Gram-Scale Purification of Dihydrorobinetin from Robinia pseudoacacia L. Wood by Centrifugal Partition Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extraction Method
2.4. HPLC Analysis
2.5. HPLC-MS Analyses
2.6. Centrifugal Partition Chromatography Purification
3. Results and Discussion
3.1. Extraction Optimisation
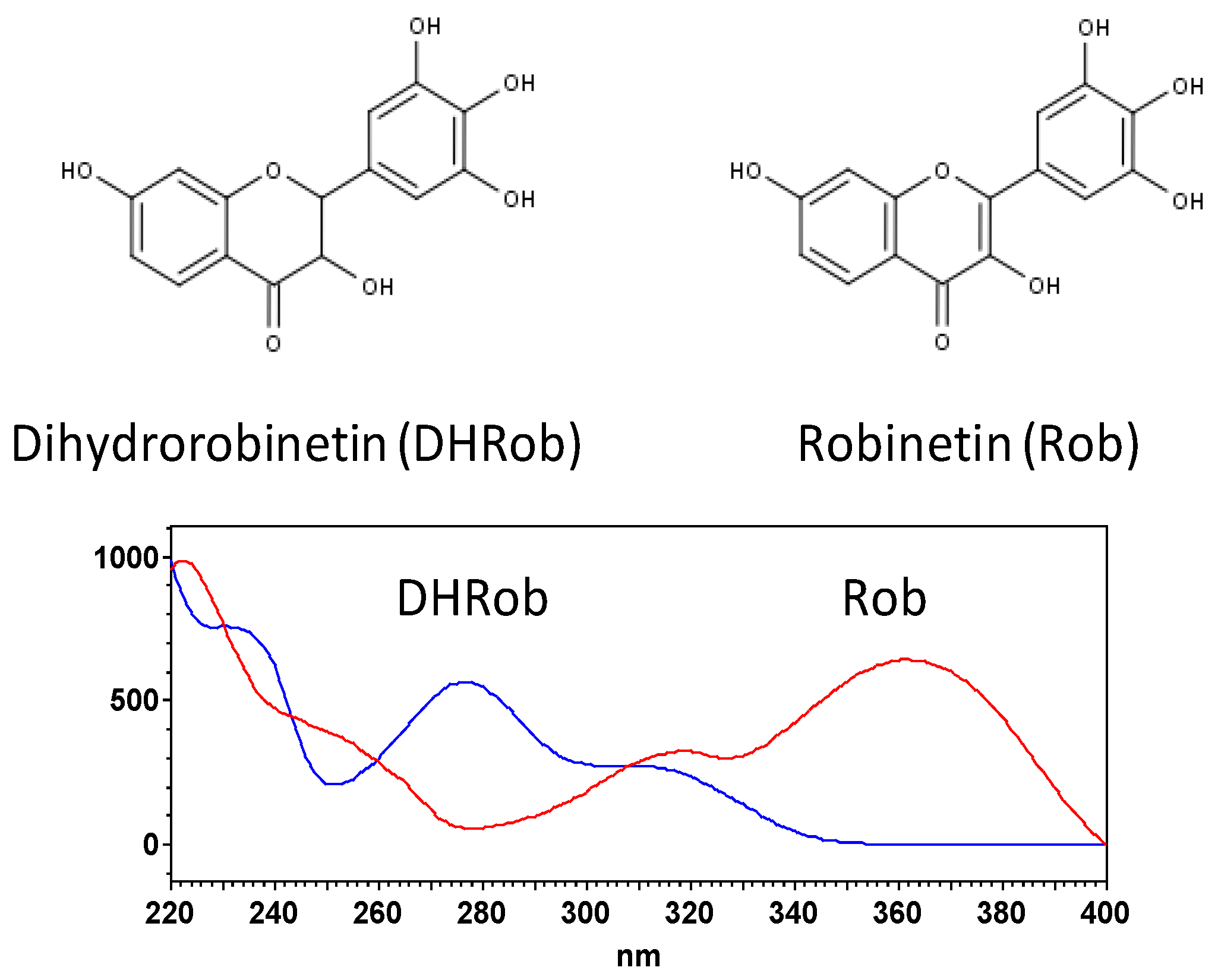
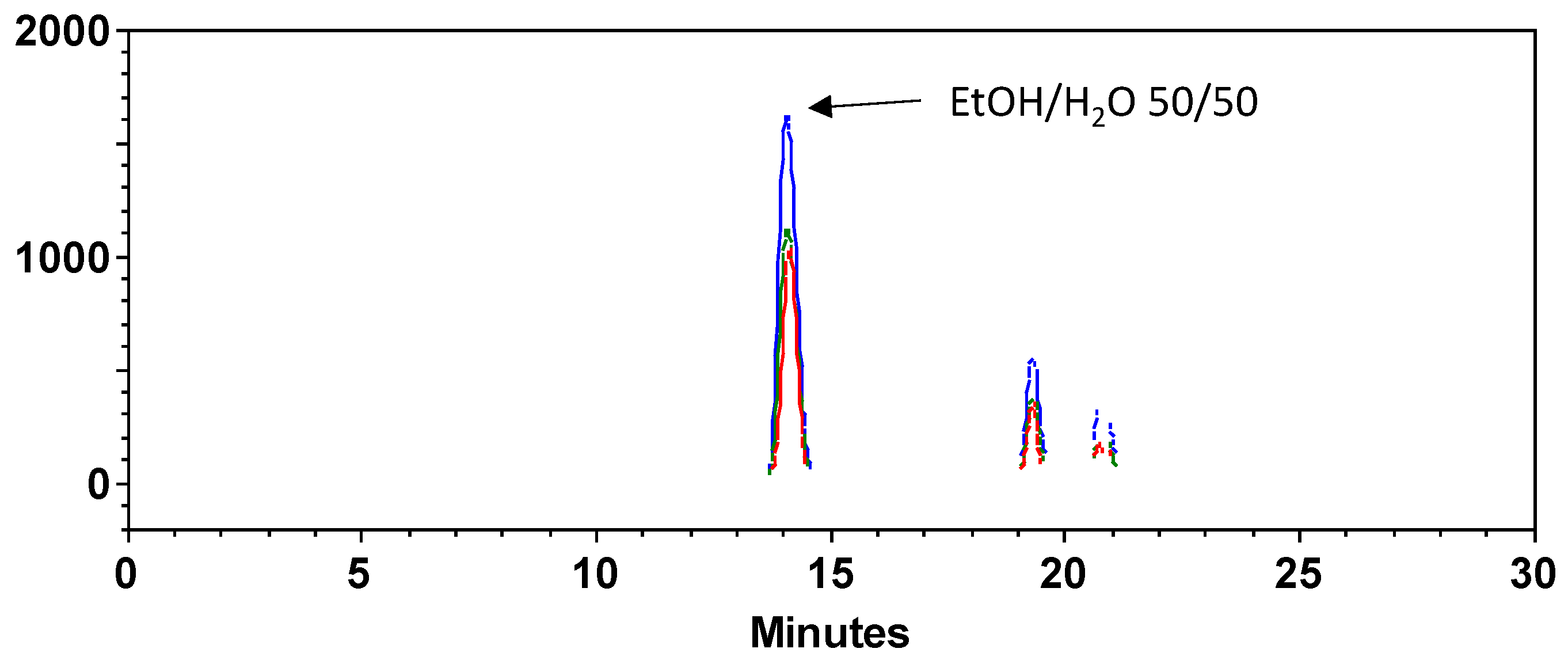
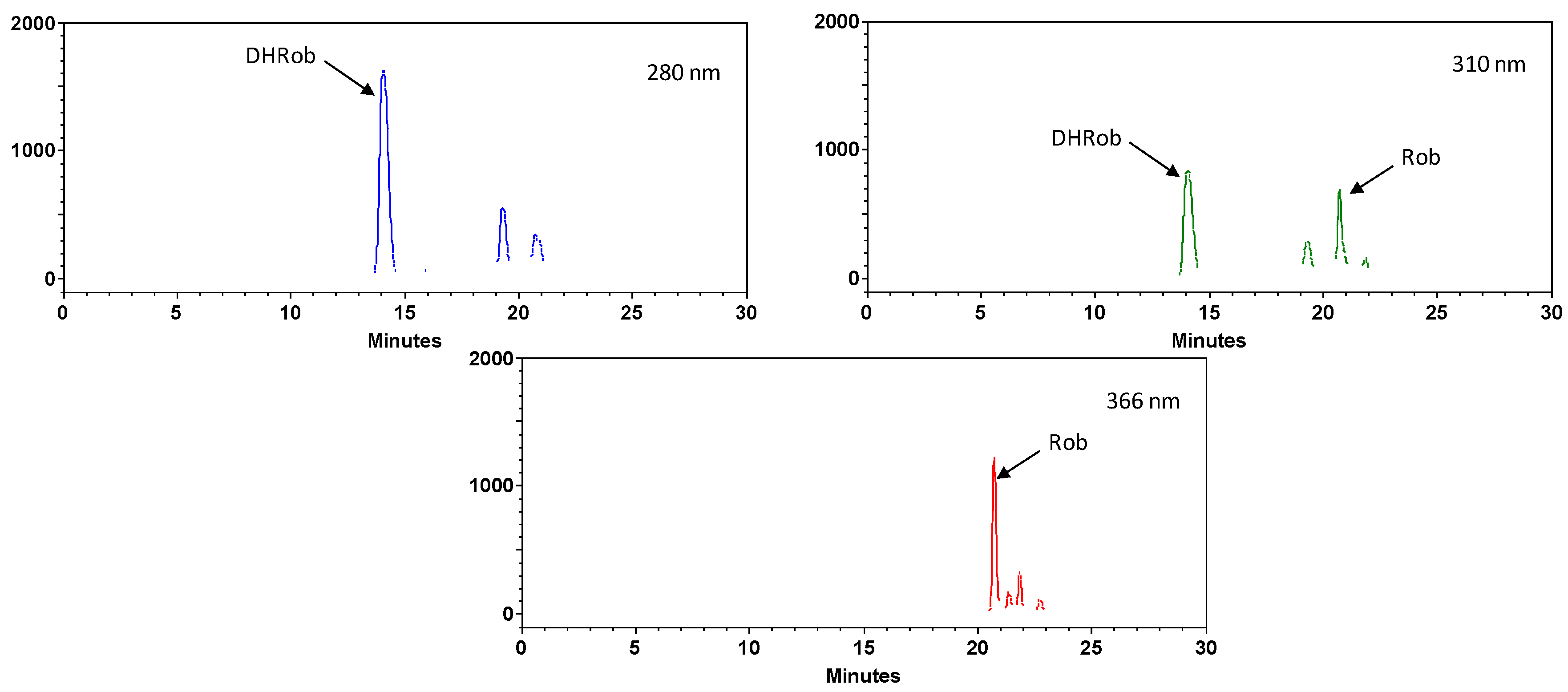
3.2. HPLC Analysis of the Optimized Extract
3.3. DHRob Purification by Centrifugal Partition Chromatography
3.4. HPLC-MS Analysis of CPC Fractions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barbier, C.; Merzeau, D.; Pastuszka, P.; Charpentier, J.-P. Une première collection nationale de robiniers. Forêt-Entreprise 2016, 226, 10–19. [Google Scholar]
- Benesperi, R.; Giuliani, C.; Zanetti, S.; Gennai, M.; Lippi, M.M.; Guidi, T.; Nascimbene, J.; Foggi, B. Forest plant diversity is threatened by Robinia pseudoacacia (black-locust) invasion. Biodivers. Conserv. 2012, 21, 3555–3568. [Google Scholar] [CrossRef]
- Weber, E.; Gut, D. Assessing the risk of potentially invasive plant species in central europe. J. Nat. Conserv. 2004, 12, 171–179. [Google Scholar] [CrossRef]
- Calina, D.; Olah, N.K.; Pătru, E.; Docea, A.; Popescu, H.; Bubulica, M.V. Chromatographic analysis of the flavonoids from Robinia pseudoacacia species. Curr. Health Sci. J. 2013, 39, 232–236. [Google Scholar]
- Veitch, N.C.; Elliott, P.C.; Kite, G.C.; Lewis, G.P. Flavonoid glycosides of the black locust tree, Robinia pseudoacacia (leguminosae). Phytochemistry 2010, 71, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Kocak, M.S.; Tepe, B.; Uren, M.C. An alternative antioxidative and enzyme inhibitory agent from turkey: Robinia pseudoacacia L. Ind. Crops Prod. 2015, 78, 110–115. [Google Scholar] [CrossRef]
- Marinas, I.C.; Oprea, E.; Geana, E.-I.; Chifiriuc, C.; Lazar, V. Antimicrobial and antioxidant activity of the vegetative and reproductive organs of Robinia pseudoacacia. J. Serbian Chem. Soc. 2014, 79, 1363–1378. [Google Scholar] [CrossRef]
- Zhang, L.B.; Lv, J.L.; Zhang, H. Geranyl flavonoids from Robinia pseudoacacia. Nat. Prod. Commun. 2013, 8, 335–336. [Google Scholar] [PubMed]
- Patra, J.K.; Kim, E.S.; Oh, K.; Kim, H.J.; Dhakal, R.; Kim, Y.; Baek, K.H. Bactericidal effect of extracts and metabolites of Robinia pseudoacacia L. On streptococcus mutans and porphyromonas gingivalis causing dental plaque and periodontal inflammatory diseases. Molecules 2015, 20, 6128–6139. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; McLaughlin, J.L. Bioactive flavonoids from the black locust tree, Robinia pseudoacacia. Pharm. Biol. 2000, 38, 229–234. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Dai, G.H.; Zhuge, Y.Y.; Li, Y.B. Protective effect of Robinia pseudoacacia Linn1 extracts against cucumber powdery mildew fungus, Sphaerotheca fuliginea. Crop Prot. 2008, 27, 920–925. [Google Scholar] [CrossRef]
- Sanz, M.; de Simon, B.F.; Esteruelas, E.; Munoz, A.M.; Cadahia, E.; Hernandez, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; de Simon, B.F.; Esteruelas, E.; Munoz, A.M.; Cadahia, E.; Hernandez, T.; Estrella, I.; Pinto, E. Effect of toasting intensity at cooperage on phenolic compounds in acacia (Robinia pseudoacacia) heartwood. J. Agric. Food Chem. 2011, 59, 3135–3145. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Espartero, J.L.; Winterhalter, P.; Garcia-Parrilla, M.C.; Troncoso, A.M. (+)-Dihydrorobinetin: A marker of vinegar aging in acacia (Robinia pseudoacacia) wood. J. Agric. Food Chem. 2009, 57, 9551–9554. [Google Scholar] [CrossRef] [PubMed]
- Pollet, C.; Jourez, B.; Hebert, J. Natural durability of black locust (Robinia pseudoacacia L.) wood grown in wallonia, belgium. Can. J. For. Res. 2008, 38, 1366–1372. [Google Scholar] [CrossRef]
- Sergent, T.; Kohnen, S.; Jourez, B.; Beauve, C.; Schneider, Y.-J.; Vincke, C. Characterization of black locust (Robinia pseudoacacia L.) heartwood extractives: Identification of resveratrol and piceatannol. Wood Sci. Technol. 2014, 48, 1005–1017. [Google Scholar] [CrossRef]
- Beritognolo, I.; Magel, E.; Abdel-Latif, A.; Charpentier, J.P.; Jay-Allemand, C.; Breton, C. Expression of genes encoding chalcone synthase, flavanone 3-hydroxylase and dihydroflavonol 4-reductase correlates with flavanol accumulation during heartwood formation in juglans nigra. Tree Physiol. 2002, 22, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Dünisch, O.; Latorraca, J.V.d.F. The assimilate partitioning importance for heartwood extractives formation in Robinia pseudoacacia L. of different ages. Floresta e Ambient. 2015, 22, 400–407. [Google Scholar] [CrossRef]
- Roux, D.G.; Paulus, E. Condensed tannins 13. Interrelationships of flavonoid components from the heartwood of Robinia pseudoacacia. Biochem. J. 1962, 82, 324–330. [Google Scholar] [PubMed]
- Magel, E.; Jay-Allemand, C.; Ziegler, H. Formation of heartwood substances in the stemwood of Robinia pseudoacacia L. II. Distribution of nonstructural carbohydrates and wood extractives across the trunk. Trees 1994, 8, 165–171. [Google Scholar] [CrossRef]
- Latorraca, J.V.; Dunisch, O.; Koch, G. Chemical composition and natural durability of juvenile and mature heartwood of Robinia pseudoacacia L. Anais Acad Bras. Cienc. 2011, 83, 1059–1068. [Google Scholar] [CrossRef]
- Smith, A.L.; Campbell, C.L.; Walker, D.B.; Hanover, J.W. Extracts from black locust äs wood preservatives: Extraction of decay resistance from black locust heartwood. Holzforschung 1989, 43, 293–296. [Google Scholar] [CrossRef]
- Foucault, A.P.; Chevolot, L. Counter-current chromatography: Instrumentation, solvent selection and some recent applications to natural product purification. J. Chromatogr A 1998, 808, 3–22. [Google Scholar] [CrossRef]
- Ito, Y. Chromatography: Liquid/countercurrent liquid chromatography. In Encyclopedia of Separation Science; Academic Press: Oxford, UK, 2000; pp. 573–583. [Google Scholar]
- Challal, S.; Queiroz, E.F.; Debrus, B.; Kloeti, W.; Guillarme, D.; Gupta, M.P.; Wolfender, J.L. Rational and efficient preparative isolation of natural products by MPLC-UV-ELSD based on HPLC to MPLC gradient transfer. Planta Med. 2015, 81, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, A.; Favre-Godal, Q.; Zhang, J.; Marcourt, L.; Ebrahimi, S.N.; Wang, S.; Fan, P.; Lou, H.; Guillarme, D.; Queiroz, E.F.; et al. Preparative scale MS-guided isolation of bioactive compounds using high-resolution flash chromatography: Antifungals from chiloscyphus polyanthos as a case study. Planta Med. 2016, 82, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Guo, Q.S.; Wang, G.S. Preparative separation and purification of the total flavonoids in scorzonera austriaca with macroporous resins. Molecules 2016, 21, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Hamburger, M. Gram-scale purification of dehydroevodiamine from evodia rutaecarpa fruits, and a procedure for selective removal of quaternary indoloquinazoline alkaloids from evodia extracts. Fitoterapia 2014, 94, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Ganzera, M. Supercritical fluid chromatography-theoretical background and applications on natural products. Planta Med. 2015, 81, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr A 2006, 1112, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.S.; Wan, J.Z.; Ding, W.J.; Wu, X.F.; Xie, Z.Y. One-step separation and purification of two chromones and one pyrone from aloe barbadensis miller: A comparison between reversed-phase flash chromatography and high-speed counter current chromatography. Phytochem. Anal. 2014, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Borie, N.; Chollet, S.; Perret, J.; Barbet-Massin, C.; Berger, M.; Dayde, J.; Renault, J.H. Intensified separation of steviol glycosides from a crude aqueous extract of stevia rebaudiana leaves using centrifugal partition chromatography. Planta Med. 2015, 81, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Bouju, E.; Berthod, A.; Faure, K. Scale-up in centrifugal partition chromatography: The "Free-space between peaks" Method. J. Chromatogr. A 2015, 1409, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Himbert, F.; Dreux, M.; Pennanec, R.; Chaimbault, P.; Elfakir, C.; Lafosse, M. Le split en mode actif: Une approche innovante du couplage de la chromatographie liquide en phase normale à la spectrométrie de masse. Spectra Analyse 2003, 32, 29–31. (In French) [Google Scholar]
- Berthod, A.; Hassoun, M.; Harris, G. Using the liquid nature of the stationary phase: The elution-extrusion method. J. Liquid Chromatogr. Related Technol. 2005, 28, 1851–1866. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. On-line hyphenation of centrifugal partition chromatography and high pressure liquid chromatography for the fractionation of flavonoids from Hippophaë rhamnoides L. Berries. J. Chromatogr. A 2011, 1218, 6173–6178. [Google Scholar] [CrossRef] [PubMed]
- Toribio, A.; Destandau, E.; Elfakir, C.; Lafosse, M. Hyphenation of centrifugal partition chromatography with electrospray ionization mass spectrometry using an active flow-splitter device for characterization of flavonol glycosides. Rapid Commun. Mass Spectrom. 2009, 23, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive molecules in Kalanchoe pinnata leaves: Extraction, purification, and identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Hassoun, M.; Ruiz-Angel, M. Alkane effect in the arizona liquid systems used in countercurrent chromatography. Anal. Bioanal. Chem. 2005, 383, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.; Steynberg, J.P.; Steynberg, P.J.; Brandt, E.V.; Ferreira, D. Oligomeric fiavanoids. Part 18 a dimeric prorobinetinidins from robiniapseudacacia. Tetrahedron 1995, 51, 2339–2352. [Google Scholar] [CrossRef]
- Roux, D.G.; Paulus, E. Condensed tannins. 14. Formation of (−)-3′,4′,5′,7-tetrahydroxyflavanone and (+)-3′,4′,5′,7-tetrahydroxyflavan-4-ol by interconversion from (+)-dihydrorobinetin, and synthesis of their racemates. Biochem. J. 1962, 8, 416–421. [Google Scholar] [CrossRef]
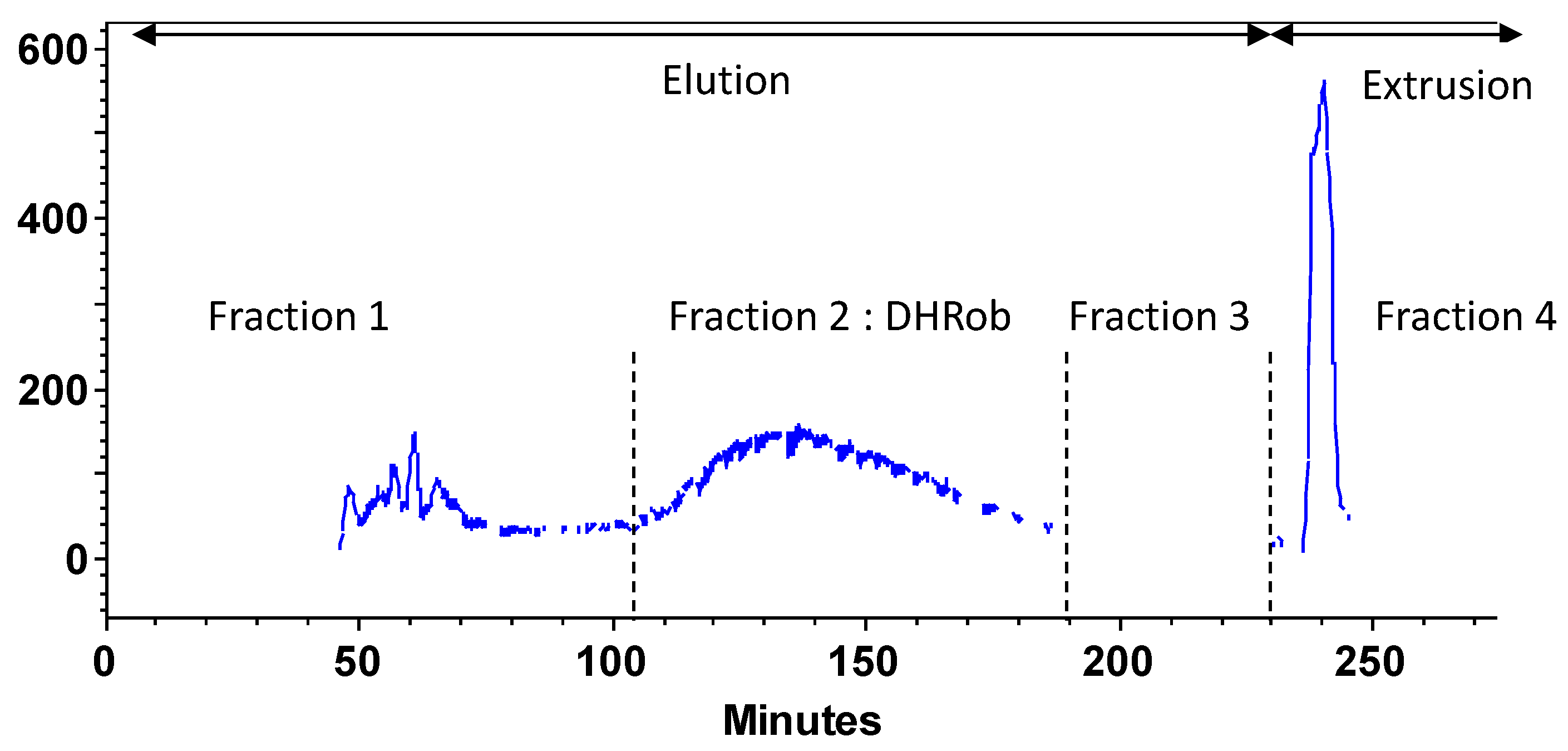
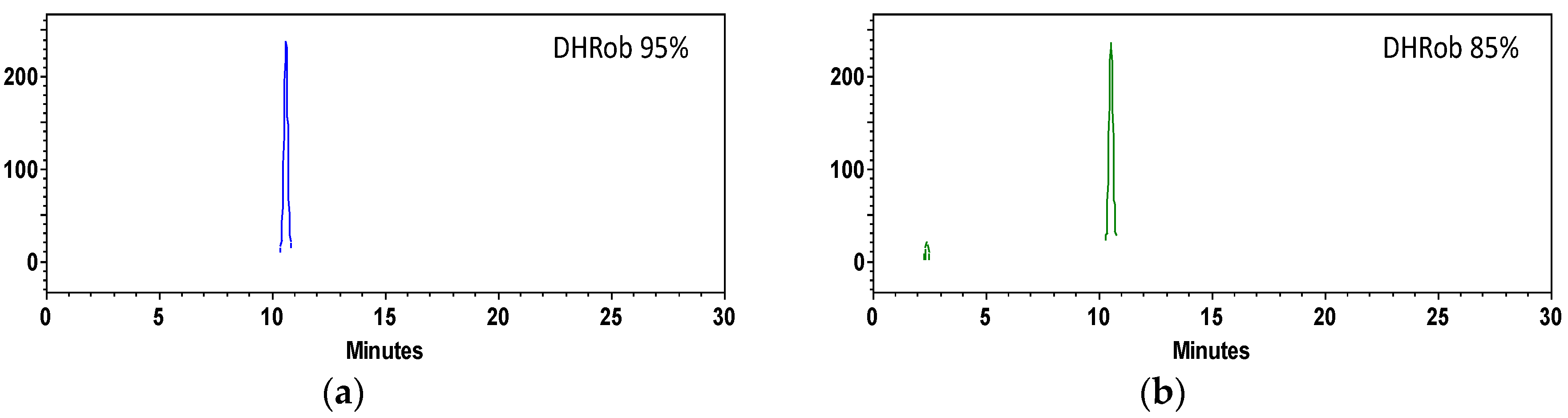
| Parameter | Value | DHRob Concentration mg·L−1 |
|---|---|---|
| Preliminary solvent screening | H2O | 240 |
| MeOH/H2O 80/20 | 820 | |
| EtOH/H2O 80/20 | 870 | |
| Acetone/H2O 80/20 | 780 | |
| Optimization in EtOH/H2O 80/20 | ||
| Temperature | 15–40 °C | 740 ± 29 |
| Rw/s | 1% | 120 |
| 5% | 770 | |
| 9% | 1380 | |
| 13% | 1782 | |
| Time | 1 h | 1050 |
| 2 h | 1500 | |
| 4 h | 2000 | |
| 6 h | 1950 | |
| 24 h | 2023 | |
| Optimized extraction | ||
| EtOH/H2O 50/50 | 2500 | |
| Sphase | 38% | |
| Dphase | 5.5% |
| CPC Column | Biphasic System | Partition Coefficient | Sample Loading | DHRob Recovery |
|---|---|---|---|---|
| 50 mL | Hept/EtOAc/MeOH/H2O 1:4:1:4 | KDHRob = 1.4 KRob = 3.8 | 35 mg crude extract | 11.3 mg |
| 50 mL | Hept/EtOAc.MeOH/H2O 1:4:1:4 | 500 mg crude extract Low solubility | - | |
| 50 mL | EtOAc/MeOH/H2O 1:0.05:1 | KDHRob = 3.2 KRob = ∞ | 495 mg S phase | 170 mg purity 93% |
| 200 mL | EtOAc/MeOH/H2O 1:0.05:1 | 5 g S phase | 1.308 g purity 95% 0.675 g purity 85% |
| Fraction | RT (min) | UV (nm) | MS (m/z) | Compound |
|---|---|---|---|---|
| 1 | 3.4 | 281–320 | 181 | Hydroxycinamic acid derivative |
| 4.3 | 280 | 303, 285, 167, 137 | Leucorobinetinidin isomer | |
| 5.3 | 280 | 303, 285, 167, 137 | Leucorobinetinidin isomer | |
| 9.4 | 280 | 319, 301 | Pentahydroxydihydroflavonol | |
| 10.6 | 280–320 | 303, 285 | Tetrahydroxydihydroflavonol | |
| 21.6 | 288 | 589, 449, 301 | Dimeric prorobinetinidin | |
| 22.4 | 280–310 | 605, 421 | ||
| 23.2 | 280–310 | 589, 419 | Dimeric prorobinetinidin | |
| 23.9 | 280–310 | 603, 585, 567, 457 | ||
| 24.1 | 290 | 607, 467, 301 | ||
| 2 | 14.8 | 280–310 | 303, 285 | Dihydrorobinetin |
| 17.1 | 280–310 | 303, 285 | Tetrahydroxydihydroflavonol | |
| 3 | 27.9 | 575, 423, 287 | ||
| 28.8 | 589, 419 | Dimeric prorobinetinidin | ||
| 4 | 19.4 | 290 | 319 | Pentahydroxydihydroflavonol |
| 21.9 | 290 | 319 | Pentahydroxydihydroflavonol | |
| 23.6 | 280–310 | 317, 299, 289, 284, 274 | Trihydroxymethoxydihydroflavonol | |
| 23.8 | 280–310 | 287, 269, 259, 243, 225 | Fustin | |
| 25.4 | 280–310 | 287 | Robtin | |
| 27.9 | 260–360 | 301 | Robinetin | |
| 29.1 | 260–396 | 285, 149 | Tetrahydroxyaurone | |
| 29.4 | 280–310 | 271 | Butin | |
| 30.0 | 260–384 | 287, 269, 151 | Robtein | |
| 30.3 | 280–310 | 255 | Liquiritigenin | |
| 30.9 | 260–380 | 271, 253, 135 | Butein | |
| 31.8 | 370 | 255 | Isoliquiritigenin |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Destandau, E.; Charpentier, J.-P.; Bostyn, S.; Zubrzycki, S.; Serrano, V.; Seigneuret, J.-M.; Breton, C. Gram-Scale Purification of Dihydrorobinetin from Robinia pseudoacacia L. Wood by Centrifugal Partition Chromatography. Separations 2016, 3, 23. https://doi.org/10.3390/separations3030023
Destandau E, Charpentier J-P, Bostyn S, Zubrzycki S, Serrano V, Seigneuret J-M, Breton C. Gram-Scale Purification of Dihydrorobinetin from Robinia pseudoacacia L. Wood by Centrifugal Partition Chromatography. Separations. 2016; 3(3):23. https://doi.org/10.3390/separations3030023
Chicago/Turabian StyleDestandau, Emilie, Jean-Paul Charpentier, Stéphane Bostyn, Sandrine Zubrzycki, Valérie Serrano, Jean-Marc Seigneuret, and Christian Breton. 2016. "Gram-Scale Purification of Dihydrorobinetin from Robinia pseudoacacia L. Wood by Centrifugal Partition Chromatography" Separations 3, no. 3: 23. https://doi.org/10.3390/separations3030023