins in Marquette, Frontenac, and St. Croix cold-hardy grape cultivars. Fermentation 2017, 3, 47. [Google Scholar] [CrossRef]
- University of Minnesota, Minnesota Hardy. Available online: http://mnhardy.umn.edu/varieties/fruit/grapes (accessed on 28 April 2016).
- Chateau Stripmine, Frontenac Parentage. Available online: http://chateaustripmine.info/Parentage/Frontenac.gif (accessed on 10 May 2017).
- Chateau Stripmine, Marquette Parentage. Available online: http://chateaustripmine.info/Parentage/Marquette.gif (accessed on 10 May 2017).
- Leffingwell & Associates. Odor & Flavor Detection Thresholds in Water. Available online: http://www.leffingwell.com/odorthre.htm (accessed on 29 December 2017).
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of wines and spirits by phthalates: Types of contaminants present, contamination sources and means of prevention. Food Addit. Contam. A 2014, 31, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: www.flavornet.org (accessed on 14 February 2018).
- Mansfield, A.K.; Schirle-Keller, J.P.; Reineccius, G.A. Identification of odor-impact compounds in red table wines produced from Frontenac grapes. Am. J. Enol. Vitic. 2011, 62, 169–176. [Google Scholar] [CrossRef]
- Mansfield, A.K.; Vickers, Z.M. Characterization of the aroma of red Frontenac table wines by descriptive analysis. Am. J. Enol. Vitic. 2009, 60, 435–441. [Google Scholar]
- The Good Scents Company. Available online: www.thegoodscentscompany (accessed on 14 February 2018).
- Patterson, M.Q.; Stevens, J.C.; Cain, W.S.; Cometto-Muniz, J.E. Detection thresholds for an olfactory mixture and its three constituent compounds. Chem. Sens. 1993, 18, 723–734. [Google Scholar] [CrossRef]
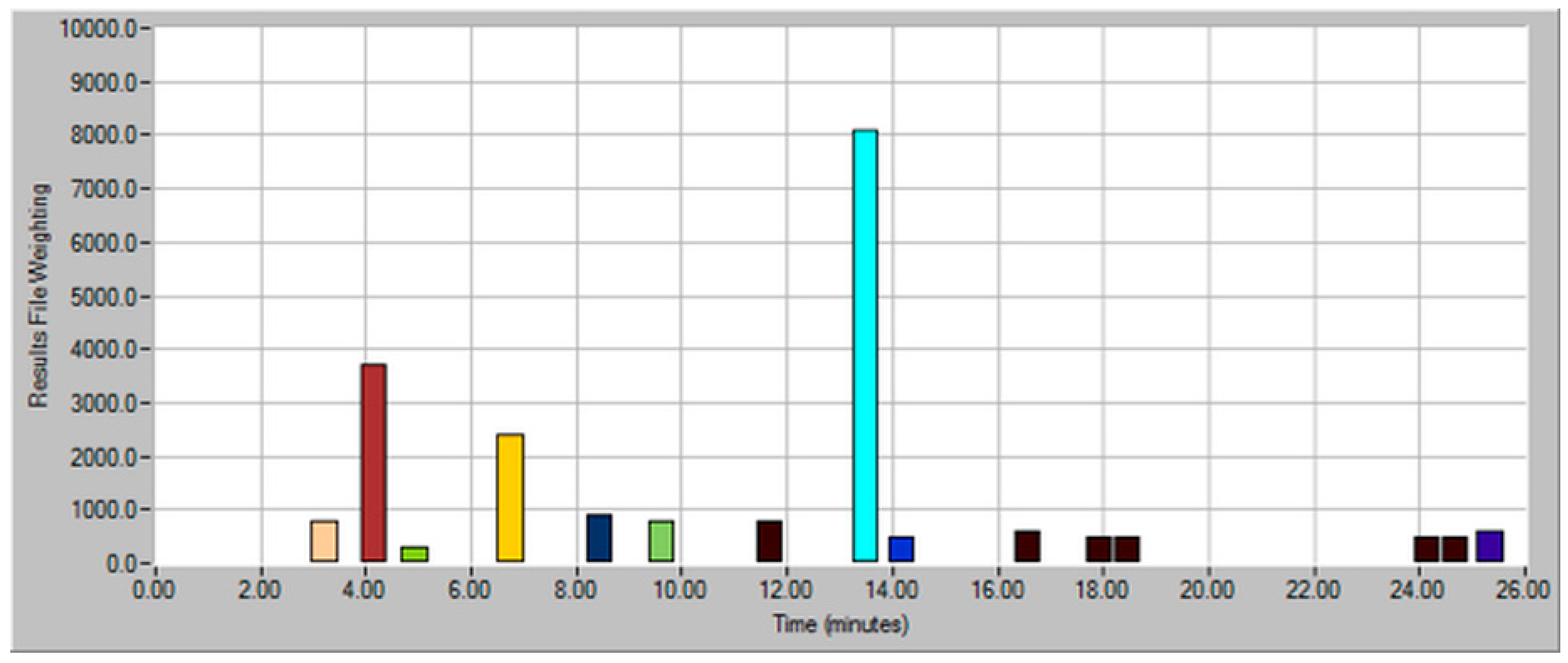
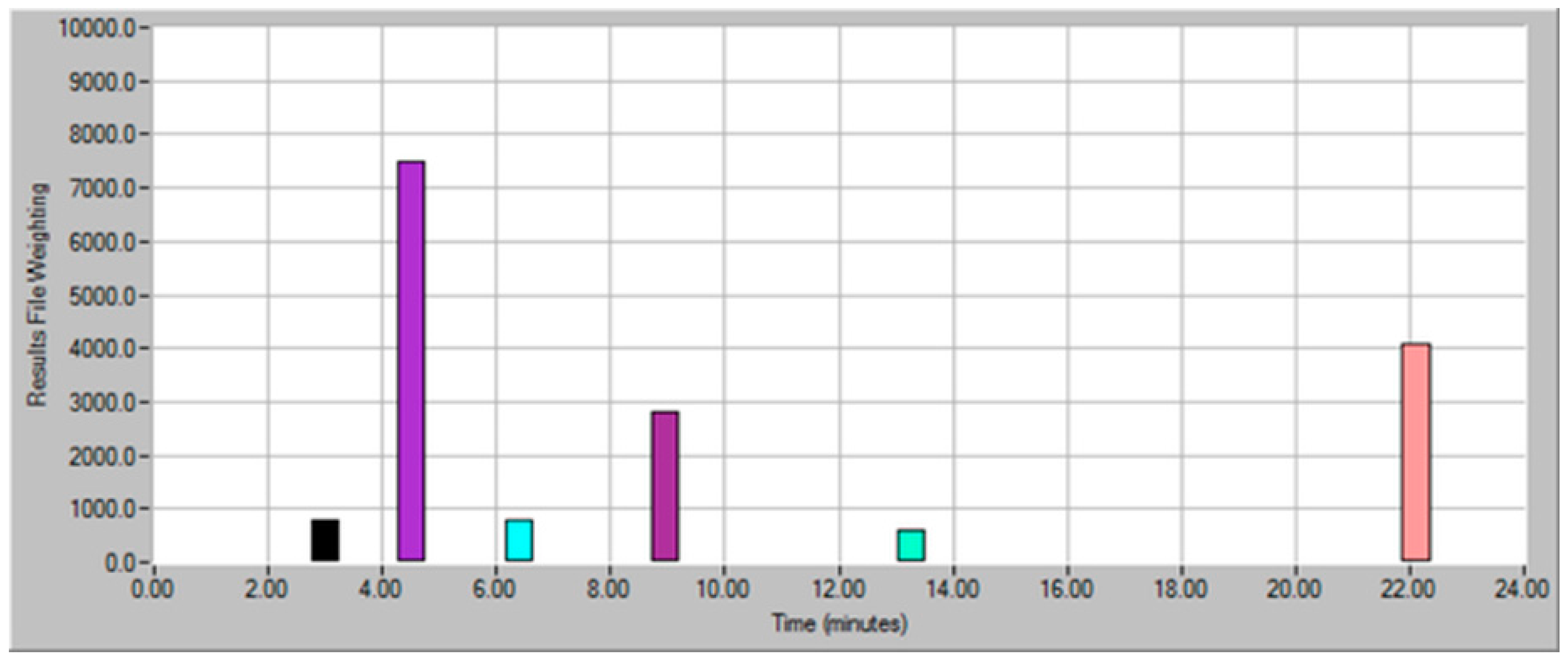
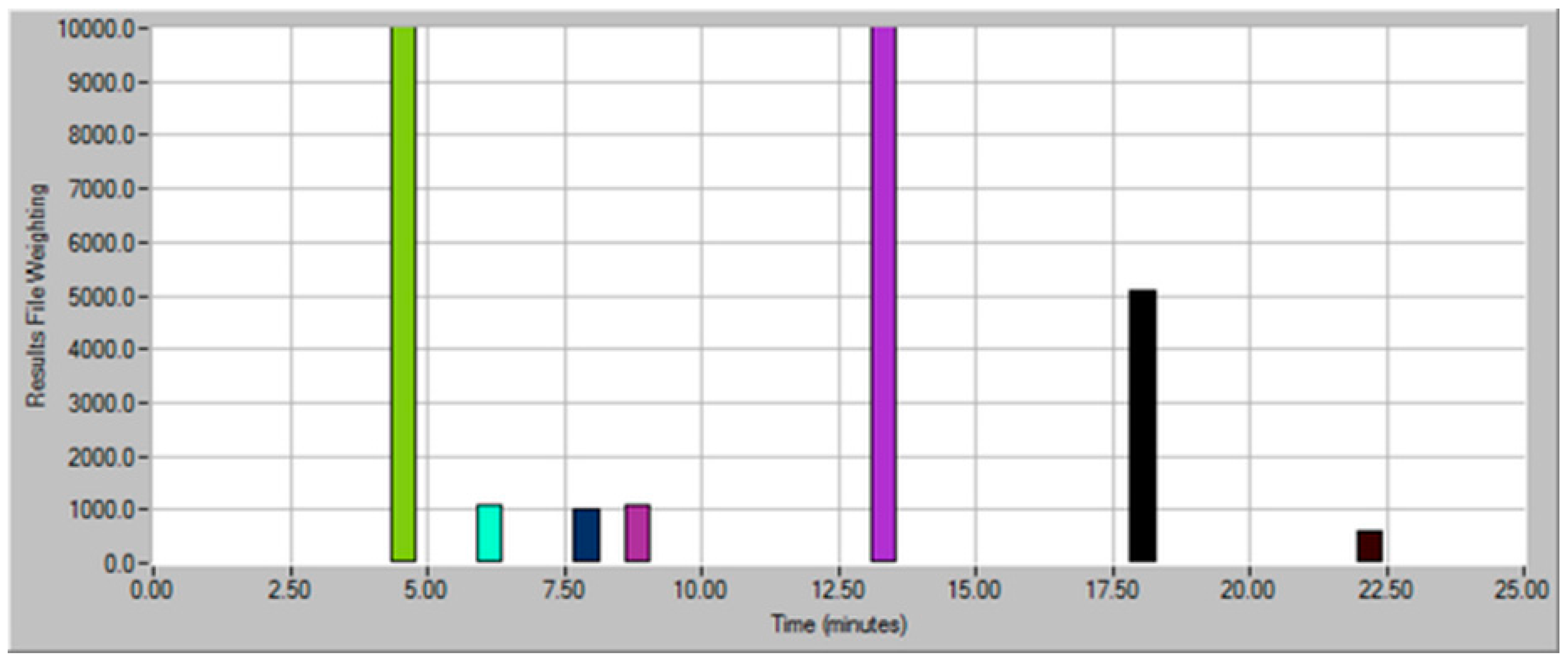
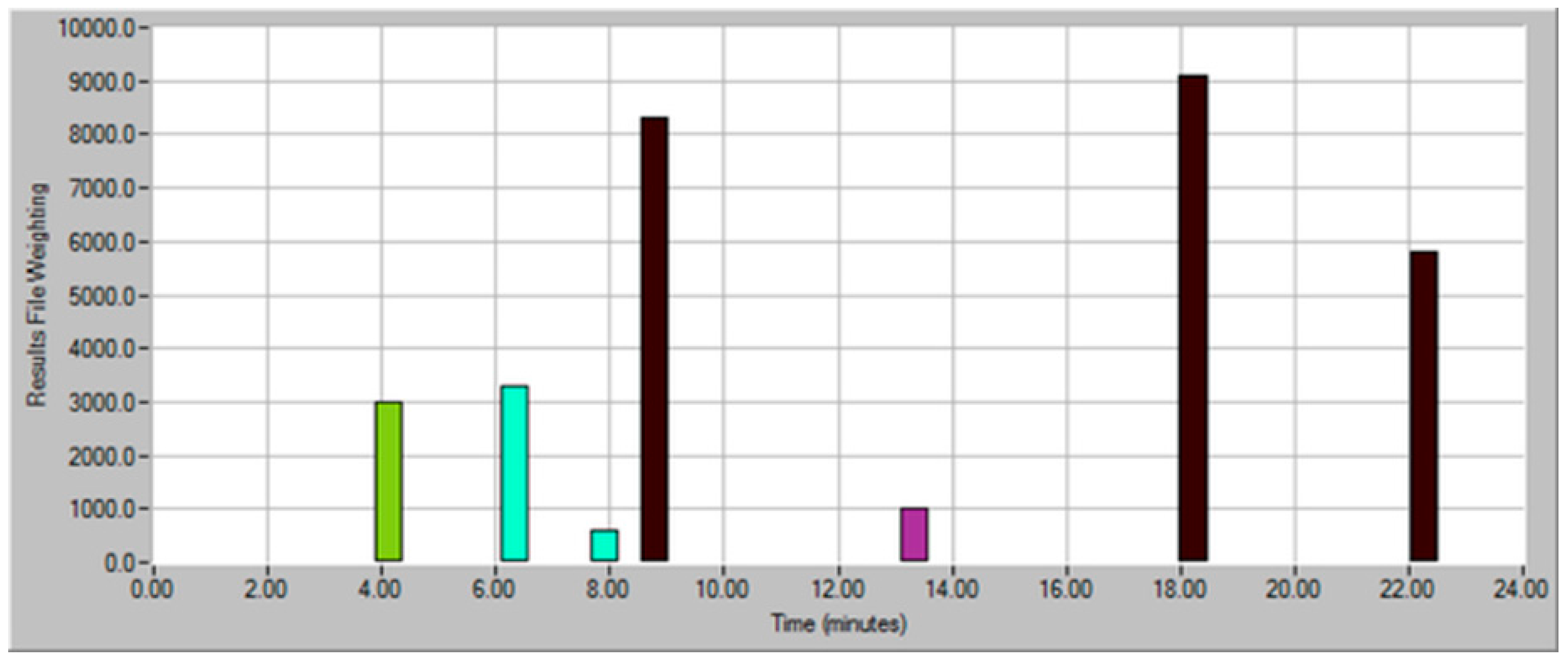
| Dilution Factor | Weighting Factor | Sample Volume (mL) | Model Wine Volume (mL) | Total Volume (mL) |
|---|---|---|---|---|
| 0 | 1.000 | 4.000 | 0.000 | 4 |
| 2 | 0.500 | 2.000 | 2.000 | 4 |
| 4 | 0.250 | 1.000 | 3.000 | 4 |
| 8 | 0.125 | 0.500 | 3.500 | 4 |
| 16 | 0.063 | 0.250 | 3.750 | 4 |
| 32 | 0.031 | 0.125 | 3.875 | 4 |
| No. | Compound | Relative Retention 1 |
|---|---|---|
| 1 | Acetaldehyde | 0.18 |
| 2 | Methyl acetate | 0.22 |
| 3 | 2-nitropropane | 0.22 |
| 4 | Isobutyraldehyde | 0.22 |
| 5 | Ethanol | 0.25 |
| 6 | Ethyl acetate | 0.27 |
| 7 | Acetic acid ethenyl ester | 0.30 |
| 8 | Methylbutanal | 0.31 |
| 9 | 1,1-dimethyl-hydrazine | 0.33 |
| 10 | n-Propyl acetate | 0.38 |
| 11 | Isobutanol | 0.39 |
| 12 | Ethyl isobutyrate | 0.42 |
| 13 | 1-butanol | 0.46 |
| 14 | Isobutyl acetate | 0.46 |
| 15 | Ethyl butyrate | 0.51 |
| 16 | Isoamyl alcohol | 0.54 |
| 17 | 3-methylpentane | 0.55 |
| 18 | Isocyanatomethane | 0.55 |
| 19 | Ethyl methylbutyrate | 0.58 |
| 20 | Ethyl 3-methylbutanoate | 0.59 |
| 21 | Isoamyl acetate | 0.65 |
| 22 | Ethyl lactate | 0.72 |
| 23 | Styrene | 0.73 |
| 24 | 1-hexanol | 0.76 |
| 25 | Acetic acid | 0.79 |
| 26 | 1,2-dimethyl hydrazine | 0.79 |
| 27 | Ethyl hexanoate | 0.85 |
| 28 | 1-Heptanol | 0.92 |
| 29 | 2,3,4-trimethylpentane | 0.94 |
| 30 | Isoamyl butyrate | 0.94 |
| 31 | Undecane | 0.97 |
| 32 | Benzaldehyde | 0.97 |
| 33 | 3-Nonanone (IS) | 1.00 |
| 34 | Methyl octanoate | 1.06 |
| 35 | 1-Octanol | 1.06 |
| 36 | Octyl formate | 1.06 |
| 37 | Linalool | 1.08 |
| 38 | Phenylethanal | 1.11 |
| 39 | Ethyl octanoate | 1.16 |
| 40 | 1-Nonanol | 1.20 |
| 41 | Pentanoic acid | 1.25 |
| 42 | Propyl octanoate | 1.28 |
| 43 | Ethyl nonanoate | 1.30 |
| 44 | Methyl salicylate | 1.30 |
| 45 | Methyl acetylsalicylate | 1.30 |
| 46 | Phenylethyl alcohol | 1.34 |
| 47 | Phenethyl isobutyrate | 1.37 |
| 48 | Phenethyl phenyl acetate | 1.37 |
| 49 | Ethyl decanoate | 1.42 |
| 50 | Octanoic Acid | 1.47 |
| 51 | β-damascenone | 1.47 |
| 52 | Isoamyl octanoate | 1.49 |
| 53 | Ethyl laurate | 1.66 |
| 54 | 2,4-di-tert-butylphenol | 1.76 |
| 55 | Diethyl phthalate | 1.85 |
| 56 | Ethyl tetradecanoate | 1.89 |
| 57 | Ethyl hexadecanoate | 2.14 |
| 58 | Dibutyl phthalate | 2.37 |
| Compound | Quantitation Range (ppm) | R2 |
|---|---|---|
| Ethyl hexanoate | 2.89 × 10−2–1.16 × 102 | 0.998 |
| Ethyl isobutyrate | 2.86 × 10−2–1.15 × 102 | 0.999 |
| Ethyl octanoate | 2.88 × 10−2–1.15 × 102 | 0.998 |
| Ethyl decanoate | 2.79 × 10−2–1.12 × 102 | 0.997 |
| Ethyl butyrate | 2.93 × 10−2–1.17 × 102 | 0.999 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rice, S.; Lutt, N.; Koziel, J.A.; Dharmadhikari, M.; Fennell, A. Determination of Selected Aromas in Marquette and Frontenac Wine Using Headspace-SPME Coupled with GC-MS and Simultaneous Olfactometry. Separations 2018, 5, 20. https://doi.org/10.3390/separations5010020
Rice S, Lutt N, Koziel JA, Dharmadhikari M, Fennell A. Determination of Selected Aromas in Marquette and Frontenac Wine Using Headspace-SPME Coupled with GC-MS and Simultaneous Olfactometry. Separations. 2018; 5(1):20. https://doi.org/10.3390/separations5010020
Chicago/Turabian StyleRice, Somchai, Nanticha Lutt, Jacek A. Koziel, Murlidhar Dharmadhikari, and Anne Fennell. 2018. "Determination of Selected Aromas in Marquette and Frontenac Wine Using Headspace-SPME Coupled with GC-MS and Simultaneous Olfactometry" Separations 5, no. 1: 20. https://doi.org/10.3390/separations5010020