Photocatalytic Membranes in Photocatalytic Membrane Reactors
Abstract
:1. Introduction
2. Operating Parameters and Limits of Photocatalytic Membranes (PMs)
2.1. Operating Mode
2.2. Typology of Photocatalyst Immobilization
2.3. Photocatalyst Type and Its Characteristics
2.4. Ligth Source
2.5. Feed Characteristics
2.6. Flow Rate over and across the Membrane
3. Preparation and Choice of Materials to Manufacture PMs
3.1. Dip Coating with Photocatalyst Particles
3.2. Electrosprying of Photocatalyst Particles
3.3. Sputtering of Photocatalyst Particles
3.4. Deposition of Gas Phase Photocatalyst Nanoparticles
3.5. Blended and Free-Standing PMs
4. Selected Case Studies
4.1. Ti- and Ag-Based Photocatalytic Membrane Reactors
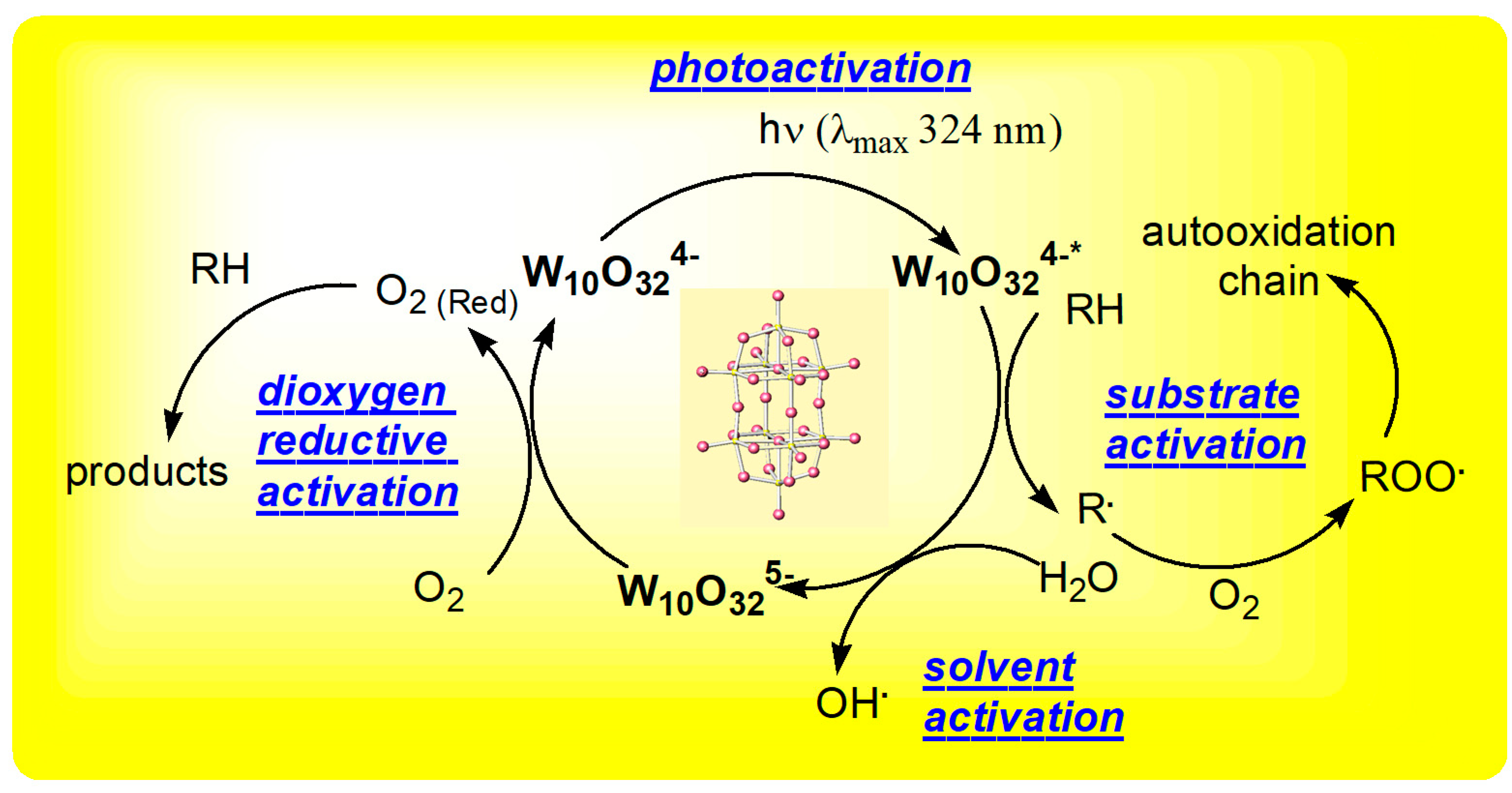
4.2. Polyoxometalates-Based Photocatalytic Membranes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| AOPs | advanced oxidation processes |
| BAT | best available technology |
| BCFM | binary composite fiber membrane |
| BPA | bisphenol A |
| BET | Brunauer-Emmet-Teller |
| CHD | chlorhexidine digluconate |
| CMD | cimetidine |
| CB | conduction band |
| DCF | diclofenac |
| DB | direct black 168 |
| DW | distilled water |
| DMF | N,N-dimethylformamide |
| GO-TiO2 | graphene oxide doped TiO2 |
| HF | hollow fiber |
| IBU | ibuprofen |
| MBR | membrane bioreactor |
| MR | membrane reactor |
| MB | methylene blue |
| MO | methyl orange |
| MWCO | molecular weight cut off |
| MWCNT | multi-walled carbon nanotubes |
| NP | nanoparticle |
| NT | nanotube |
| N-TiO2 | nitrogen doped TiO2 |
| PM | photocatalytic membrane |
| PMR | photocatalytic membrane reactor |
| PAN | polyacrylonitrile |
| PDMS | polydimethylsiloxane |
| PES | polyethersulfone |
| PET | polyethylene terephthalate |
| POMs | polyoxometalates |
| PTFE | polytetrafluoroethylene |
| PU | polyurethane |
| PVC | polyvinylchloride |
| PVDF | polyvinylidene fluoride |
| P(VDF-TrFE) | poly(vinylidenefluoride–trifluoroethylene) |
| PI | process intensification |
| (RfN)4 W10O32 | [CF3(CF2)7(CH2)3]3CH3N)4W10O32 |
| RhB | rhodamine B |
| SEM | scanning electron microscopy |
| SBW | simulated brackish water |
| SSF | stainless steel filter |
| TBAW10 | (n-C4H9N)4W10O32 |
| TCFMs | ternary composite fiber membranes |
| TiO2NTs | TiO2 nanotubes |
| TNF | titanium dioxide nanofiber |
| TOC | total organic carbon |
| UF | ultrafiltration |
| UF-PM | photocatalytic UF membrane |
| UV | ultraviolet |
| VB | valence band |
| XRD | X-ray diffraction |
| WSC | water-soluble chitosan |
References
- Sanchez Marcano, J.G.; Tsotsis, T.T. Membrane Reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005; ISBN 9783527303854. [Google Scholar]
- Wöltinger, J.; Karau, A.; Leuchtenberger, W.; Drauz, K. Membrane Reactors at Degussa. In Technology Transfer in Biotechnology. Advances in Biochemical Engineering; Kragl, U., Ed.; Springer: Berlin, Germany, 2005; Volume 92, ISBN 978-3-540-22412-9. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van der Bruggen, B. CO2 Capture Using Hollow Fiber Membranes: A Review of Membrane Wetting. Energy Fuels 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Charpentier, J.-C. Modern Chemical Engineering in the Framework of Globalization, Sustainability, and Technical Innovation. Ind. Eng. Chem. Res. 2007, 46, 3465–3485. [Google Scholar] [CrossRef]
- Stankiewicz, A. Reactive separations for process intensification: An industrial perspective. Chem. Eng. Process. 2003, 42, 137–144. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Shanbhag, P.V.; Kovvali, A.S. Membrane in a Reactor: A Functional Perspective. Ind. Eng. Chem. Res. 1999, 38, 3715–3737. [Google Scholar] [CrossRef]
- Fontananova, E.; Drioli, E. Catalytic Membranes and Membrane Reactors. In Comprehensive Membrane Science and Engineering; Drioli, E., Giorno, L., Eds.; Elsevier: Oxford, UK, 2010; Volume 3, pp. 109–133. ISBN 978-0-08-093250-7. [Google Scholar]
- Vankelecom, I.F.J. Polymeric Membranes in Catalytic Reactors. Chem. Rev. 2002, 102, 3779–3810. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrogen Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-Y. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Dioos, B.M.L.; Vankelecom, I.F.J.; Jacobs, P.A. Aspects of Immobilisation of Catalysts on Polymeric Supports. Adv. Synth. Catal. 2006, 348, 1413–1446. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Photocatalytic reduction of acetophenone in membrane reactors under UV and visible light using TiO2 and Pd/TiO2 catalysts. Chem. Eng. J. 2015, 274, 307–316. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Phenol and nitrophenol photodegradation using aqueous TiO2 dispersions. In Aquatic and Surface Photochemistry; Helz, G.R., Zepp, R.G., Crosby, D.G., Eds.; Lewis Publishers: London, UK, 1994; p. 419. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Kadhum, A.A.H. Effect of various zinc oxide nanoparticles in membrane photocatalytic reactor for Congo red dye treatment. Sep. Purif. Technol. 2014, 137, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Molinari, R.; Caruso, A.; Argurio, P.; Poerio, T. Degradation of the drugs Gemfibrozil and Tamoxifen in pressurized and de-pressurized membrane photoreactors using suspended polycrystalline TiO2 as catalyst. J. Membr. Sci. 2008, 319, 54–63. [Google Scholar] [CrossRef]
- Lavorato, C.; Argurio, P.; Mastropietro, T.F.; Pirri, G.; Poerio, T.; Molinari, R. Pd/TiO2 doped faujasite photocatalysts for acetophenone transfer hydrogenation in a photocatalytic membrane reactor. J. Catal. 2017, 353, 152–161. [Google Scholar] [CrossRef]
- Brunetti, A.; Barbieri, G.; Drioli, E. Upgrading of a syngas mixture for pure hydrogen production in a Pd–Ag membrane reactor. Chem. Eng. Sci. 2009, 64, 3448–3454. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG–TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Wang, W.Y.; Irawan, A.; Ku, Y. Photocatalytic degradation of Acid Red 4 using a titanium dioxide membrane supported on a porous ceramic tube. Water Res. 2008, 42, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic Photocatalytic Membranes for Water Filtration Under UV and Visible Light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Pastrana-Martìnez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene Oxide Based Ultrafiltration Membranes for Photocatalytic Degradation of Organic Pollutants in Salty Water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.J.; Das, S.; Habeeb Rahman, A.P.; Das, B.; Jayabalan, R.; Behera, S.K.; Suar, M.; Tamhankar, A.J.; Mishra, A.; Lundborg, C.S.; et al. Doped ZnO nanoparticles impregnated on Kaolinite (Clay): A reusable nanocomposite for photocatalytic disinfection of multidrug resistant Enterobacter sp. under visible light. J. Colloid Interface Sci. 2018, 530, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Najma, B.; Kasi, A.K.; Khan Kasi, J.; Akbar, A.; Bokhari, S.M.A.; Stroe, I.R. ZnO/AAO photocatalytic membranes for efficient water disinfection: Synthesis, characterization and antibacterial assay. Appl. Surf. Sci. 2018, 448, 104–114. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Khalili, S. Fouling of WO3 nanoparticle-incorporated PSf membranes in ultrafiltration of landfill leachate and dairy a combined wastewaters: An investigation using model. Chin. J. Chem. Eng. 2017, 25, 741–751. [Google Scholar] [CrossRef]
- Alzahrani, E. Chitosan membrane embedded with ZnO/CuO nanocomposites for the photodegradation of fast green dye under artificial and solar irradiation. Anal. Chem. Insights 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, D.E. A Survey of Applications of Polyoxometalates. Chem. Rev. 1998, 98, 359–388. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.-M. Heterogeneous Photocatalysis: State of the Art and Present Applications. Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Brosillon, S.; Lhomme, L.; Vallet, C.; Bouzaza, A.; Wolbert, D. Gas Phase Photocatalysis and Liquid Phase Photocatalysis: Interdependence and Influence of Substrate Concentration and Photon Flow on Degradation Reaction Kinetics. Appl. Catal. B Environ. 2008, 78, 232–241. [Google Scholar] [CrossRef]
- Sabaghi, V.; Davar, F.; Fereshteh, Z. ZnS nanoparticles prepared via simple reflux and hydrothermal method: Optical and photocatalytic properties. Ceram. Int. 2018, 44, 7545–7556. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, L.; Luo, J.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Reddy, N.L.; Rao, V.N.; Kumari, M.M.; Kakarla, R.R.; Ravi, P.; Sathish, M.; Karthik, M.; Venkatakrishnan, S.M.; Inamuddin. Nanostructured semiconducting materials for efficient hydrogen generation. Environ. Chem. Lett. 2018, 16, 765–796. [Google Scholar] [CrossRef]
- Yamani, Z.H. Comparative Study on Photocatalytic Degradation of Methylene Blue by Degussa P25 Titania: Pulsed Laser Light Versus Continuous Broad Spectrum Lamp Irradiation. Arab. J. Sci. Eng. 2018, 43, 423–432. [Google Scholar] [CrossRef]
- Hayat, K.; Gondal, M.A.; Khaleda, M.M.; Yamani, Z.H.; Ahmed, S. Laser induced photocatalytic degradation of hazardous dye (Safranin-O) using self synthesized nanocrystalline WO3. J. Hazard. Mater. 2011, 186, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Q.; Liu, X.; Yang, L.; Ding, J.; Dong, F.; Li, Y.; Irfana, M.; Zhang, P. Fast photocatalytic degradation of dyes using low power laser-fabricated Cu2O–Cu nanocomposites. RSC Adv. 2018, 8, 20277–20286. [Google Scholar] [CrossRef]
- Dominguez, S.; Laso, J.; Margallo, M.; Aldaco, R.; Rivero, M.J.; Irabien, Á.; Ortiz, I. LCA of greywater management within a water circular economy restorative thinking framework. Sci. Total Environ. 2018, 621, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.Y.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mat. 2018, 344, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Utama, R.; Cox, S.; Le-Clech, P. Performances of submerged membrane photocatalysis reactor during treatment of humic substances. Membr. Water Treat. 2010, 1, 283–296. [Google Scholar] [CrossRef]
- Song, L.; Zhu, B.; Jegatheesan, V.; Gray, S.; Duke, M.; Muthukumaran, S. Treatment of secondary effluent by sequential combination of photocatalytic oxidation with ceramic membrane filtration. Environ. Sci. Pollut. Res. 2018, 25, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Lu, G.; Wang, W.; Wang, R.; Huang, K.; Qiu, Z.; Tao, X.; Dang, Z. Photocatalytic removal of organic phosphate esters by TiO2: Effect of inorganic ions and humic acid. Chemosphere 2018, 206, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, P.; Das, M.R. Hydrothermal assisted decoration of NiS2 and CoS nanoparticles on the reduced graphene oxide nanosheets for sunlight driven photocatalytic degradation of azo dye: Effect of background electrolyte and surface charge. J. Colloid Interface Sci. 2018, 516, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The Combination of Heterogeneous Photocatalysis With Chemical and Physical Operations: A Tool for Improving the Photoprocess Performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Darowna, D.; Wróbel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Gladysz, J.A. Recoverable catalysts. Ultimate goals, criteria of evaluation, and the green chemistry interface. Pure Appl. Chem. 2001, 73, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.L. Homogeneous catalysis. Controlled green oxidation. Nature 1999, 401, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Daels, N.; Radoicic, M.; Radetic, M.; Van Hulle, S.W.; De Clerck, K. Functionalisation of electrospun polymer nanofibre membranes with TiO2 nanoparticles in view of dissolved organic matter photodegradation. Sep. Purif. Technol. 2014, 133, 282–290. [Google Scholar] [CrossRef]
- Wu, G.; Cui, L.; Xu, Y.; Lu, X. Photocatalytic membrane reactor for degradation of phenol in aqueous solution. Fresenius Environ. Bull. 2007, 16, 812–816. [Google Scholar]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Latif, A.A.; Griebel, J.; Prager, A.; Schulze, A. Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Artoshina, O.V.; Rossouw, A.; Semina, V.K.; Nechaev, A.N.; Apel, P.Y. Structural and physicochemical properties of titanium dioxide thin films obtained by reactive magnetron sputtering, on the surface of track-etched membranes. Pet. Chem. 2015, 55, 759–768. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Li, Y.; Qu, J.; Yu, Z.-Z. Fabrication of PAN@TiO2/Ag nanofibrous membrane with high visible light response and satisfactory recyclability for dye photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 622–629. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhang, J.; Sun, Z.; Zhao, J.; Zhang, J.; Zuo, W. Precisely-controlled modification of PVDF membranes with 3D TiO2/ZnO nanolayer: Enhanced anti-fouling performance by changing hydrophilicity and photocatalysis under visible light irradiation. J. Membr. Sci. 2017, 528, 359–368. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Wróbel, R.; Morawski, A.W. A study on the stability of polyethersulfone ultrafiltration membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2015, 495, 176–186. [Google Scholar] [CrossRef]
- Drioli, E.; Fontananova, E. Catalytic membranes embedding selective catalysts: Preparation and applications. In Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Materials and Processes; Barbaro, P., Liguori, F., Eds.; Springer: Berlin, Germany, 2010; Chapter 6; ISBN 978-90-481-3696-4. [Google Scholar]
- Strathmann, H.; Giorno, L.; Drioli, E. An Introduction to Membrane Science and Technology; CNR: Rome, Italy, 2006; ISBN 88-8080-063-9. [Google Scholar]
- Wang, X.; Shi, F.; Huang, W.; Fan, C. Synthesis of high quality TiO2 membranes on alumina supports and their photocatalytic activity. Thin Solid Films 2012, 520, 2488–2492. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.A.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Mohamed, M.A.; Rahman, M.A.; Othman, M.H.D.; Lau, W.J.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Kaijun, Z.; Qingshan, L.; Yu, W. Preparation and performance of PMMA/R-TiO2 and PMMA/A-TiO2 electrospun fibrous films. Integr. Ferroelectr. 2018, 188, 31–43. [Google Scholar] [CrossRef]
- Fischer, K.; Gläser, R.; Schulze, A. Nanoneedle and nanotubular titanium dioxide–PES mixed matrix membrane for photocatalysis. Appl. Catal. B Environ. 2014, 160, 456–464. [Google Scholar] [CrossRef]
- Della Foglia, F.; Chiarello, G.L.; Dozzi, M.V.; Piseri, P.; Bettini, L.G.; Vinati, S.; Ducati, C.; Milani, P.; Selli, E. Hydrogen production by photocatalytic membranes fabricated by supersonic cluster beam deposition on glass fiber filters. Int. J. Hydrogen Energy 2014, 39, 13098–13104. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, L.; Zhang, B.; Xie, Y.; Sun, C.; Jiang, C.; Zhang, Y.; Wang, G. Modification of polyvinylidene fluoride membrane with different shaped α-Fe2O3 nanocrystals for enhanced photocatalytic oxidation performance. Mater. Chem. Phys. 2018, 214, 41–47. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H.; Yang, X.; Li, F.; Yang, S.; He, Y. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci.-Mater. Electron. 2017, 28, 3865–3874. [Google Scholar] [CrossRef]
- Hoseini, S.N.; Pirzaman, A.K.; Aroon, M.A.; Pirbazari, A.E. Photocatalytic degradation of 2,4-dichlorophenol by Co-doped TiO2(Co/TiO2) nanoparticles and Co/TiO2 containing mixed matrix membranes. J. Water Proc. Eng. 2017, 17, 124–134. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Chang, W.; Huang, Z.; Feng, X.; Qi, X.; Li, Z. Efficient photocatalytic degradation of gaseous N,N-dimethylformamide in tannery waste gas using doubly open-ended Ag/TiO2 nanotube array membranes. Appl. Surf. Sci. 2018, 444, 610–620. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate Fibers as Photocatalyst Immobilizing Agents Applied in Hybrid Photocatalytic/Ultrafiltration Water Treatment Processes. Water Res. 2012, 46, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Gieselmann, M.J.; Xu, Q.J. Titania and Alumina Ceramic Membranes. J. Membr. Sci. 1988, 39, 243–258. [Google Scholar] [CrossRef]
- Moosemiller, M.D.; Hill, C.G., Jr.; Anderson, M.A. Physicochemical Properties of Supported γ-Al2O3 and TiO2 Ceramic Membranes. Sep. Sci. Technol. 1989, 24, 641–657. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on Various Reactor Configurations for Coupling Photocatalysis and Membrane Processes in Water Purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. Fabrication of Photocatalytic Membrane and Evaluation Its Efficiency in Removal of Organic Pollutants From Water. Sep. Purif. Technol. 2006, 50, 147–155. [Google Scholar] [CrossRef]
- Wang, Z.-B.; Guan, Y.-J.; Chen, B.; Bai, S.-L. Retention and Separation of 4BS Dye from Wastewater by the N-TiO2 Ceramic Membrane. Desalin. Water Treat. 2016, 57, 16963–16969. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Shen, J.-S.; Zhao, F.; Shao, Z.-D.; Zhong, L.-B.; Zheng, Y.-M. Flexible electrospun MWCNTs/Ag3PO4/PAN ternary composite fiber membranes with enhanced photocatalytic activity and stability under visible-light irradiation. J. Mater. Sci. 2018, 53, 10147–10159. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Yoo, H.N.; Song, K.G.; Lee, J.; Choi, K.J.; Hong, S.W. Titanium Dioxide Nanofibers Integrated Stainless Steel Filter for Photocatalytic Degradation of Pharmaceutical Compounds. J. Hazard. Mater. 2013, 258–259, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Georgi, A.; Kopinke, F.-D. Interaction of Adsorption and Catalytic Reactions in Water Decontamination Processes: Part I. Oxidation of Organic Contaminants with Hydrogen Peroxide Catalyzed by Activated Carbon. Appl. Catal. B Environ. 2005, 58, 9–18. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent Filtration and Solar Photocatalytic Disinfection/Degradation Using High-Performance Ag/TiO2 Nanofiber Membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive Microfiltration Membranes via Directed Synthesis of TiO2 Nanoparticles on the Polymer Surface for Removal of Drugs From Water. J. Membr. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Fischer, K.; Kuhnert, M.; Glaser, R.; Schulze, A. Photocatalytic Degradation and Toxicity Evaluation of Diclofenac by Nanotubular Titanium Dioxide–PES Membrane in a Static and Continuous Setup. RSC Adv. 2015, 5, 16340–16348. [Google Scholar] [CrossRef]
- Pope, M. Heteropoly and Isopoly Oxometalates; Springer: New York, NY, USA, 1983; ISBN 978-3-662-12006-4. [Google Scholar]
- Maldotti, A.; Molinari, A.; Amadelli, R. Photocatalysis with Organized Systems for the Oxofunctionalization of Hydrocarbons by O2. Chem. Rev. 2002, 102, 3811–3836. [Google Scholar] [CrossRef] [PubMed]
- Bonchio, M.; Carraro, M.; Scorrano, G.; Fontananova, E.; Drioli, E. Heterogeneous Photooxidation of Alchols in Water by Photocatalytic Membranes Incorporating Decatungstate. Adv. Synth. Catal. 2003, 345, 1119–1126. [Google Scholar] [CrossRef]
- Mylonas, A.; Papaconstantinou, E. Photocatalytic degradation of phenol and p-cresol by polyoxotungstates. Mechanistic implications. Polyhedron 1996, 15, 3211–3217. [Google Scholar] [CrossRef]
- Texier, I.; Giannotti, C.; Malato, S.; Richter, C.; Delaire, J. Solar photodegradation of pesticides in water by sodium decatungstate. Catal. Today 1999, 54, 297–307. [Google Scholar] [CrossRef]
- Bonchio, M.; Carraro, M.; Gardan, M.; Scorrano, G.; Drioli, E.; Fontananova, E. Hybrid photocatalytic membranes embedding decatungstate for heterogeneous photooxygenation. Top. Catal. 2006, 40, 133–140. [Google Scholar] [CrossRef]
- Fontananova, E.; Donato, L.; Drioli, E.; Lopez, L.; Favia, P.; d’Agostino, R. Heterogenization of Polyoxometalates on the Surface of Plasma-Modified Polymeric Membranes. Chem. Mater. 2006, 18, 1561–1568. [Google Scholar] [CrossRef]
- Drioli, E.; Fontananova, E.; Bonchio, M.; Carraro, M.; Gardan, M.; Scorrano, G. Catalytic Membranes and Membrane Reactors: An Integrated Approach to Catalytic Process with a High Efficiency and a Low Environmental Impact. Chin. J. Catal. 2008, 29, 1152–1158. [Google Scholar] [CrossRef]
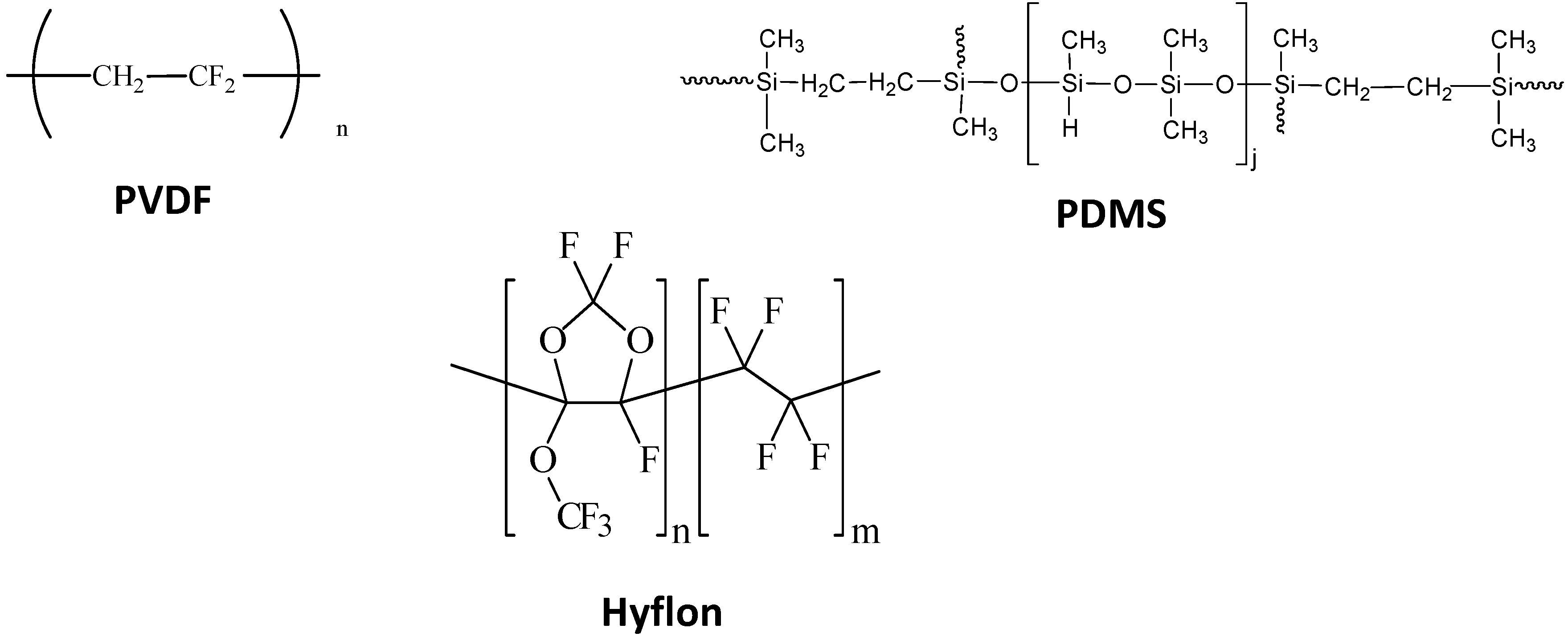
- Carraro, M.; Gardan, M.; Scorrano, G.; Drioli, E.; Fontananova, E.; Bonchio, M. Solvent-free, heterogeneous photooxygenation of hydrocarbons by Hyflon® membranes embedding a fluorous-tagged decatungstate: The importance of being fluorous. Chem. Commun. 2006, 43, 4533–4535. [Google Scholar] [CrossRef]
- Lopez, L.C.; Buonomenna, M.G.; Fontananova, E.; Iacoviello, G.; Drioli, E.; d’Agostino, R.; Favia, P. A New Generation of Catalytic Poly(vinylidene fluoride) Membranes: Coupling Plasma Treatment with Chemical Immobilization of Tungsten-Based Catalysts. Adv. Funct. Mater. 2006, 16, 1417–1424. [Google Scholar] [CrossRef]
- Lopez, L.C.; Buonomenna, M.G.; Fontananova, E.; Drioli, E.; Favia, P.; d’Agostino, R. Immobilization of Tungsten Catalysts on Plasma Modified Membranes. Plasma Processes Polym. 2007, 4, 326–333. [Google Scholar] [CrossRef]
- Favia, P.; Sardella, E.; Gristina, R.; d’Agostino, R. Novel plasma processes for biomaterials: Micro-scale patterning of biomedical polymers. Surf. Coat. Technol. 2003, 169–170, 707–711. [Google Scholar] [CrossRef]
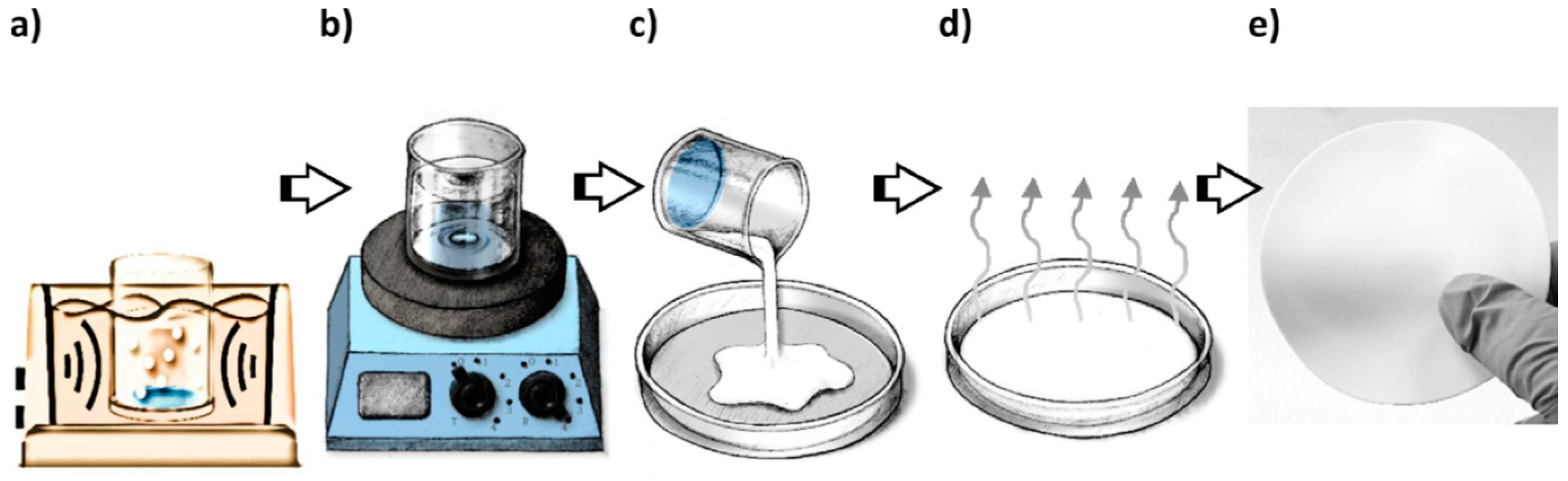
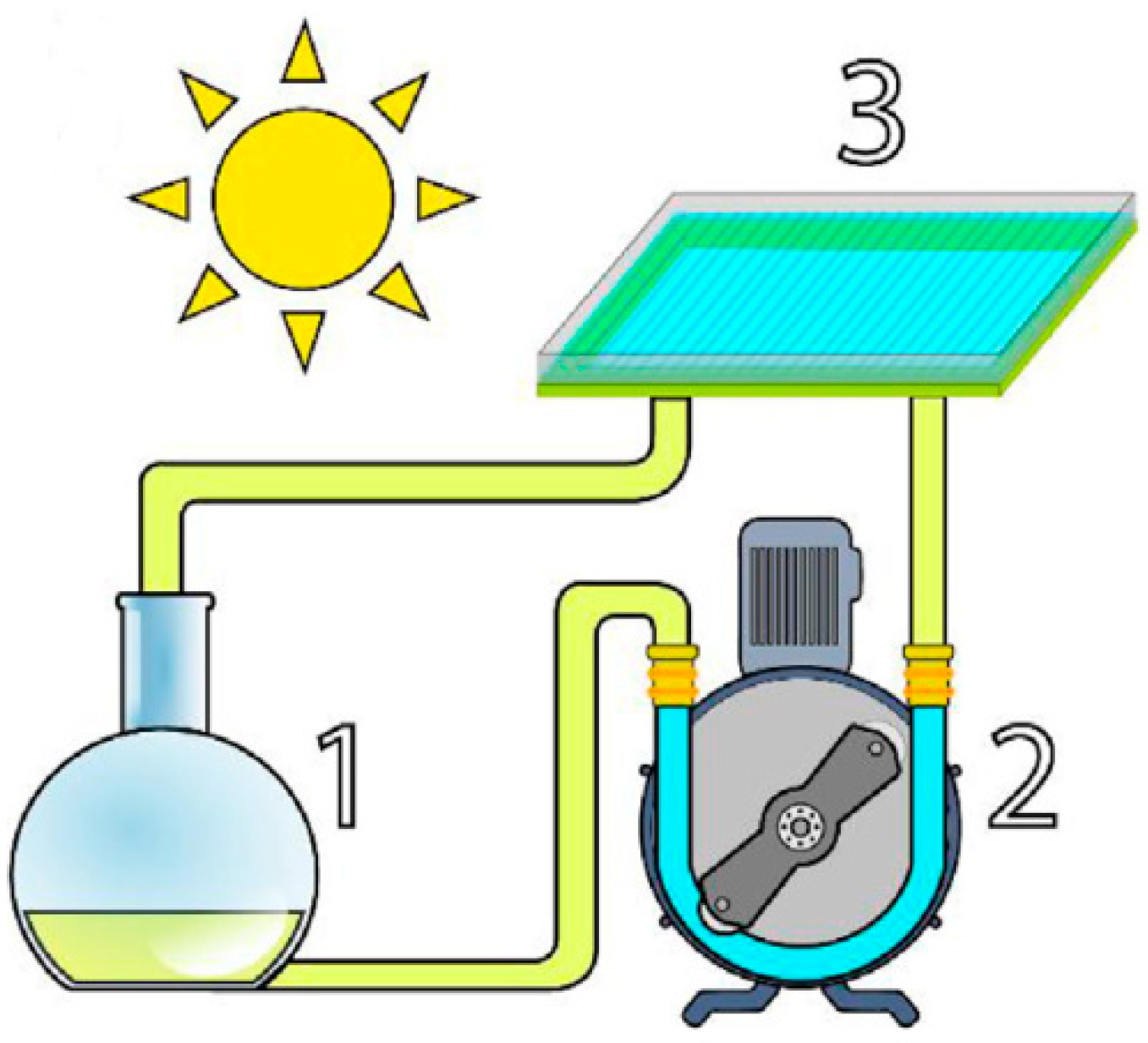
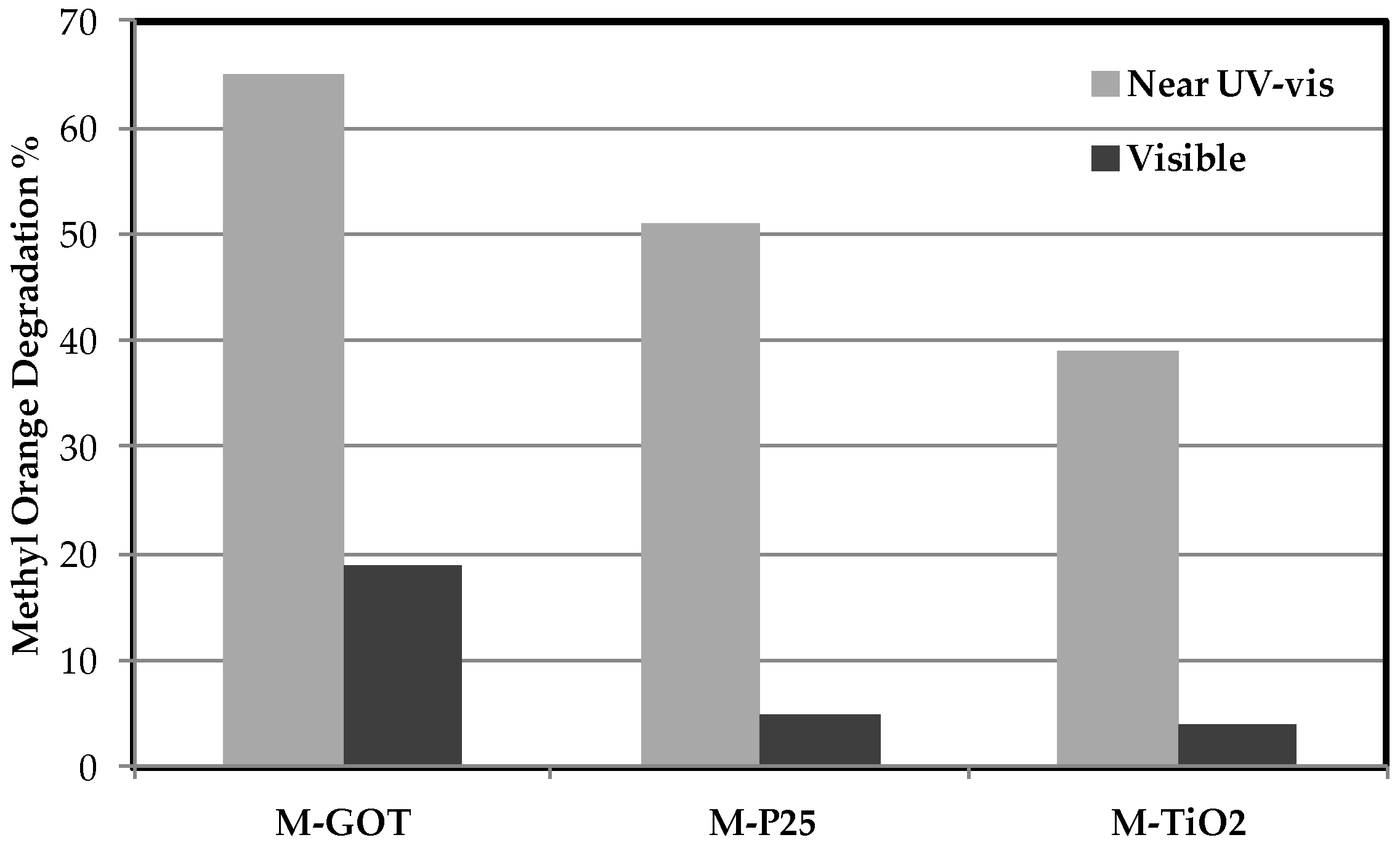

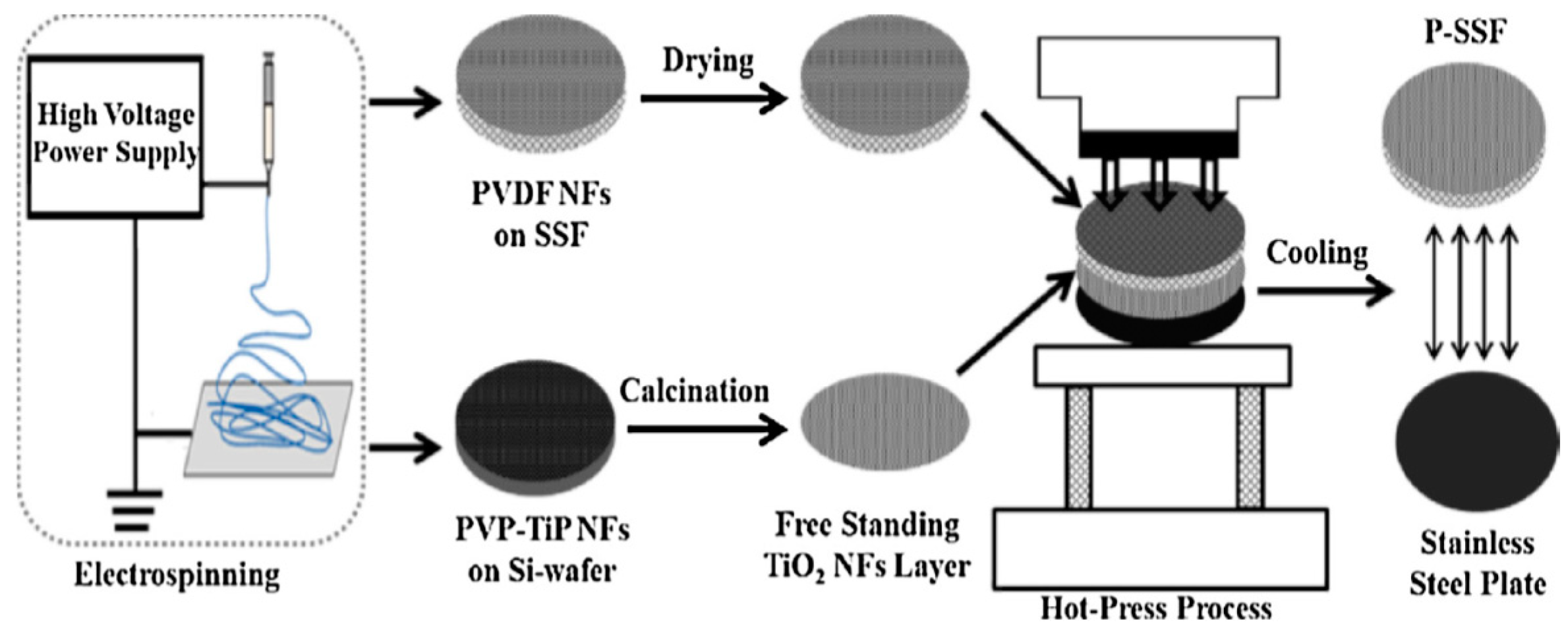
| Flow Rate (mL·min−1) | Feed RhB Concentration (mg·L−1) | Removal Percentage (%) |
|---|---|---|
| 10 | 2 | 88.3 |
| 30 | 2 | 89.4 |
| 50 | 2 | 96.9 |
| 30 | 1 | 89.9 |
| 30 | 4 | 96.4 |
| 30 | 8 | 54.4 |
| BET Specific Surface Area (m2·g−1) | MB Degradation Rate Constant (min−1) | |
|---|---|---|
| Ag/TiO2-nanofiber membrane | 102.3 | 0.0211 |
| TiO2-nanofiber membrane | 85.6 | 0.0137 |
| P25 membrane | / | 0.0076 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. https://doi.org/10.3390/pr6090162
Argurio P, Fontananova E, Molinari R, Drioli E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes. 2018; 6(9):162. https://doi.org/10.3390/pr6090162
Chicago/Turabian StyleArgurio, Pietro, Enrica Fontananova, Raffaele Molinari, and Enrico Drioli. 2018. "Photocatalytic Membranes in Photocatalytic Membrane Reactors" Processes 6, no. 9: 162. https://doi.org/10.3390/pr6090162







