Comparison of Separation of Seed Oil Triglycerides Containing Isomeric Conjugated Octadecatrienoic Acid Moieties by Reversed-Phase HPLC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Seed Material
2.2. Oil Extraction and Purification
2.3. Chromatography
2.4. Calculations and Designations
3. Results
3.1. UV Spectroscopic Properties of Conjugated Octadecatrienoic Acids
3.2. Retention Analysis of TGs of Plant Sources Seeds with Isomeric Octadecatrienoic Acids
3.2.1. Triglycerides of Oil with (9Z,11E,13E)-octadeca-9,11,13-trienoic Acid Moieties
3.2.2. Triglycerides of Oil with (9Z,11E,13Z)-octadeca-9,11,13-trienoic Acid Moieties
3.2.3. Triglycerides of Oil with (8E,10E,12Z)-octadeca-8,10,12-trienoic Acid Moieties
3.2.4. Triglycerides of Oil with (9E,11E,13Z)-octadeca-9,11,13-trienoic Acid Moieties
3.2.5. Triglycerides of Oil with (8Z,10E,12Z)-octadeca-8,10,12-trienoic Acid Moieties
3.2.6. Triglycerides of Oil with (9E,11E,13E)-octadeca-9,11,13-trienoic Acid Moieties
3.2.7. Triglycerides of Three Plant’s Seed Oil
4. Discussion
4.1. Some Remarks About Increment Approach
4.2. Comparison of Retention of TGs with Isomeric Conjugated Octadecatrienoic Acid Moieties
4.3. Minor Seed Oil Components Identification
4.3.1. Momordica charantia Seed Oil
4.3.2. Trichosanthus anguina Seed Oil
4.3.3. Calendula Officinalis Seed Oil
4.3.4. Catalpa ovata Seed Oil
4.3.5. Jacaranda mimosifolia Seed Oil
4.4. Relative Retention Analysis of TGs with Conjugated Seed Oils
5. Conclusions
Author Contributions
Conflicts of Interest
References
- De Carvalho, E.B.T.; de Melo, I.L.P.; Mancini-Filho, J. Chemical and physiological aspects of isomers of conjugated fatty acids. Ciênc. Tecnol. Aliment. Campinas 2010, 30, 295–307. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.-F.; Chena, X.-E.; Li, D. Conjugated linolenic acids and their bioactivities: A Review. Food Funct. 2014, 5, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hosokawa, M.; Yasui, Y.; Ishigamori, R.; Miyashita, K. Cancer Chemopreventive Ability of Conjugated Linolenic Acids. Int. J. Mol. Sci. 2011, 12, 7495–7509. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Tokuyama, Y.; Igarashi, M.; Miyazawa, T. Tumor growth suppression by α-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis 2004, 25, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R.P. Health Benefits of Punicic Acid: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 16–27. [Google Scholar] [CrossRef]
- Suzuki, R.; Yasui, Y.; Kohno, H.; Miyamoto, S.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Catalpa seed oil rich in 9t,11t,13c-conjugated linolenic acid suppresses the development of colonic aberrant crypt foci induced by azoxymethane in rats. Oncol. Rep. 2006, 16, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Ito, J.; Tsuduki, T.; Honma, T.; Kijima, R.; Sugawara, S.; Arai, T.; Yamasaki, M.; Ikezaki, A.; Yokoyama, M.; et al. Jacaric acid, a linolenic acid isomer with a conjugated triene system, reduce stearoyl-CoA desaturase expression in liver of mice. J. Oleo Sci. 2012, 61, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metabol. 2009, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.Y.; Chisholm, M.J. A Survey of the Conjugated Fatty Acids of Seed Oils. J. Am. Oil Chem. Soc. 1968, 45, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Frede, E. Improved HPLC of Triglycerides by Special Tempering Procedures. Chromatographia 1988, 21, 29–36. [Google Scholar] [CrossRef]
- Barrón, L.J.R.; Santa-Maria, G. Non-Aqueous Reverse-Phase HPLC Analysis of Triglycerides. Chromatographia 1987, 23, 209–214. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Wang, Z.; Liu, J.; Wei, J.; Xiong, S.; Zhao, Z. Mass Spectrometry Methodology in Lipid Analysis. Int. J. Mol. Sci. 2014, 15, 10492–10507. [Google Scholar] [CrossRef] [PubMed]
- Podlaha, O.; Toregerd, B. A System for Identification of Triglycerides in Reversed Phase HPLC Chromatograms Based on Equivalent Carbon Numbers. J. Sep. Sci. 1982, 5, 553–558. [Google Scholar] [CrossRef]
- Sempoore, G.; Bezard, J. Qualitative and quantitative analysis of peanut oil triacylglycerols by reversed-phase liquid chromatography. J. Chromatogr. 1986, 366, 261–282. [Google Scholar] [CrossRef]
- Deineka, V.I. Experimental substantiation of a method of relative analysis of retention in HPLC. Russ. J. Phys. Chem. 2006, 80, 425–428. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, P.R.; Fitzgerald, G.F.; Stanton, C. Sources and Bioactive Properties of Conjugated Dietary Fatty Acids. Lipids 2016, 51, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.Y.; Chisholm, M.J.; Orgodnik, J.A. Identity and Configuration of Conjugated Fatty Acids in Certain Seed Oils. Lipids 1969, 4, 89–92. [Google Scholar] [CrossRef]
- Chisholm, M.J.; Hopkin, C.Y. Fatty Acid Composition of Some Cucurbitaceae Seed Oils. Can. J. Chem. 1964, 42, 560–564. [Google Scholar] [CrossRef]
- Chang, M.-K.; Conkerton, E.J.; Chapital, D.C.; Wan, P.J.; Vadhwa, O.P.; Spiers, J.M. Chinese Melon (Momordica charantia L.) Seed: Composition and Potential Use. J. Am. Oil Chem. Soc. 1996, 73, 263–265. [Google Scholar] [CrossRef]
- Joh, Y.-G.; Kim, S.-J. Analysis of Molecular Species of Triacylglycerols from Vegetable Oils Containing Fatty Acids with Non-Methylene-Interrupted Double Bonds, by HPLC in the Silver-ion Mode. J. Jpn. Oil Chem. Soc. 1998, 47, 927–936. [Google Scholar] [CrossRef]
- Joh, Y.-G.; Kim, S.-L.; Christie, W.W. The Structure of the Triacylglycerols, Containing Punicic Acid, in the Seed Oil of Trichosanthes kirilowii. J. Am. Oil Chem. Soc. 1995, 72, 1037–1042. [Google Scholar] [CrossRef]
- Król, B.; Paszko, T.; Król, A. Conjugated linolenic acid content in seeds of some pot marigold (Calendula officinalis) cultivars grown in Poland. Farmacia 2016, 64, 881–886. [Google Scholar]
- Takagi, T.; Itabashi, Y. Occurrence of mixtures of geometrical isomers of conjugated octadecatrienoic acids in some seed oils: Analysis by open-tubular gas liquid chromatography and high performance liquid chromatography. Lipids 1981, 16, 546–551. [Google Scholar] [CrossRef]
- Yamasaki, M.; Motonaga, C.; Yokoyama, M.; Ikezaki, A.; Kakihara, T.; Hayasegawa, R.; Yamasaki, K.; Sakono, M.; Sakakibara, Y.; Suiko, M.; et al. Induction of apoptotic call death in HL-60 cells by Jacaranda seed oil derived fatty acids. J. Oleo Sci. 2013, 62, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.Y.; Chisholm, M.J. Identification of conjugated triene fatty acids in certain seed oils. Can. J. Chem. 1962, 40, 2078–2082. [Google Scholar] [CrossRef]
- Radunz, A.; Heb, P.; Schmid, G.H. Analysis of the Seed Lipids of Aleurites montana. Z. Naturforsch. 1998, 53c, 305–310. [Google Scholar]
- Tulloch, A.P. 13C Nuclear Magnetic Resonance Spectroscopic Analysis of Seed Oils Containing Conjugated Unsaturated Acids. Lipids 1982, 17, 544–550. [Google Scholar] [CrossRef]
- Turtygin, A.V.; Deineka, V.I.; Deineka, L.A. Determination of the triglyceride composition of pomegranate seed oil by reversed-phase HPLC and spectrophotometry. J. Anal. Chem. 2013, 68, 558–563. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranat seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430–442. [Google Scholar]
- Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of the Triglyceride Composition of Pomegranate Seed Oil by RP-HPLC Followed by GC-MS. J. Am. Oil Chem. Soc. 2015, 92, 791–800. [Google Scholar] [CrossRef]
- Deineka, V.I. Relative retention analysis in HPLC: The correlation between incremental relationships. Russ. J. Phys. Chem. 2006, 80, 605–608. [Google Scholar] [CrossRef]
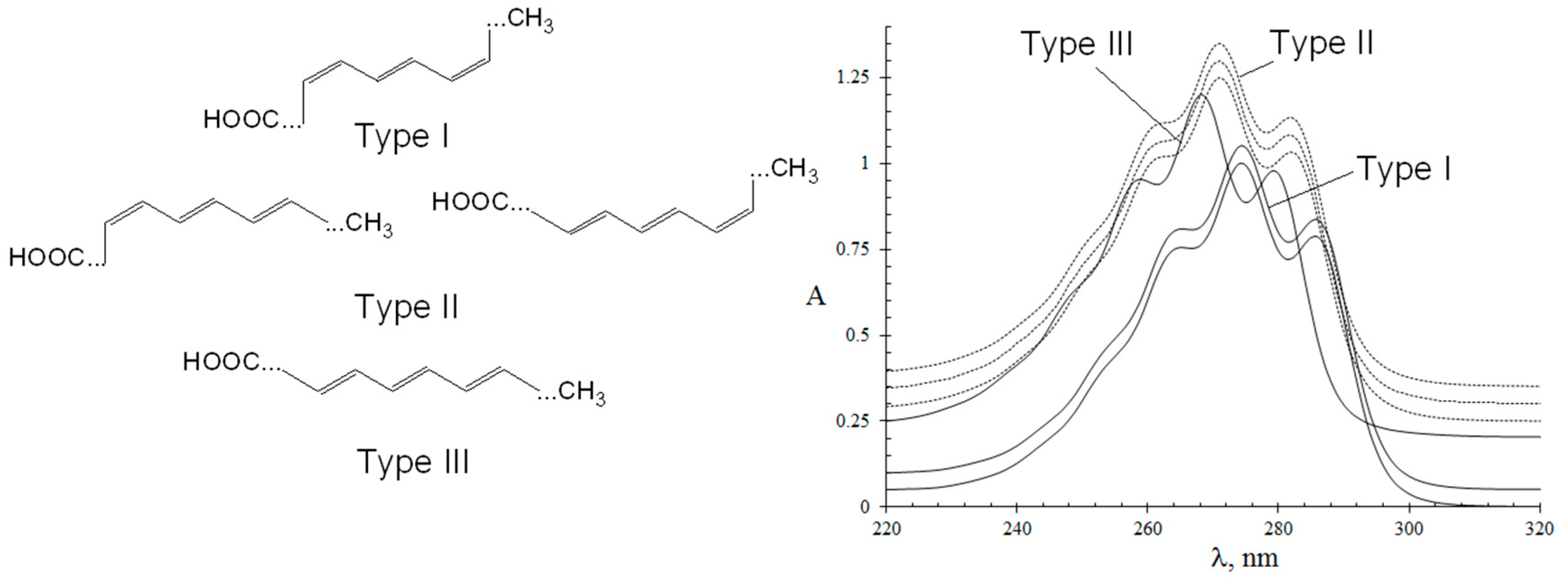
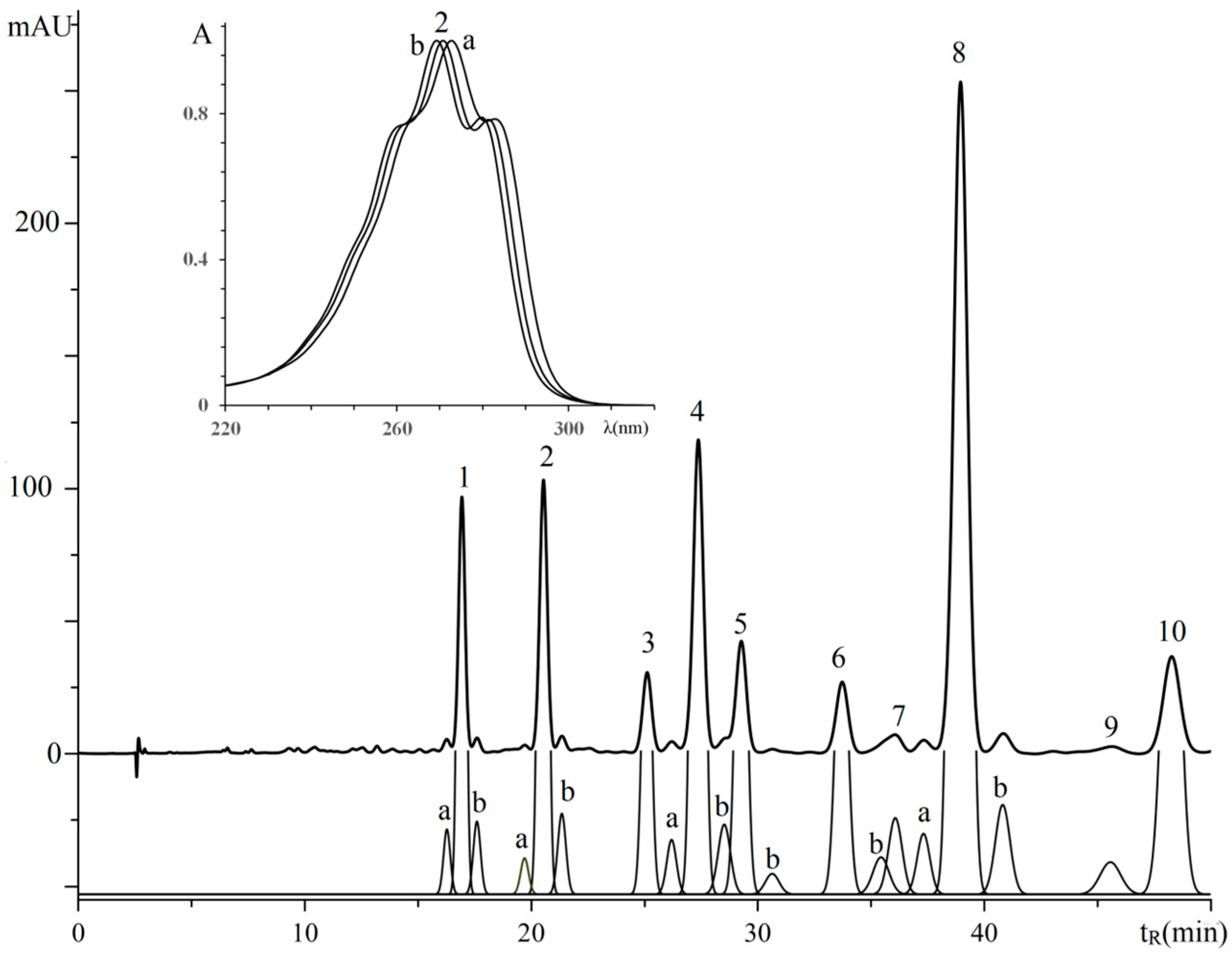
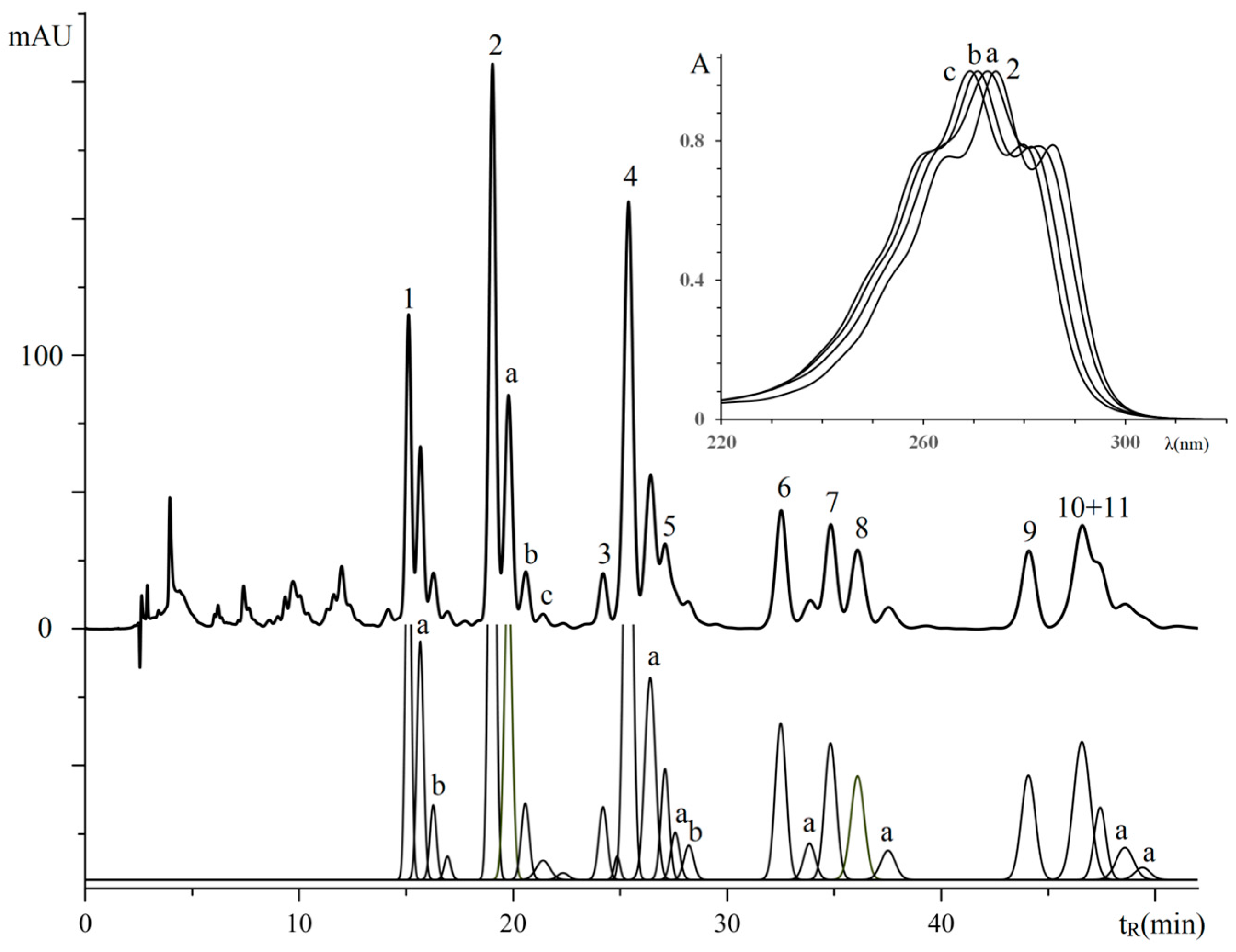
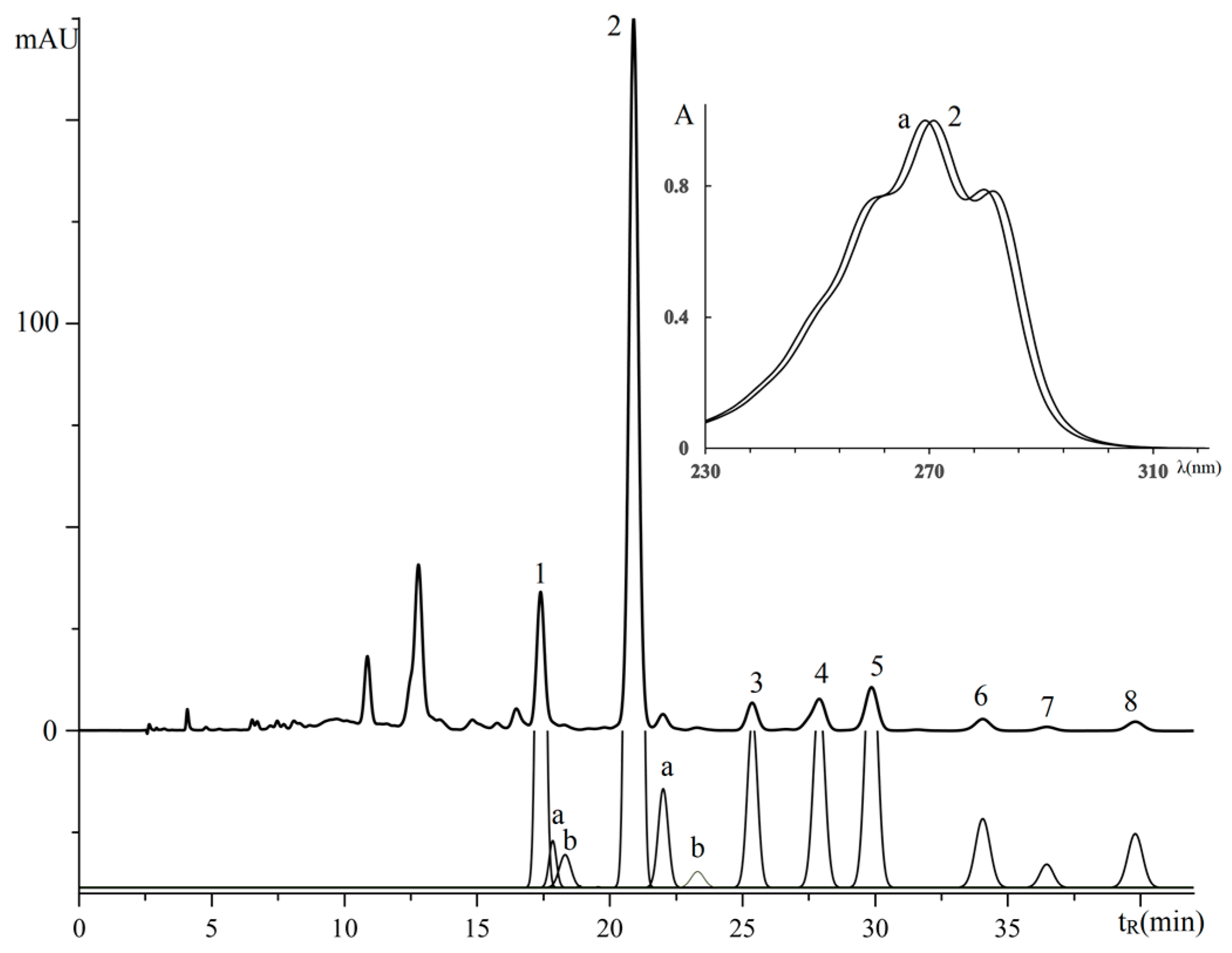
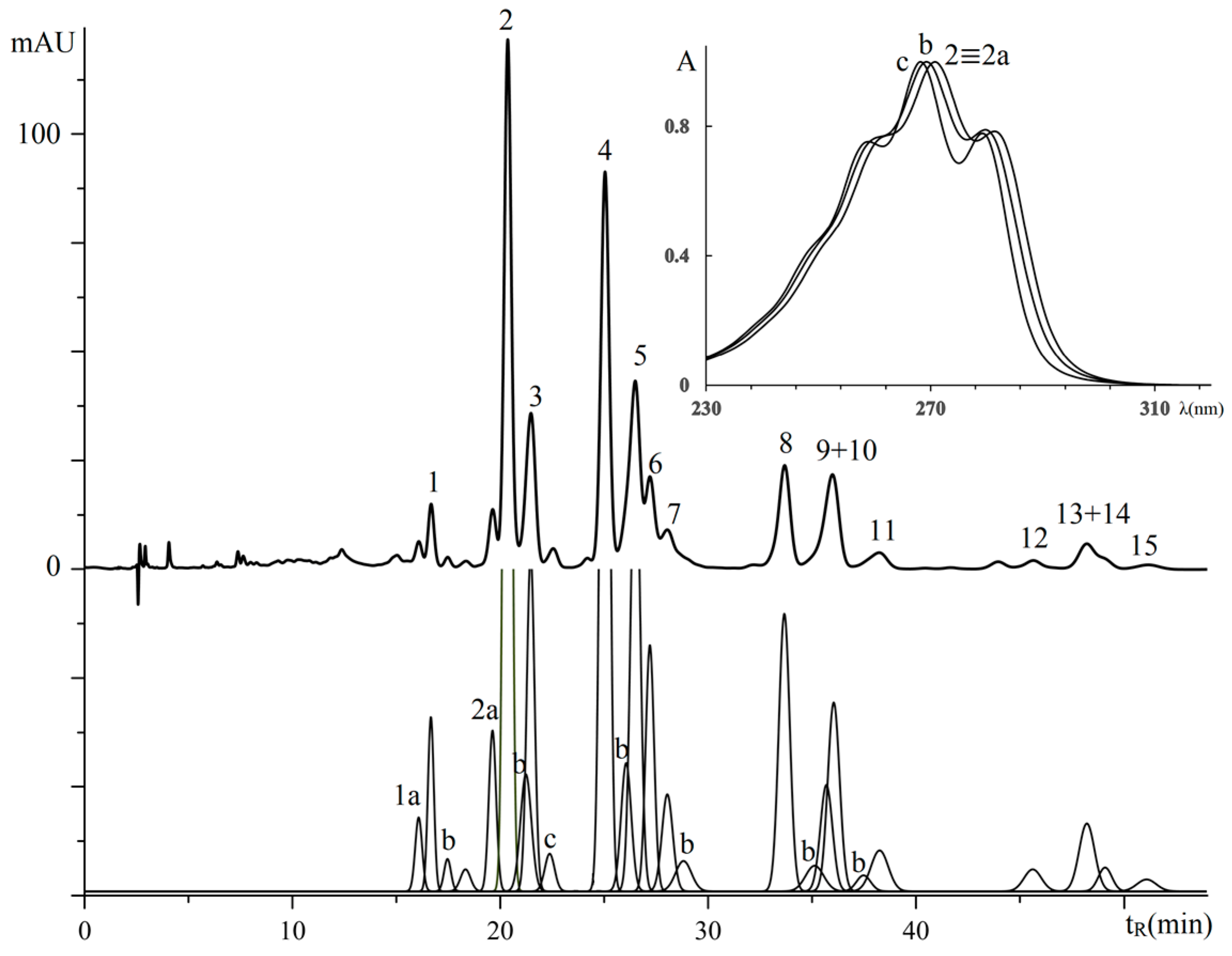
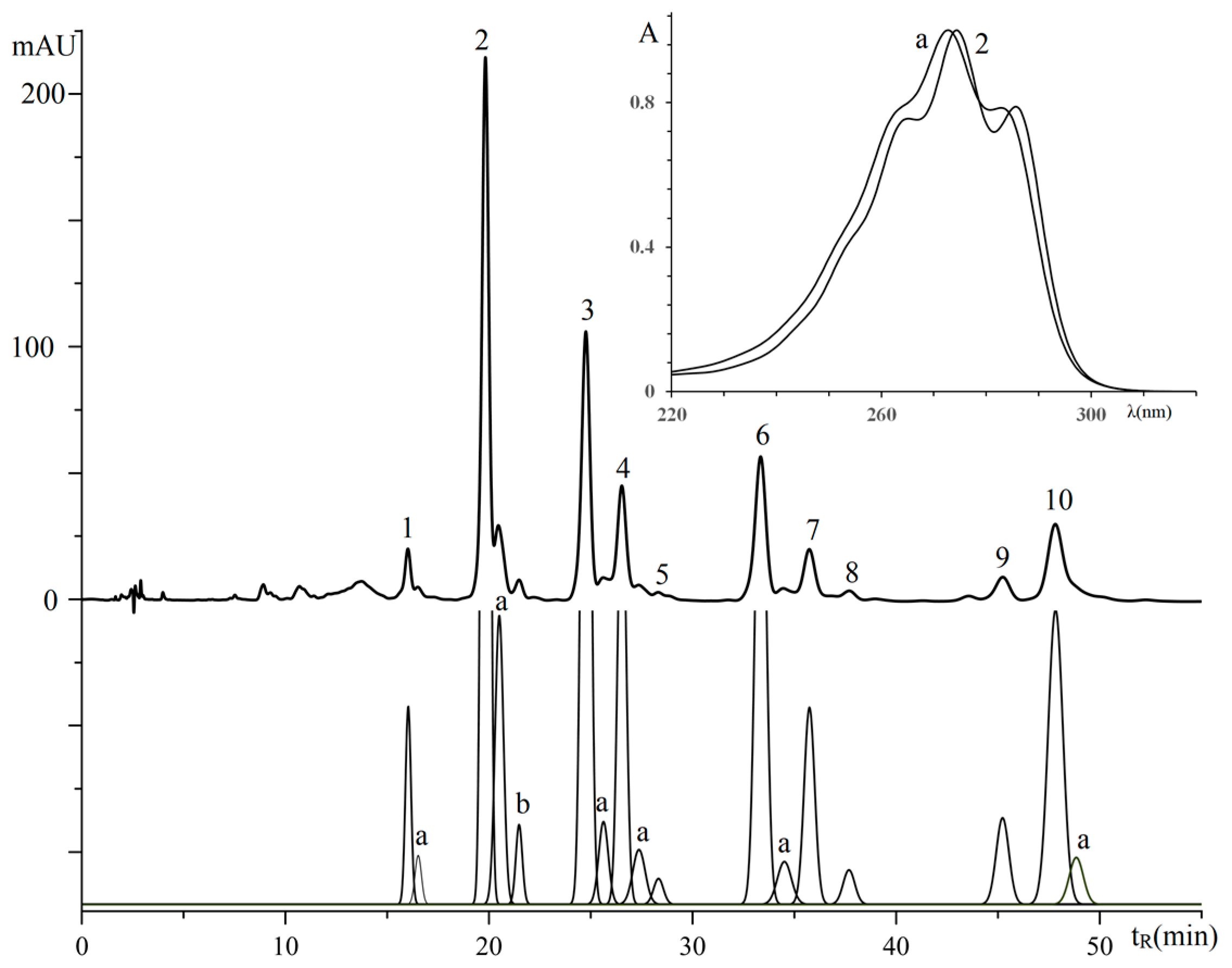
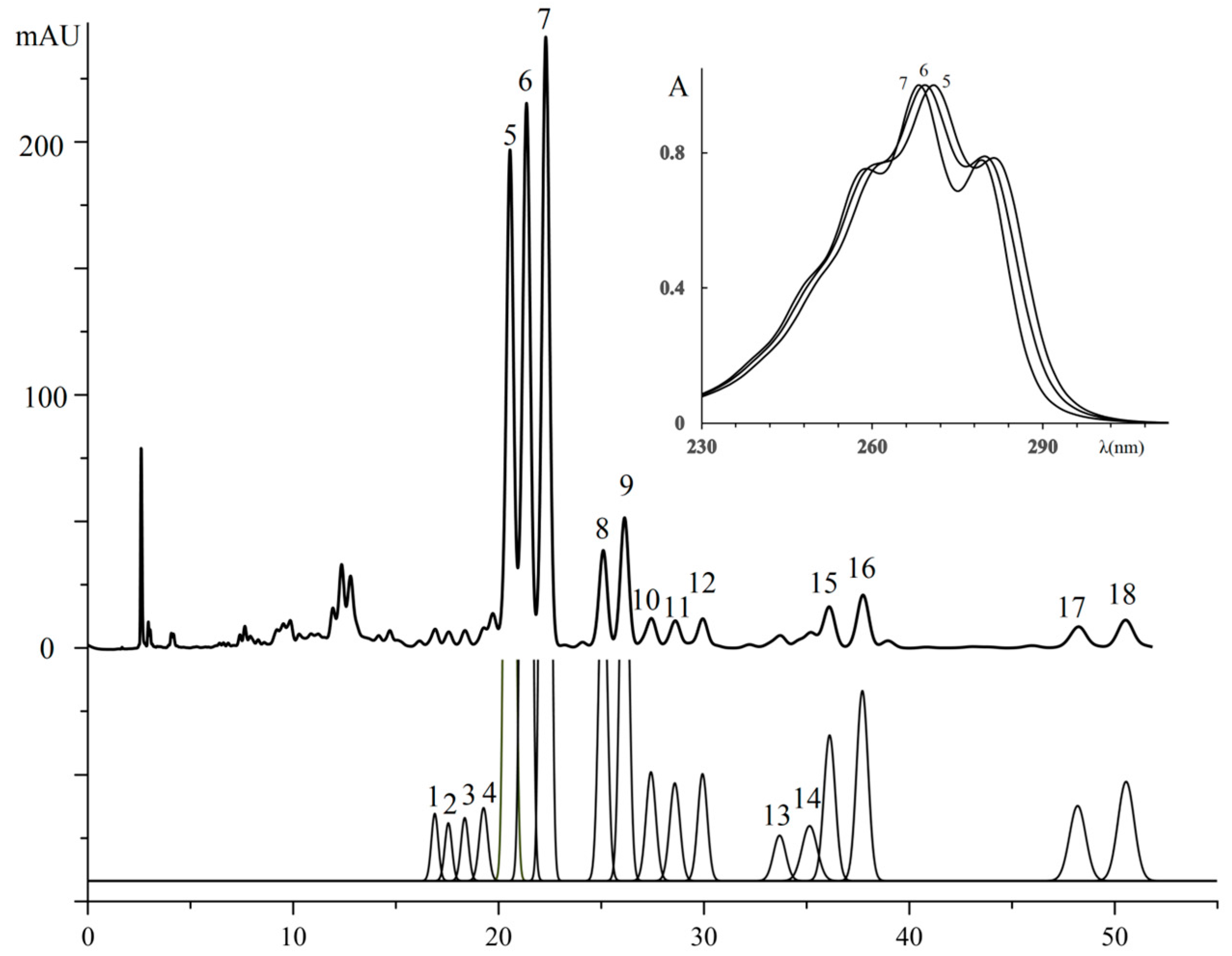
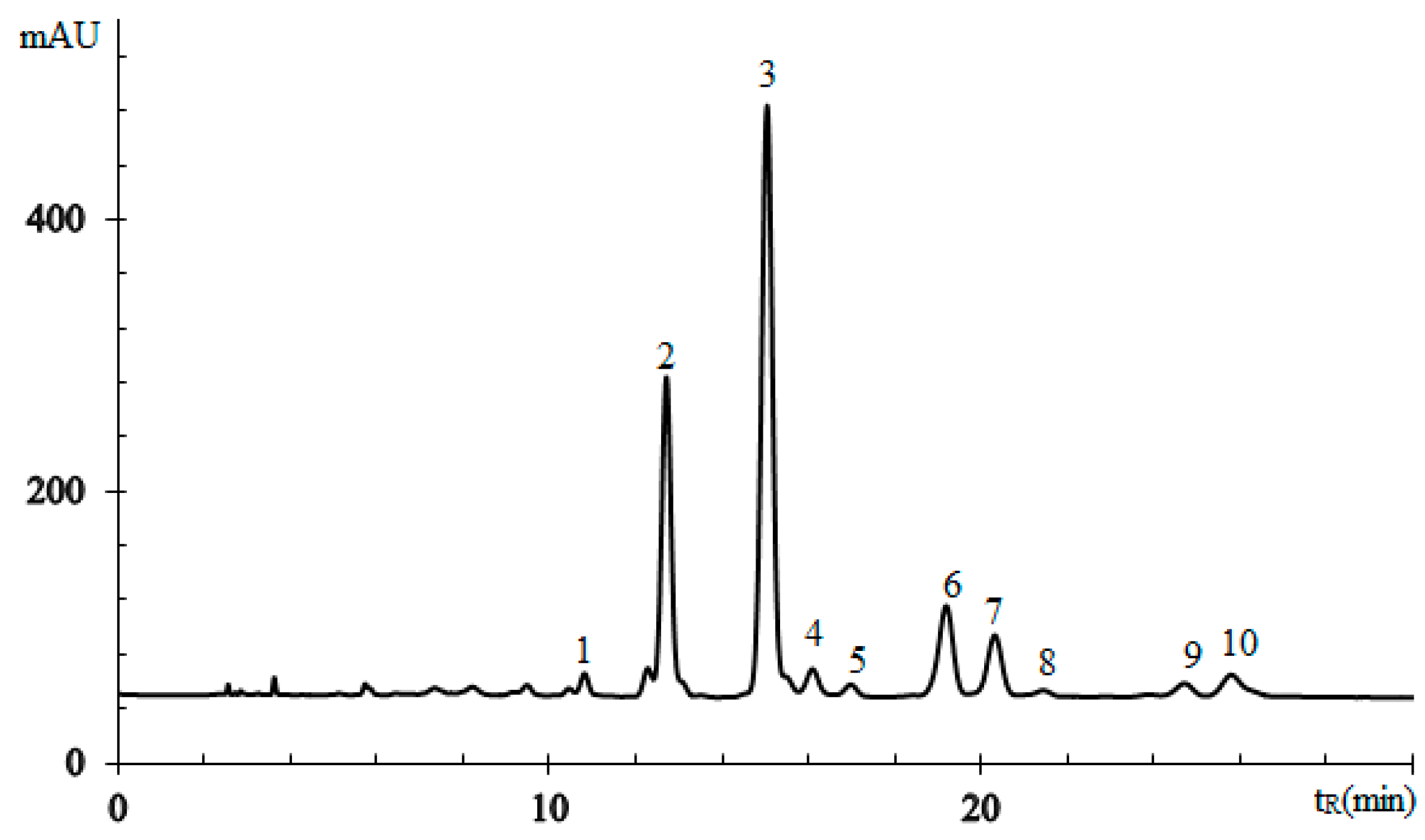
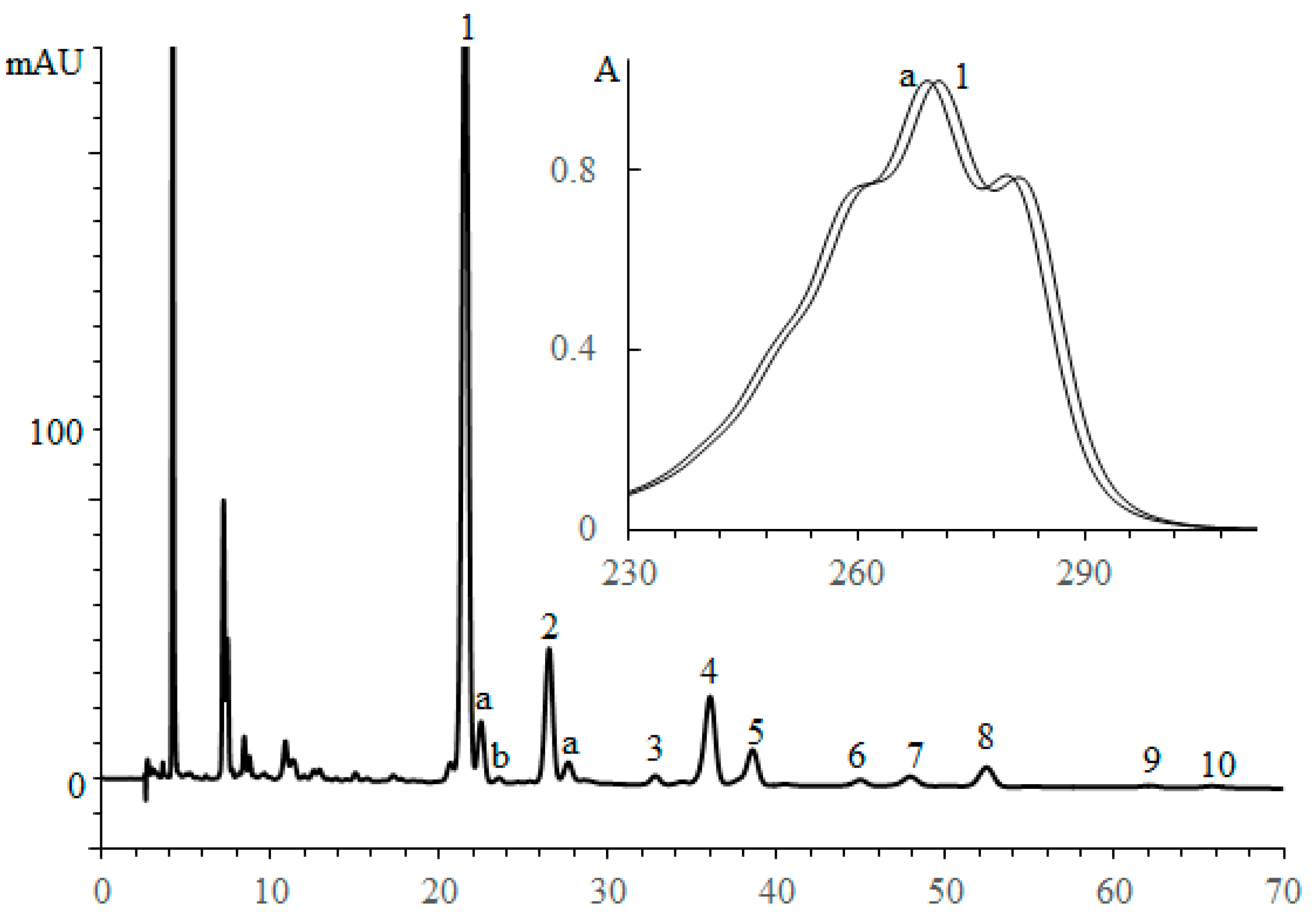
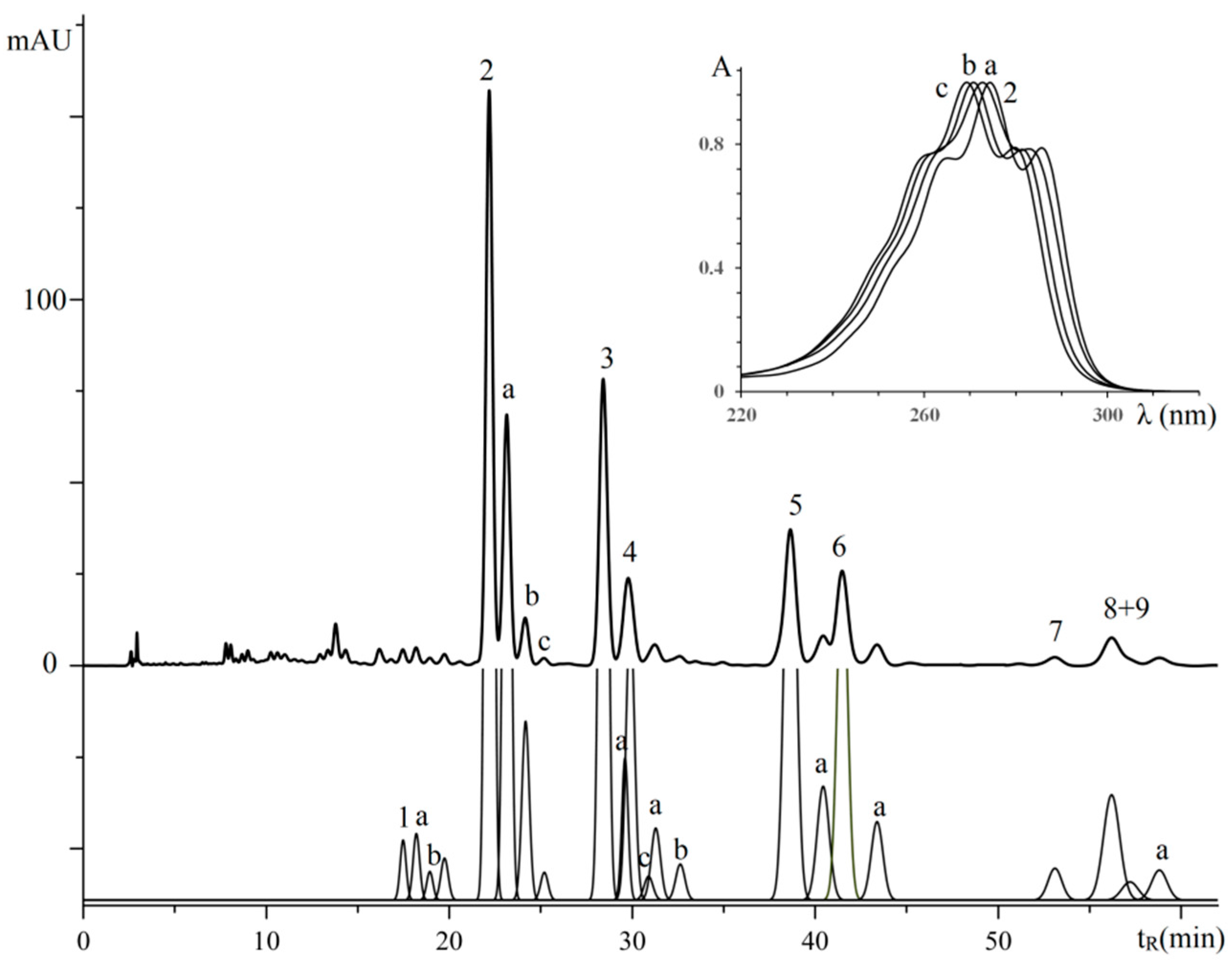
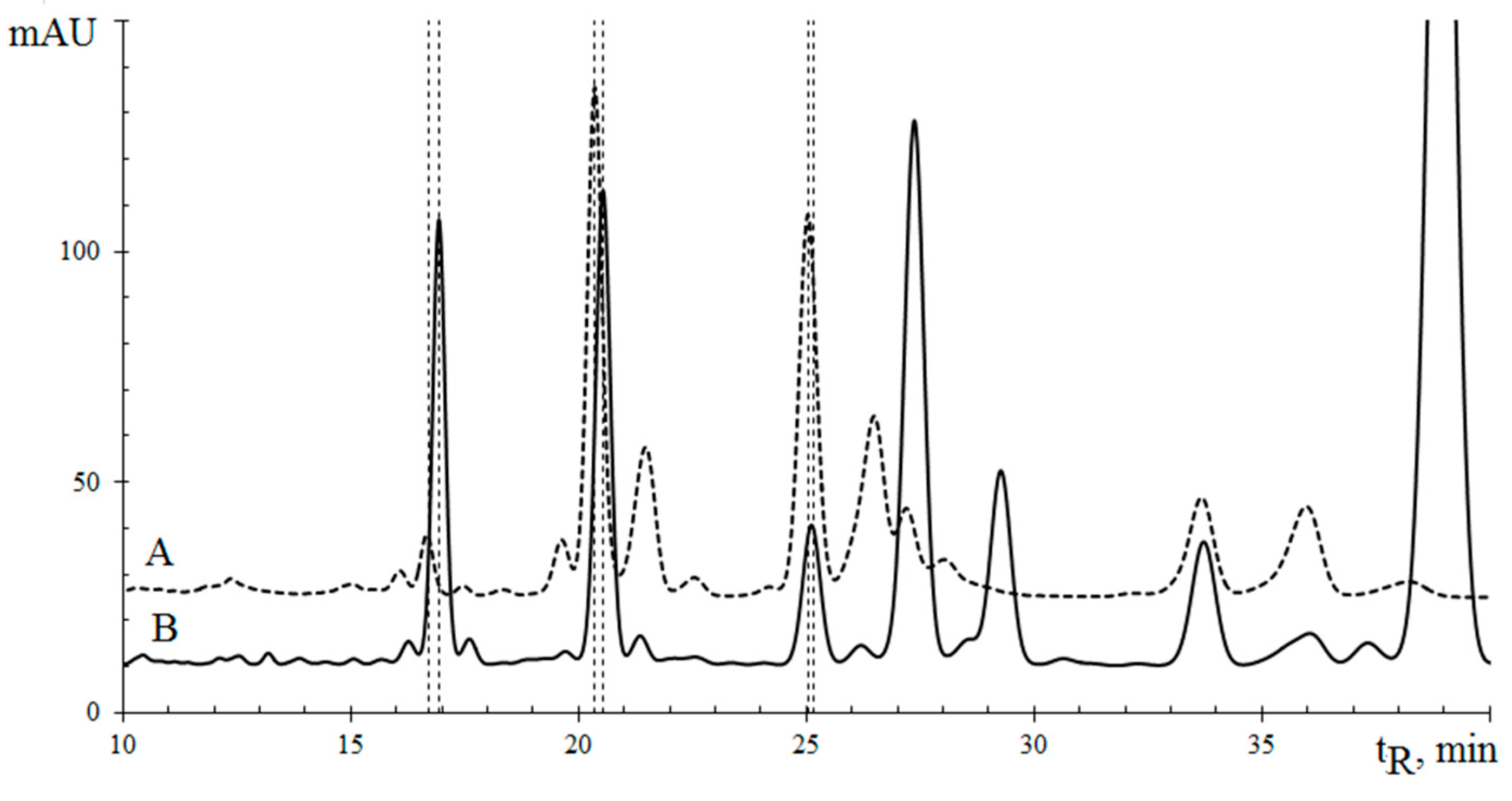
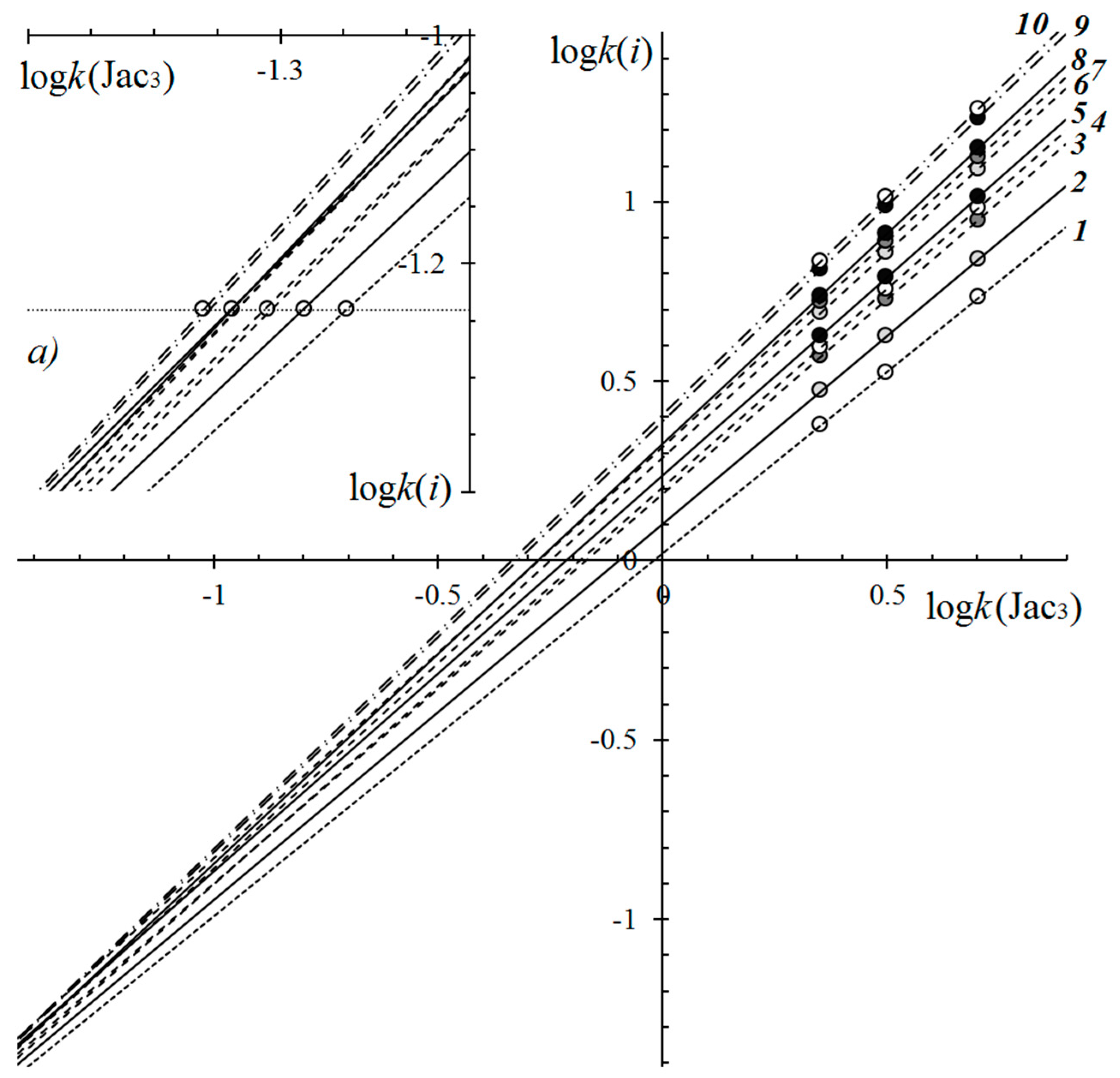
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | |||
|---|---|---|---|---|---|---|---|---|---|
| X→L | L→O | O→P | P→S | ||||||
| 1 | X b3 | 16.94 | 0.761 | 4.8 | 873.8 | ||||
| 2 | X2L | 20.54 | 0.858 | 0.097 | 9.1 | 875.8 | |||
| 3 | XL2 | 25.11 | 0.956 | 0.098 | 5.6 | 877.7 | |||
| 4 | X2O | 27.37 | 0.998 | 0.140 | 13.3 | 877.7 | |||
| 5 | X2P | 29.27 | 1.030 | 0.032 | 4.6 | 851.8 | |||
| 6 | XLO | 33.72 | 1.097 | 0.099 | 0.140 | 6.9 | 879.8 | ||
| 7 | XLP | 36.05 | 1.128 | 0.098 | 0.031 | 2.8 | n.d. c | ||
| 8 | X2S | 38.95 | 1.164 | 0.134 | 38.2 | 879.8 | |||
| 9 | XO2 | 45.65 | 1.237 | 0.141 | 1.5 | n.d. | |||
| 10 | XLS(+XOP) | 48.27 | 1.263 | 0.099 | 0.135 | 13.2 | 881.7 + 855.7 | ||
| Middle value | 0.098 | 0.140 | 0.031 | 0.134 | |||||
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | |||
|---|---|---|---|---|---|---|---|---|---|
| X→L | L→O | O→P | P→S | ||||||
| 1 | X b3 | 15.11 | 0.703 | 6.18 | 873.8 | ||||
| 2 | X2L | 19.04 | 0.820 | 0.118 | 18.43 | 875.8 | |||
| 3 | XL2 | 24.20 | 0.939 | 0.118 | 2.96 | 877.7 | |||
| 4 | X2O | 25.39 | 0.962 | 0.141 | 17.18 | 877.7 | |||
| 5 | X2P | 27.10 | 0.993 | 0.031 | 4.33 | 851.8 | |||
| 6 | XLO | 32.52 | 1.080 | 0.118 | 10.25 | 879.8 | |||
| 7 | XLP | 34.84 | 1.112 | 0.032 | 8.14 | 853.7 | |||
| 8 | X2S | 36.09 | 1.128 | 0.135 | 4.26 | 879.8 | |||
| 9 | XO2 | 44.09 | 1.221 | 0.142 | 7.39 | 881.8 | |||
| 10 | XLS(+XOP) | 46.59 | 1.246 | 0.118 | 0.135 | 20.24 | 881.7 + 855.7 | ||
| Mean value: | 0.118 | 0.141 | 0.032 | 0.135 | |||||
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | |||
|---|---|---|---|---|---|---|---|---|---|
| X→L | L→O | O→P | P→S | ||||||
| 1 | X b3 | 17.39 | 0.775 | 8.25 | 873.7 | ||||
| 2 | X2L | 20.90 | 0.867 | 0.092 | 68.91 | 875.8 | |||
| 3 | XL2 | 25.36 | 0.961 | 0.094 | 6.06 | 877.7 | |||
| 4 | X2O | 27.89 | 1.007 | 0.140 | 4.66 | 877.7 | |||
| 5 | X2P | 29.86 | 1.039 | 0.032 | 5.42 | 851.8 | |||
| 6 | XLO | 34.05 | 1.101 | 0.094 | 0.140 | 3.75 | 879.8 | ||
| 7 | XLP | 36.46 | 1.133 | 0.032 | 1.37 | 853.8 | |||
| 8 | X2S | 39.80 | 1.174 | 0.135 | 1.58 | 879.8 | |||
| 0.093 | 0.140 | 0.032 | 0.135 | ||||||
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| X→L | L→Y | L→O | O→P | P→S | ||||||
| 1a | LnX b2 | 16.08 | 0.735 | 0.56 | 873.7 | |||||
| 1 | X3 | 16.67 | 0.753 | 1.08 | ||||||
| 2a | LnXL | 19.63 | 0.836 | 2.56 | 875.8 | |||||
| 2 | X2L | 20.36 | 0.854 | 0.100 | 12.02 | |||||
| 3 | X2Y c | 21.50 | 0.881 | 0.027 | 2.87 | |||||
| 4 | XL2 | 25.04 | 0.955 | 0.101 | 42.24 | 877.8 | ||||
| 5 | XYL | 26.50 | 0.982 | 0.027 | 9.74 | |||||
| 6 | X2O | 27.19 | 0.995 | 0.141 | 2.28 | 877.8 | ||||
| 7 | XY2 | 28.05 | 1.009 | 0.027 | 4.62 | 877.8 | ||||
| 8 | XLO | 33.67 | 1.096 | 0.101 | 7.69 | 879.7 | ||||
| 9 | XYO | 35.69 | 1.123 | 0.027 | 2.84 | |||||
| 10 | XLP | 36.03 | 1.127 | 0.032 | 5.40 | 853.7 | ||||
| 11 | XYP | 38.20 | 1.155 | 0.027 | 1.56 | |||||
| 12 | XO2 | 45.63 | 1.237 | 0.141 | 0.84 | 881.7 | ||||
| 13 | XLS | 48.11 | 1.261 | 0.134 | 2.31 | 881.7 | ||||
| 14 | XOP | 48.91 | 1.269 | 0.032 | 0.87 | 855.8 | ||||
| 15 | XYS | 51.10 | 1.289 | 0.028 | 0.5 | 881.7 | ||||
| 0.101 | 0.027 | 0.141 | 0.032 | 0.134 | ||||||
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | |||
|---|---|---|---|---|---|---|---|---|---|
| X→L | L→O | O→P | P→S | ||||||
| 1 | X b3 | 16.02 | 0.733 | 1.36 | 873.8 | ||||
| 2 | X2L | 19.83 | 0.841 | 0.108 | 22.50 | 875.8 | |||
| 3 | XL2 | 24.76 | 0.950 | 0.109 | 24.78 | 877.7 | |||
| 4 | X2O | 26.52 | 0.983 | 0.142 | 6.25 | 877.7 | |||
| 5 | X2P | 28.31 | 1.014 | 0.031 | 0.42 | 851.8 | |||
| 6 | XLO | 33.34 | 1.091 | 0.108 | 0.142 | 18.01 | 879.8 | ||
| 7 | XLP | 35.73 | 1.124 | 0.110 | 0.032 | 6.42 | 853.7 | ||
| 8 | X2S | 37.69 | 1.148 | 0.135 | 0.75 | n.d. c | |||
| 9 | XO2 | 45.33 | 1.234 | 0.143 | 3.83 | 879.7 | |||
| 10 | XLS(+XOP) | 47.82 | 1.258 | 0.110 | 0.135 | 15.67 | 881.7 + 855.7 | ||
| Mean value: | 0.109 | 0.142 | 0.032 | 0.135 | |||||
| No. a | TG Composition | tR (min) | Logk | Increment Values | Mole Fraction of TG, % | M/z [M + H+] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| αEl→βEl | αEl→L | L→O | O→P | P→S | ||||||
| 1 | αEl b3 | 16.94 | 0.761 | 0.28 | 873.8 | |||||
| 2 | (αEl)2βEl | 17.62 | 0.782 | 0.020 | ||||||
| 3 | αEl(βEl)2 | 18.36 | 0.802 | 0.021 | ||||||
| 4 | βEl b3 | 19.28 | 0.827 | 0.022 | 0.41 | |||||
| 5 | αEl2L | 20.56 | 0.859 | 0.098 | 15.35 | 875.7 | ||||
| 6 | αElβElL | 21.39 | 0.878 | 0.020 | 16.66 | |||||
| 7 | βEl2L | 22.30 | 0.899 | 0.020 | 21.40 | |||||
| 8 | αElL2 | 25.10 | 0.956 | 0.097 | 6.88 | 877.7 | ||||
| 9 | βElL2 | 26.14 | 0.976 | 0.020 | 10.19 | |||||
| 10 | αEl2O | 27.44 | 0.999 | 0.140 | 2.34 | 877.7 | ||||
| 11 | αElβElO | 28.60 | 1.019 | 0.020 | 0.140 | 2.28 | ||||
| 12 | βEl2O | 29.94 | 1.040 | 0.022 | 3.17 | |||||
| 13 | αElLO | 33.71 | 1.096 | 0.097 | 1.41 | 879.8 | ||||
| 14 | βElLO | 35.21 | 1.117 | 0.020 | 2.07 | |||||
| 15 | αElLP | 36.10 | 1.128 | 0.032 | 4.09 | 853.7 | ||||
| 16 | βElLP | 37.74 | 1.149 | 0.021 | 0.032 | 5.52 | ||||
| 17 | αElLS | 48.24 | 1.262 | 0.134 | 3.01 | 881.8 | ||||
| 18 | βElLS | 50.53 | 1.284 | 0.021 | 0.134 | 4.35 | ||||
| Mean value: | 0.021 | 0.097 | 0.140 | 0.032 | 0.134 | |||||
| No. a | TG Composition | tR (min) | Logk(i) | Content, Mol. % | |
|---|---|---|---|---|---|
| Experimental | Calculated | ||||
| 1 | X b3 | 10.81 | 0.522 | 0.521 | 0.6 |
| 2 | X2L | 12.71 | 0.611 | 0.609 | 13.12 |
| 3 | XL2 | 15.04 | 0.700 | 0.699 | 56.12 |
| 4 | X2O | 16.09 | 0.735 | 0.736 | 1.66 |
| 5 | X2P | 17.08 | 0.766 | 0.767 | 0.79 |
| 6 | XLO | 19.19 | 0.825 | 0.826 | 12.15 |
| 7 | XLP | 20.42 | 0.855 | 0.857 | 8.19 |
| 8 | X2S | 21.44 | 0.879 | 0.887 | 0.6 |
| 9 | XO2 | 24.82 | 0.951 | 0.953 | 2.24 |
| 10 | XLS | 26.09 | 0.975 | 0.976 | 4.52 |
| No. a | TG Composition | tR (min) | Logk(i) | Content, Mol. % | |
|---|---|---|---|---|---|
| Experimental | Calculated | ||||
| 1 | X b3 | 21.55 | 0.882 | 0.880 | 48.44 |
| 2 | X2L | 26.51 | 0.982 | 0.982 | 16.89 |
| 3 | XL2 | 32.81 | 1.084 | 1.085 | 3.63 |
| 4 | X2O | 36.05 | 1.128 | 1.129 | 12.17 |
| 5 | X2P | 38.56 | 1.159 | 1.161 | 5.28 |
| 6 | XLO | 45.09 | 1.231 | 1.233 | 2.56 |
| 7 | XLP | 48.51 | 1.265 | 1.265 | 4.52 |
| 8 | X2S | 52.62 | 1.302 | 1.303 | 4.09 |
| 9 | XO2 | 62.29 | 1.379 | 1.380 | 1.17 |
| 10 | XLS | 66.13 | 1.406 | 1.408 | 1.23 |
| No. a | TG Composition | tR (min) | Logk(i) | Content, Mol. % | |
|---|---|---|---|---|---|
| Experimental | Calculated | ||||
| 1 | X b3 | 17.46 | 0.777 | 0.777 | 1.14 |
| 2 | X2L | 22.18 | 0.896 | 0.898 | 29.14 |
| 3 | XL2 | 28.71 | 1.021 | 1.021 | 27.82 |
| 4 | X2O | 30.08 | 1.043 | 1.045 | 4.56 |
| 5 | XLO | 38.99 | 1.164 | 1.166 | 17.18 |
| 6 | XLP | 41.88 | 1.197 | 1.199 | 12.34 |
| 7 | XO2 | 53.79 | 1.312 | 1.313 | 1.18 |
| 8 + 9 | XLS + XOP | 56.82 | 1.337 | 1.339 | 6.65 |
| Pu | Jac | Cat | αEl | Cal | βEl | βCal | ||
| Pu | 9Z11E13Z | |||||||
| Jac | 8Z10E12Z | 0.009 | ||||||
| Cat | 9E11E13Z | 0.017 | 0.008 | |||||
| αEl | 9Z11E13E | 0.020 | 0.011 | 0.003 | ||||
| Cal | 8E10E12Z | 0.025 | 0.016 | 0.008 | 0.005 | |||
| βEl | 9E11E13E | 0.041 | 0.032 | 0.024 | 0.021 | 0.016 | ||
| βCal | 8E10E12E | 0.049 | 0.040 | 0.032 | 0.029 | 0.024 | 0.010 | |
| TG Structure | Coefficients of Equation (1) | X, Conjugated Octadecatrienoic Acid | ||||||
|---|---|---|---|---|---|---|---|---|
| Pu | Jac | Cat | αEl | Cal | βEl | βCal | ||
| X3 | a1 | 1.000 | 1.011 | 1.014 | 1.010 | 1.021 | 1.035 | 1.054 |
| a0 | 0.000 | 0.022 | 0.041 | 0.052 | 0.058 | 0.100 | 0.113 | |
| X2L | a1 | 1.042 | 1.048 | 1.052 | 1.047 | 1.054 | 1.062 | n.d. |
| a0 | 0.089 | 0.104 | 0.116 | 0.123 | 0.127 | 0.154 | n.d. | |
| XL2 | a1 | 1.091 | 1.086 | 1.090 | 1.085 | 1.092 | 1.087 | n.d. |
| a0 | 0.174 | 0.187 | 0.190 | 0.195 | 0.195 | 0.212 | n.d. | |
| X2O | a1 | 1.101 | 1.106 | 1.112 | 1.104 | 1.115 | 1.120 | n.d. |
| a0 | 0.189 | 0.206 | 0.215 | 0.224 | 0.225 | 0.255 | n.d. | |
| X2P | a1 | 1.105 | 1.103 | n.d. | 1.107 | 1.118 | n.d. | n.d. |
| a0 | 0.218 | 0.239 | n.d. | 0.253 | 0.256 | n.d. | n.d. | |
| XLO | a1 | 1.147 | 1.144 | 1.151 | 1.144 | 1.152 | 1.135 | n.d. |
| a0 | 0.276 | 0.281 | 0.289 | 0.295 | 0.294 | 0.317 | n.d. | |
| XLP | a1 | 1.148 | 1.146 | 1.155 | 1.146 | 1.156 | 1.146 | n.d. |
| a0 | 0.307 | 0.319 | 0.318 | 0.325 | 0.323 | 0.346 | n.d. | |
| X2S | a1 | n.d. | 1.170 | n.d. | 1.171 | 1.183 | n.d. | n.d. |
| a0 | n.d. | 0.327 | n.d. | 0.343 | 0.345 | n.d. | n.d. | |
| XO2 | a1 | 1.209 | 1.206 | 1.213 | 1.200 | n.d. | n.d. | n.d. |
| a0 | 0.374 | 0.387 | 0.387 | 0.396 | n.d. | n.d. | n.d. | |
| XLS | a1 | 1.216 | 1.210 | 1.218 | 1.214 | n.d. | 1.216 | n.d. |
| a0 | 0.394 | 0.408 | 0.408 | 0.412 | n.d. | 0.434 | n.d. | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Nguyen, A.; Deineka, V.; Deineka, L.; Vu Thi Ngoc, A. Comparison of Separation of Seed Oil Triglycerides Containing Isomeric Conjugated Octadecatrienoic Acid Moieties by Reversed-Phase HPLC. Separations 2017, 4, 37. https://doi.org/10.3390/separations4040037
Van Nguyen A, Deineka V, Deineka L, Vu Thi Ngoc A. Comparison of Separation of Seed Oil Triglycerides Containing Isomeric Conjugated Octadecatrienoic Acid Moieties by Reversed-Phase HPLC. Separations. 2017; 4(4):37. https://doi.org/10.3390/separations4040037
Chicago/Turabian StyleVan Nguyen, Anh, Victor Deineka, Lumila Deineka, and Anh Vu Thi Ngoc. 2017. "Comparison of Separation of Seed Oil Triglycerides Containing Isomeric Conjugated Octadecatrienoic Acid Moieties by Reversed-Phase HPLC" Separations 4, no. 4: 37. https://doi.org/10.3390/separations4040037




