Structure Manipulation of Carbon Aerogels by Managing Solution Concentration of Precursor and Its Application for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Characterizations
3.1.1. XRD Analysis
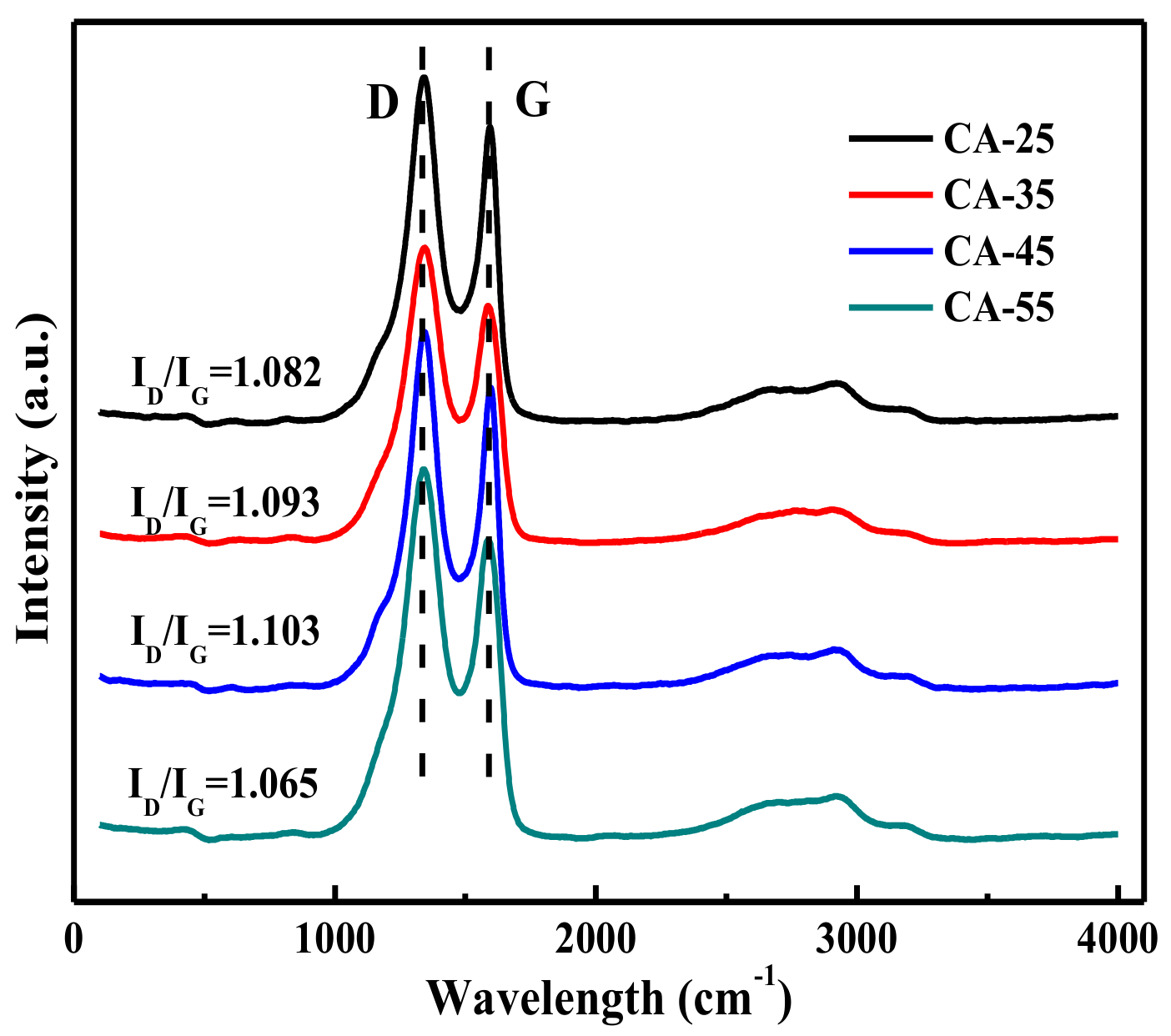
3.1.2. Raman Analysis
3.1.3. FTIR Analysis
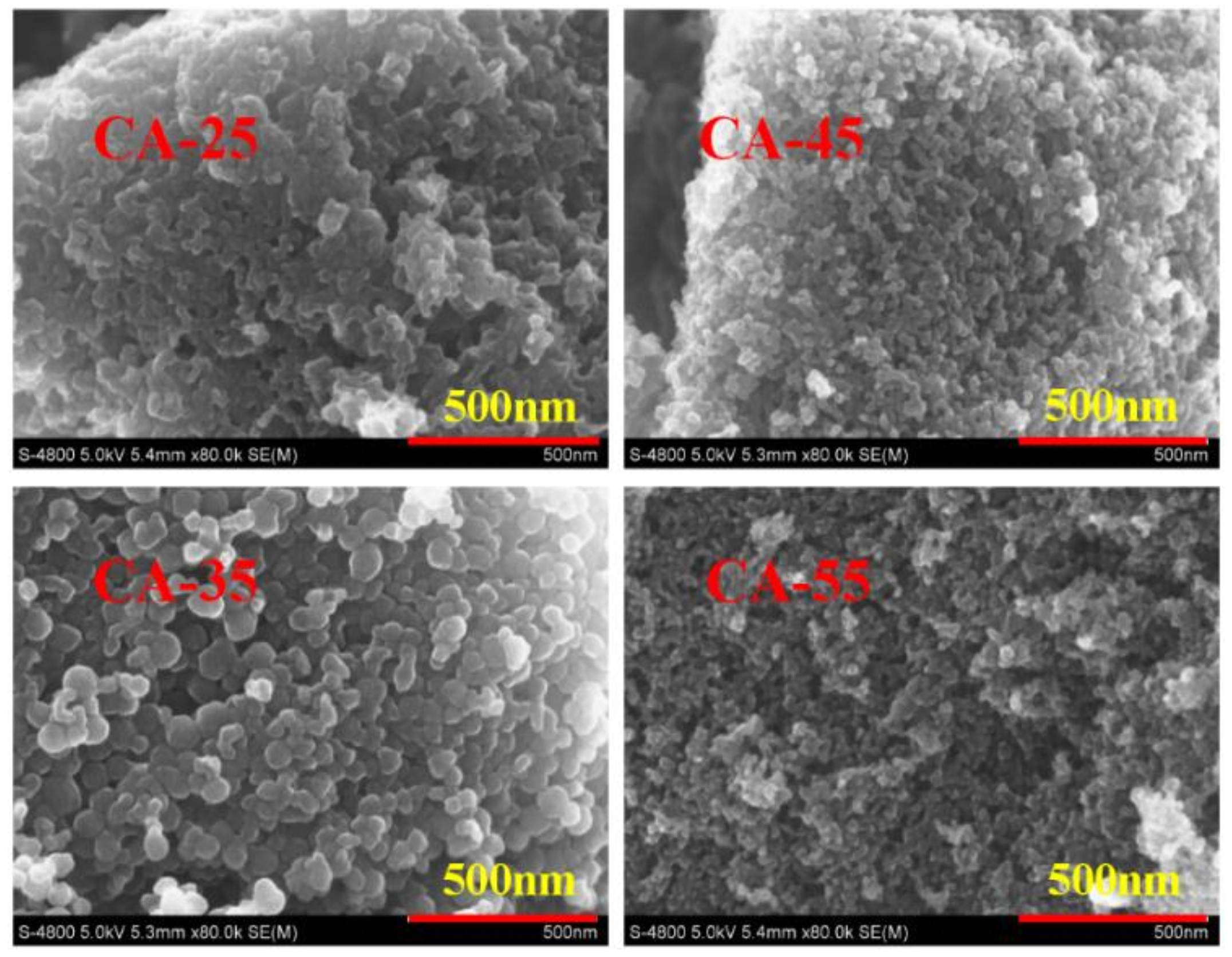
3.1.4. SEM Analysis
3.1.5. N2 Adsorption/Desorption Analysis
3.2. CO2 Adsorption
3.2.1. CO2 Adsorption Capacity
3.2.2. Isosteric Heat of Adsorption for CO2
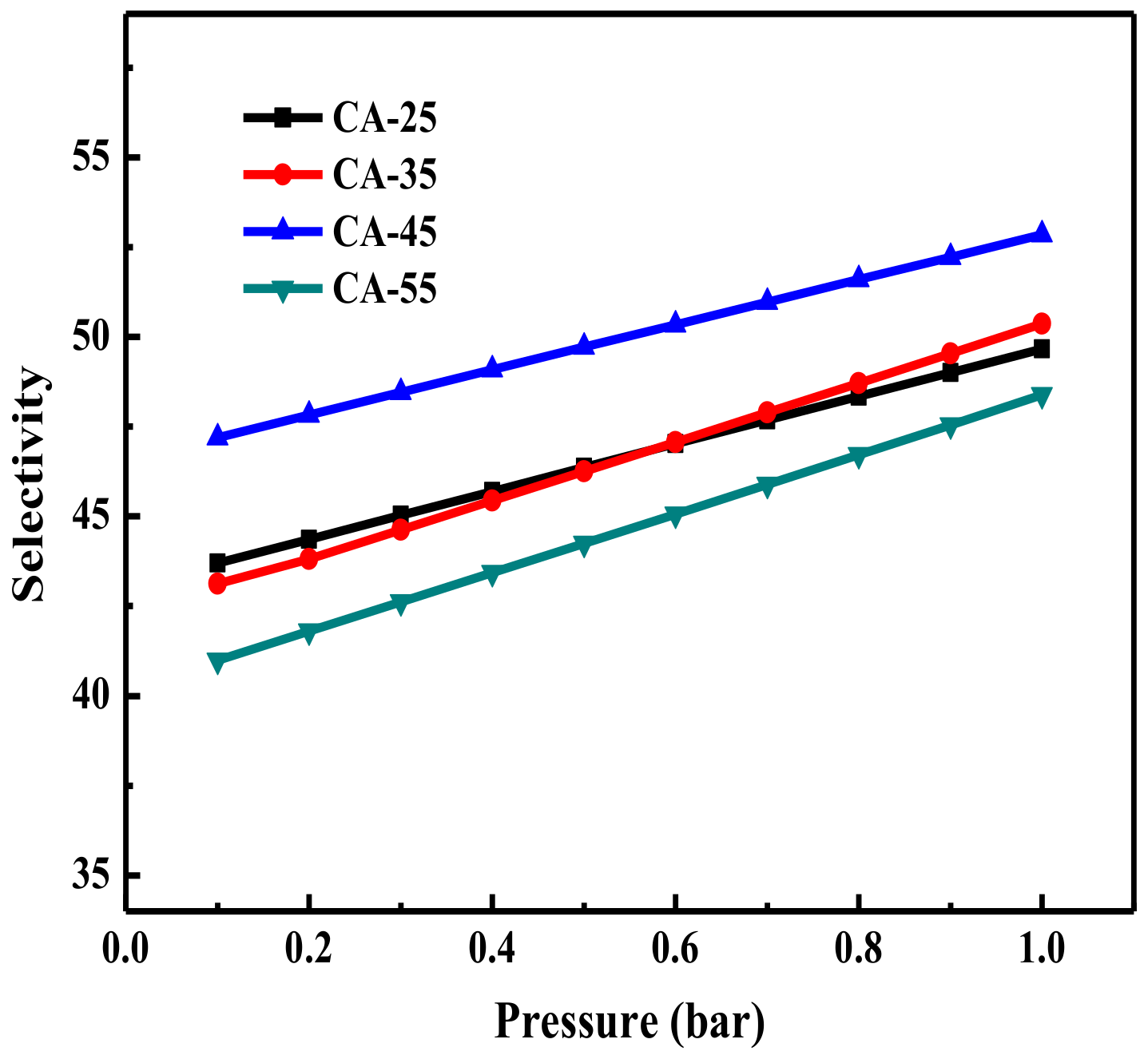
3.2.3. CO2 Adsorption Selectivity
3.2.4. Water-Resistant Experiment
3.2.5. Adsorbent Stability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernandez, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Gorak, A.; Skiborowski, M. Enzymatic reactive absorption of CO2 in MDEA by means of an innovative biocatalyst delivery system. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Lu, J.G.; Ge, H.; Chen, Y.; Ren, R.T.; Xu, Y.; Zhao, Y.X.; Zhao, X.; Qian, H. CO2 capture using a functional protic ionic liquid by membrane absorption. J. Energy Inst. 2017, 90, 933–940. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Lee, Y.R.; Ahn, W.S. Microporous amine-functionalized aromatic polymers and their carbonized products for CO2 adsorption. Chem. Eng. J. 2017, 319, 65–74. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simoes, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.Q.; Wei, X.T.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Kong, L.L.; Bashir, M.J.; Sethupathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Wu, X.B.; Wu, D.C.; Fu, R.W. Studies on the adsorption of reactive brilliant red X-3B dye on organic and carbon aerogels. J. Hazard. Mater. 2007, 147, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, P.P.; Qian, X.C.; Fei, Z.Y.; Zhang, Z.X.; Chen, X.; Tang, J.H.; Cui, M.F.; Qiao, X. Carbon Aerogels Synthesizd with Cetyltrimethyl Ammonium Bromide (CTAB) as a Catalyst and its Application for CO2 Capture. Z. Anorg. Allg. Chem. 2017, 644, 155–160. [Google Scholar] [CrossRef]
- Jeon, D.H.; Min, B.G.; Oh, J.G.; Nah, C.; Park, S.J. Influence of Nitrogen moieties on CO2 capture of Carbon Aerogel. Carbon Lett. 2015, 16, 57–61. [Google Scholar] [CrossRef]
- Kong, Y.; Jiang, G.D.; Wu, Y.; Cui, S.; Shen, X.D. Amine hybrid aerogel for high-efficiency CO2 capture: Effect of amine loading and CO2 concentration. Chem. Eng. J. 2016, 306, 362–368. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon Aerogels with Excellent CO2 Adsorption Capacity Synthesized from Clay-Reinforced Biobased Chitosan-Polybenzoxazine Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Li, Z.N.; Gadipelli, S.; Yang, Y.C.; Guo, Z.X. Design of 3D Graphene-Oxide Spheres and Their Derived Hierarchical Porous Structures for High Performance Supercapacitors. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, Y.W.; Mu, P.; Wang, X.; He, Q.; Chen, Y.; Zeng, J.H.; Wang, F.; Xu, Y.H.; Jiang, J.X. Toward High Performance Thiophene-Containing Conjugated Microporous Polymer Anodes for Lithium-Ion Batteries through Structure Design. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Ahmadi, H.; Lotfollahi-Yaghin, M.A. Stress concentration due to in-plane bending (IPB) loads in ring-stiffened tubular KT-joints of offshore structures: Parametric study and design formulation. Appl. Ocean Res. 2015, 51, 54–66. [Google Scholar] [CrossRef]
- Oh, T.H.; Oh, S.K.; Kim, H.; Lee, K.; Lee, J.M. Conceptual Design of an Energy-Efficient Process for Separating Aromatic Compounds from Naphtha with a High Concentration of Aromatic Compounds Using 4-Methyl-N-butylpyridinium Tetrafluoroborate Ionic Liquid. Ind. Eng. Chem. Res. 2017, 56, 7273–7284. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Zhang, J.Y.; Deng, K.; Xie, J.L.; Duan, W.B.; Zeng, Q.D. Solution concentration controlled self-assembling structure with host-guest recognition at the liquid-solid interface. Phys. Chem. Chem. Phys. 2015, 17, 24462–24467. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Q.; Zhou, C.J.; Wu, Y.Q.; Liu, F.Y.; Wu, Q.L. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of solid concentration on structure and properties of chitosan-caseinate blend films. Food Packag. Shelf Life 2017, 13, 76–84. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: New York, NY, USA, 2011; pp. 215–233. [Google Scholar]
- Far, H.M.; Donthula, S.; Taghvaee, T.; Saeed, A.M.; Garr, Z.; Sotiriou-Leventis, C.; Leventis, N. Air-oxidation of phenolic resin aerogels: Backbone reorganization, formation of ring-fused pyrylium cations, and the effect on microporous carbons with enhanced surface areas. RSC Adv. 2017, 7, 51104–51120. [Google Scholar] [CrossRef]
- Li, C.F.; Yang, X.Q.; Zhang, G.Q. Mesopore-dominant activated carbon aerogels with high surface area for electric double-layer capacitor application. Mater. Lett. 2015, 161, 538–541. [Google Scholar] [CrossRef]
- Kou, J.H.; Sun, L.B. Nitrogen-Doped Porous Carbons Derived from Carbonization of a Nitrogen-Containing Polymer: Efficient Adsorbents for Selective CO2 Capture. Ind. Eng. Chem. Res. 2016, 55, 10916–10925. [Google Scholar] [CrossRef]
- Liu, Q.; He, P.P.; Qian, X.C.; Fei, Z.Y.; Zhang, Z.X.; Chen, X.; Tang, J.H.; Cui, M.F.; Qiao, X.; Shi, Y. Enhanced CO2 Adsorption Performance on Hierarchical Porous ZSM-5 Zeolite. Energy Fuels 2017, 31, 13933–13941. [Google Scholar] [CrossRef]
- Jiang, S.F.; Zhang, Z.A.; Qu, Y.H.; Wang, X.W.; Li, Q.; Lai, Y.Q.; Li, J. Activated carbon aerogels with high bimodal porosity for lithium/sulfur batteries. J. Solid State Electrochem. 2014, 18, 545–551. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, H.W.; Park, S.; Song, I.K. Electrochemical properties of Mn-doped activated carbon aerogel as electrode material for supercapacitor. Curr. Appl. Phys. 2012, 12, 233–237. [Google Scholar] [CrossRef]
- Patole, A.S.; Patole, S.P.; Kang, H.; Yoo, J.B.; Kim, T.H.; Ahn, J.H. A facile approach to the fabrication of graphene/polystyrene nanocomposite by in situ microemulsion polymerization. J. Colloid Interface Sci. 2010, 350, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Patole, A.S.; Patole, S.P.; Jung, S.Y.; Yoo, J.B.; An, J.H.; Kim, T.H. Self assembled graphene/carbon nanotube/polystyrene hybrid nanocomposite by in situ microemulsion polymerization. Eur. Polym. J. 2012, 48, 252–259. [Google Scholar] [CrossRef]
- Nallusamy, S. Synthesis and Characterization of Carbon Black-Halloysite Nanotube Hybrid Composites Using XRD and SEM. J. Nano Res. 2017, 45, 208–217. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.J.; Tong, X.; Zhong, L.X.; Peng, X.W.; Sun, R.C. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crop. Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Sayari, A.; Liu, Q.; Mishra, P. Enhanced Adsorption Efficiency through Materials Design for Direct Air Capture over Supported Polyethylenimine. Chemsuschem 2016, 9, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Delgado, J.D.L.P.; Veneman, R.; Brilman, D.W.F. Stability of a Benzyl Amine Based CO2 Capture Adsorbent in View of Regeneration Strategies. Ind. Eng. Chem. Res. 2017, 56, 3259–3269. [Google Scholar] [CrossRef]
- Jiang, W.J.; Yin, Y.; Liu, X.Q.; Yin, X.Q.; Shi, Y.Q.; Sun, L.B. Fabrication of supported cuprous sites at low temperatures: An efficient, controllable strategy using vapor-induced reduction. J. Am. Chem. Soc. 2013, 135, 8137–8140. [Google Scholar] [CrossRef] [PubMed]


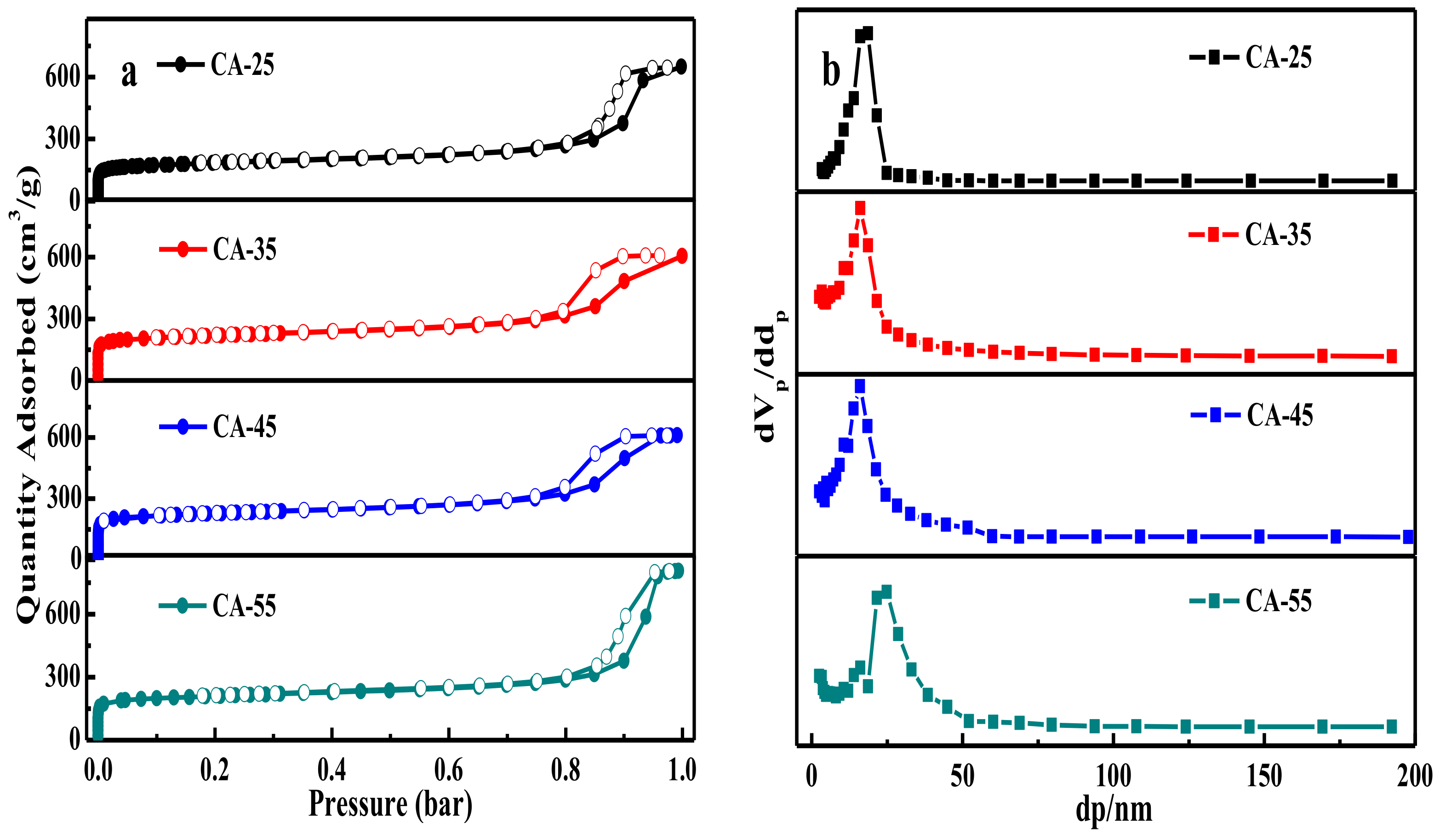



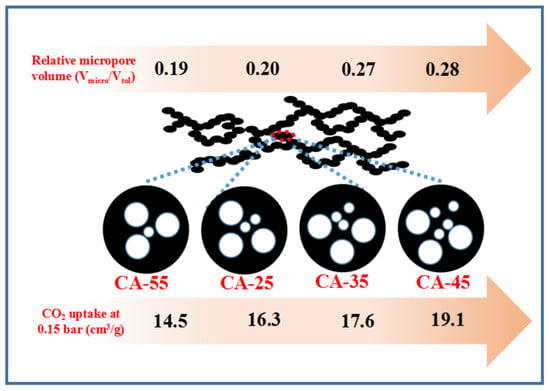
| Sample | SBET (m2/g) | Vp (cm3/g) | dp 2 (nm) | Vmicro/Vtol | ||
|---|---|---|---|---|---|---|
| Micropore 1 | Mesopore 2 | Total | ||||
| CA-25 | 638 | 0.20 | 0.79 | 0.99 | 7.37 | 0.20 |
| CA-35 | 813 | 0.25 | 0.67 | 0.92 | 4.52 | 0.27 |
| CA-45 | 848 | 0.27 | 0.68 | 0.95 | 4.46 | 0.28 |
| CA-55 | 784 | 0.24 | 1.00 | 1.24 | 6.34 | 0.19 |
| Sample | CO2 Uptake (cm3/g) | Qst 3 (kJ/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 °C | 12.5 °C | 25 °C | 50 °C | |||||
| 0.15 bar 1 | 1 bar 1 | 0.15 bar 1 | 1 bar 1 | 0.15 bar 1 | 1 bar 1 | 0.15 bar (Humid) 2 | ||
| CA-25 | 28.7 | 68.5 | 22.2 | 57.3 | 16.3 | 47.3 | 13.2 (12.1) | 25.1 |
| CA-35 | 34.1 | 79.5 | 27.5 | 68.7 | 17.6 | 49.6 | 15.1 (14.3) | 24.8 |
| CA-45 | 34.2 | 83.7 | 27.6 | 71.8 | 19.1 | 56.5 | 18.5 (16.2) | 24.3 |
| CA-55 | 26.2 | 62.7 | 22.1 | 54.4 | 14.5 | 43.3 | 13.1 (11.9) | 25.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Qian, X.; Fei, Z.; Liu, Q.; Zhang, Z.; Chen, X.; Tang, J.; Cui, M.; Qiao, X. Structure Manipulation of Carbon Aerogels by Managing Solution Concentration of Precursor and Its Application for CO2 Capture. Processes 2018, 6, 35. https://doi.org/10.3390/pr6040035
He P, Qian X, Fei Z, Liu Q, Zhang Z, Chen X, Tang J, Cui M, Qiao X. Structure Manipulation of Carbon Aerogels by Managing Solution Concentration of Precursor and Its Application for CO2 Capture. Processes. 2018; 6(4):35. https://doi.org/10.3390/pr6040035
Chicago/Turabian StyleHe, Pingping, Xingchi Qian, Zhaoyang Fei, Qing Liu, Zhuxiu Zhang, Xian Chen, Jihai Tang, Mifen Cui, and Xu Qiao. 2018. "Structure Manipulation of Carbon Aerogels by Managing Solution Concentration of Precursor and Its Application for CO2 Capture" Processes 6, no. 4: 35. https://doi.org/10.3390/pr6040035