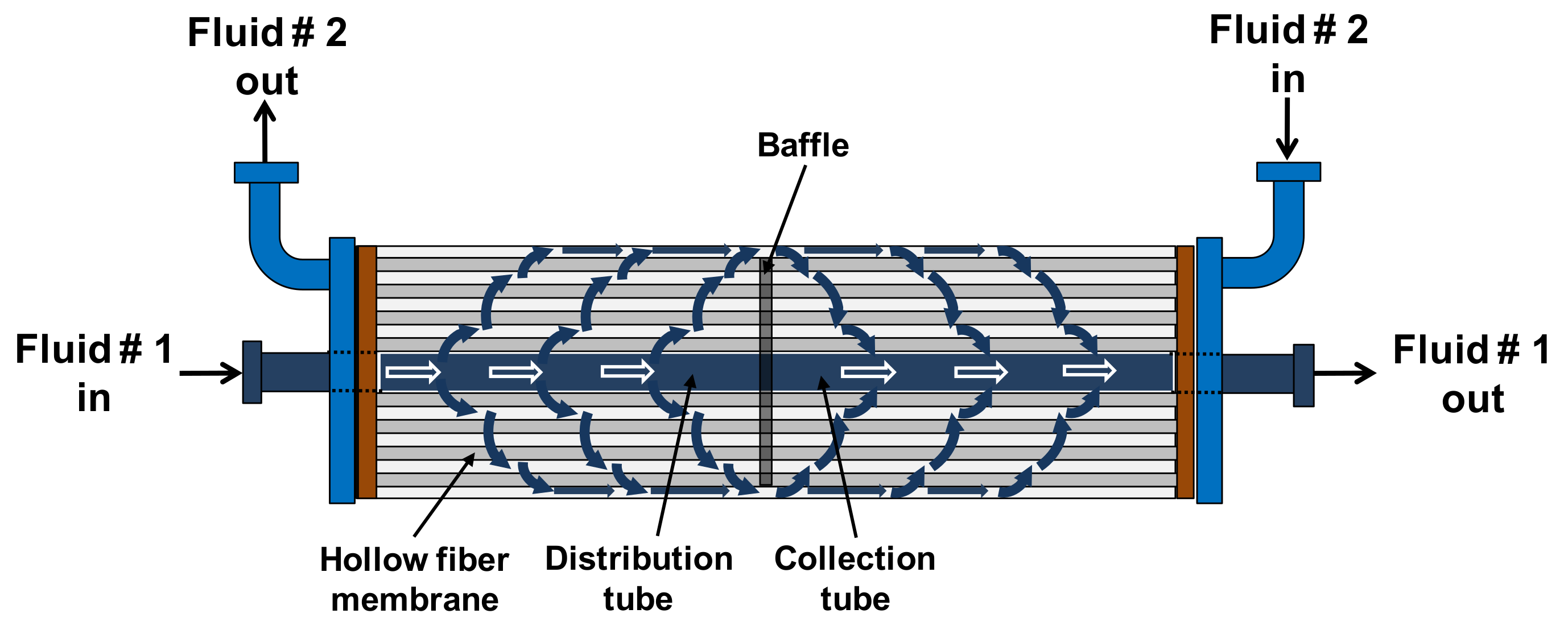
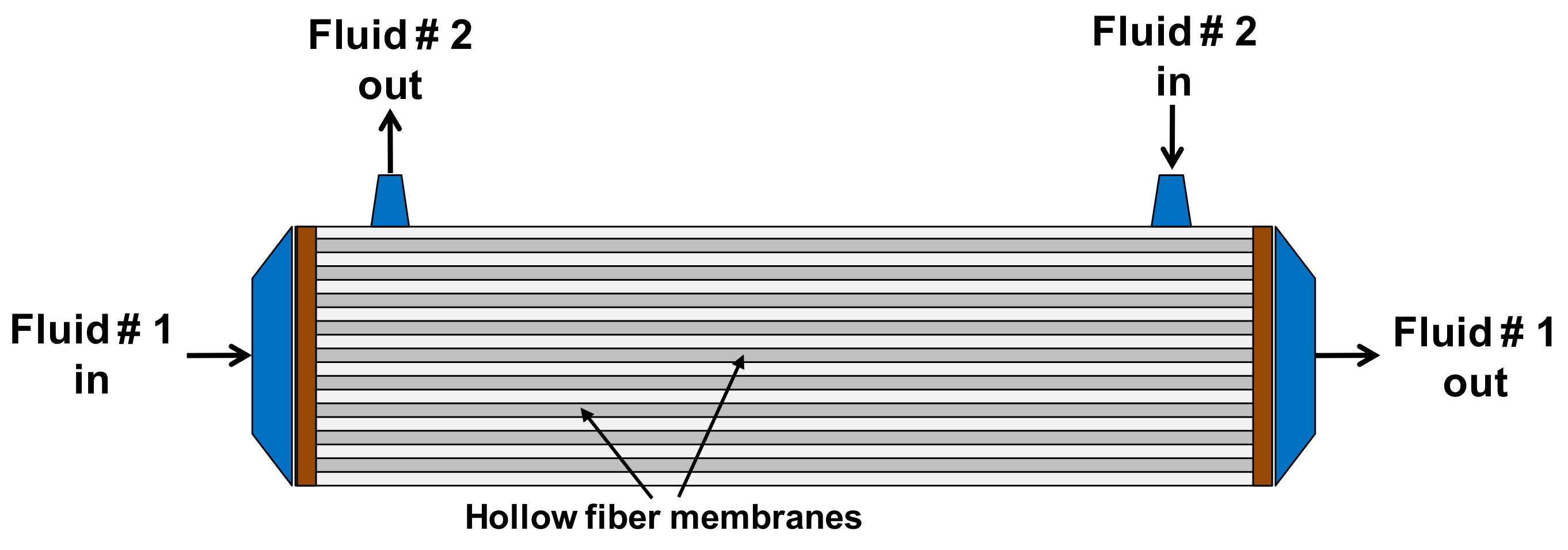
3.1. Selection of the Mass-Transfer Correlation on the Shell Side
As explained above, the estimation of the mass-transfer coefficient was performed by using the correlations described in
Table 1 for Liqui-Cel
TM modules.
Table 5 shows the obtained values for the overall mass-transfer coefficients using different mass-transfer correlations for the Liqui-Cel
TM Extra-Flow module. The results show that the three first correlations [
24,
25,
26] had similar values, fundamentally explained by similar conditions used in their respective experimental studies, such as aqueous phase flowing on the shell side, feed-flow rate and Reynolds number. Therefore, the simulations performed here can use any of these correlations. Nevertheless, it was found that the Fouad correlation [
27] overestimates the values of the overall mass-transfer coefficient with respect to those obtained from the three first correlations shown in
Table 5, because of the extremely low value of the Re number in the shell side (<0.1) at the simulated conditions of
Table 2.
In the same way, the correlation reported by Shen et al. [
28] underestimates the overall mass-transfer coefficient value. This behavior could be explained because the correlation was generated by fitting different experimental results, including gas-liquid extraction and solvent extraction systems [
11,
31].
Even though the three first correlations presented in
Table 5 present similar results, the correlation developed by Schoner and collaborators [
24] was selected to simulate the performance of the GFMA process using the industrial Extra-Flow module from Liqui-Cel
TM. This correlation was the first proposed for ExtraFlow modules. Furthermore, a simulation of the phenomenological model of the 2.5 × 8 Extra-Flow module was developed in the same conditions as the previous experimental study [
3] shown in
Table 2, in order to compare the theoretical performance of the Extra-Flow module with respect to a cylindrical configuration with parallel flow mode (
Table 2). This comparison is shown in
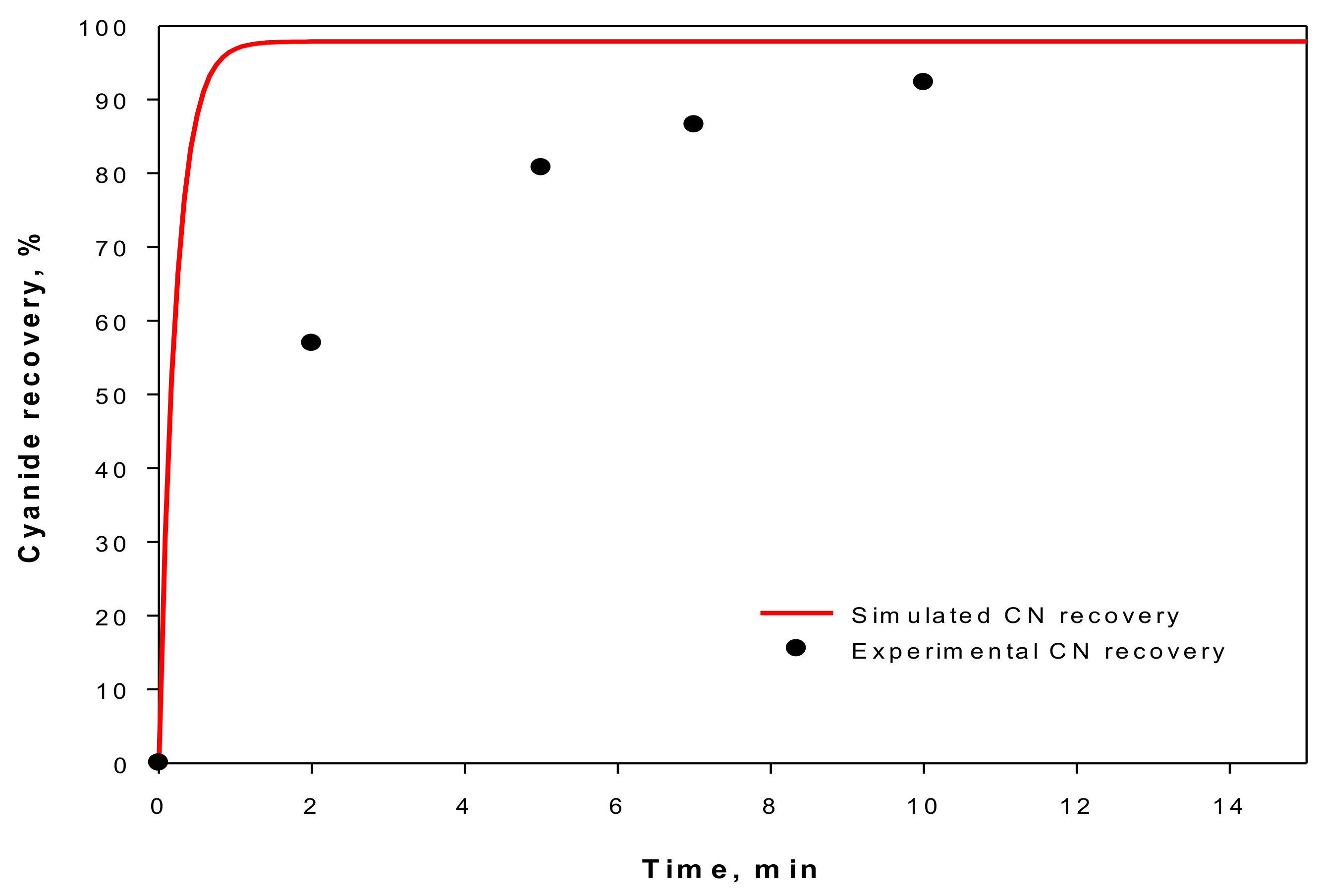
Figure 5, where the cyanide recovery estimated to the Extra-Flow module reaches 97% at 1 min in relation to the experimental results that overcome the 90% cyanide recover after 10 min [
17]. These results demonstrate the high performance that Extra-Flow Modules in this GFMA process might reach.
3.2. Performance Assessment of Industrial Extra-Flow Liqui-CelTM Modules
A phenomenological model has been developed in this work to predict the performance of the industrial membrane Liqui-Cel
TM modules in a GFMA process for cyanide recovery in a batch arrangement. This process configuration considers that the cyanide solution is fed into a membrane module and the treated solution is recirculated into the feed tank up to a complete 30 min of recirculation. Different cyanide feed-flow rates were carried out for each membrane module in order to determine the optimum capacity in each module. According to the Liqui-Cel
TM specifications, the capacity ranges for each membrane module are 16–125 m
3/h, 16–91 m
3/h, 7–28 m
3/h, and 10–57 m
3/h for 14 × 40, 14 × 28, 8 × 40, and 10 × 28 modules, respectively. Based on this information, the feed-flow rates tested in the phenomenological model ranged between 10 m
3/h and 125 m
3/h.
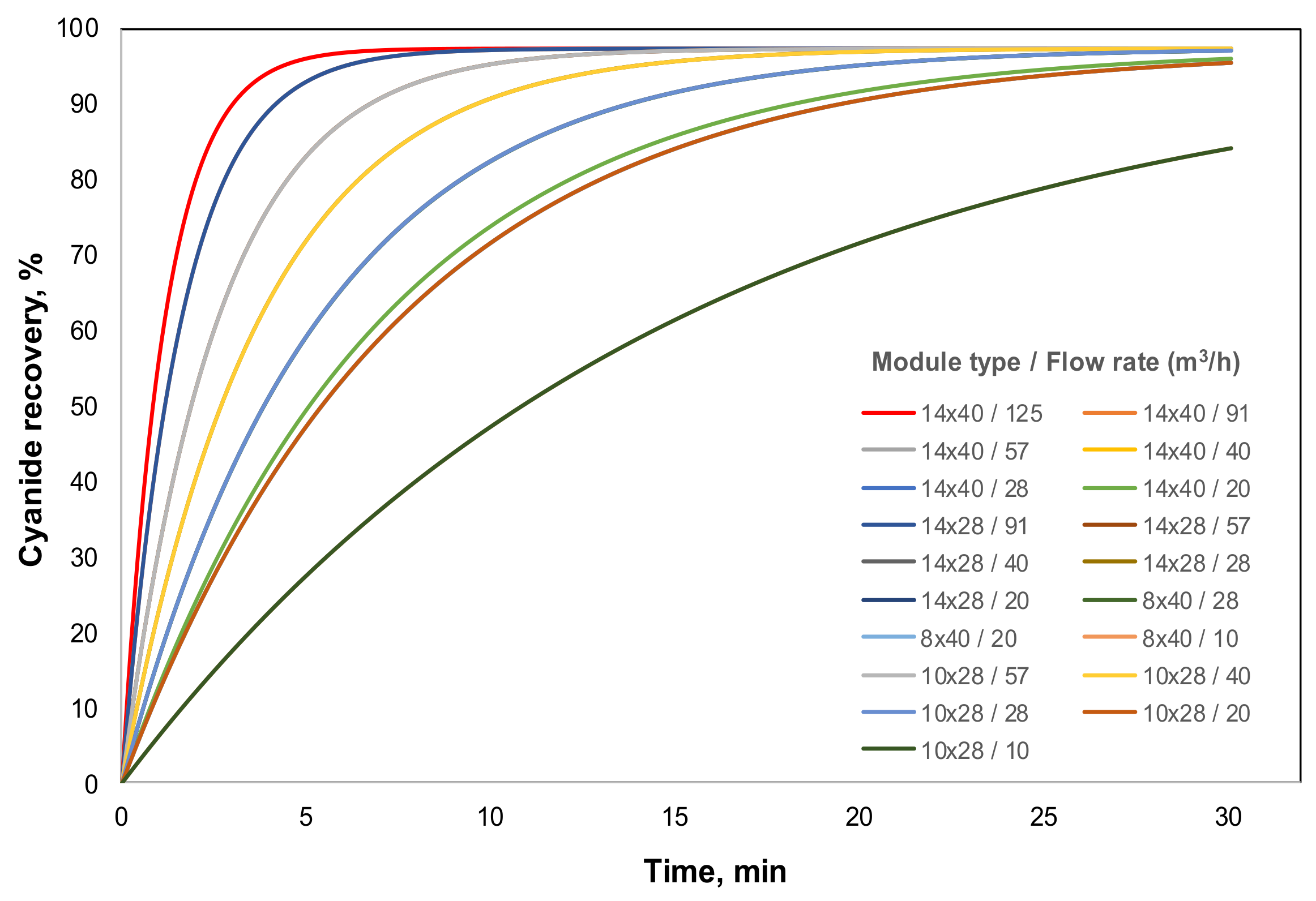
Figure 6 presents the results of these simulations, where similar kinetics of cyanide recovery were obtained for each membrane module at the same feed-flow rate, overlapping some curves. This fact is explained by a compensation effect of
K ×
A value; while
K decreases when the capacity is decreasing, the area remains higher for the larger modules. This explains the presence of 8 curves in
Figure 6 for 19 different simulated conditions.
Conversely, when the feed-flow rate is higher, the cyanide recovery increases because of the processed increase of the volume of cyanide solution. However, the expected results should have shown a better performance for a smaller membrane module while the feed-flow rate decreases; e.g., for 91 m
3/h the 14 × 28 HFMC, for 57 m
3/h the 10 × 28 HFMC and for 28 m
3/h the 8 × 40 HFMC. Nevertheless, the obtained results are contrary to this expectation, because the scaling-up criteria was based on the feed-flow rate capacity (similar
K ×
A value at the same capacity) instead of ensuring the performance at different membrane modules capacities. This criterion could affect the reproducibility of cyanide-recovery performance obtained at laboratory scale, and also complicates the methodology design for an industrial plant. A design analysis of the GFMA process, based on Equation (7), could explain the results obtained here. Equation (7) can be rewritten as follows:
where
a is the contact-specific surface area (m
2/m
3, mass-transfer area per equipment volume),
QF represents the volumetric feed flow (m
3/s),
L is the membrane fiber length, and
vs is the fluid velocity in the shell side. Thus, the effective length to promote the cyanide transfer can be defined similar to the packed bed equipment, as shown in the following equation [
32,
33]:
Therefore, the mass-transfer equipment, e.g., membrane contactors, can be sized as a relation between the number of transfer units (
NTU) and the length of transfer units (
HTU). The
NTU value is determined by operational parameters such as stream flow rates, solutes concentration and equilibrium constant value of solutes, while
HTU is defined by the equipment characteristics such as mass-transfer area, stream velocities and mass-transfer coefficient values. In this regard, the terms
NTU and
HTU are related to Equation (10) by the following expressions:
The
HTU value is the main parameter for evaluating the performance of mass-transfer equipment, since this parameter depends on equipment dimensions and hydrodynamic characteristics; instead, the
NTU value is defined by the operational conditions of the process. This supports the criterion for scaling-up based on ensuring the performance at different scales, where the
HTU parameter should be the variable to keep it fixed at the maximum capacity of a membrane contactor module. This design criterion would allow the cyanide-recovery (process performance) expected for the maximum capacity of the different membrane modules to be ensured.
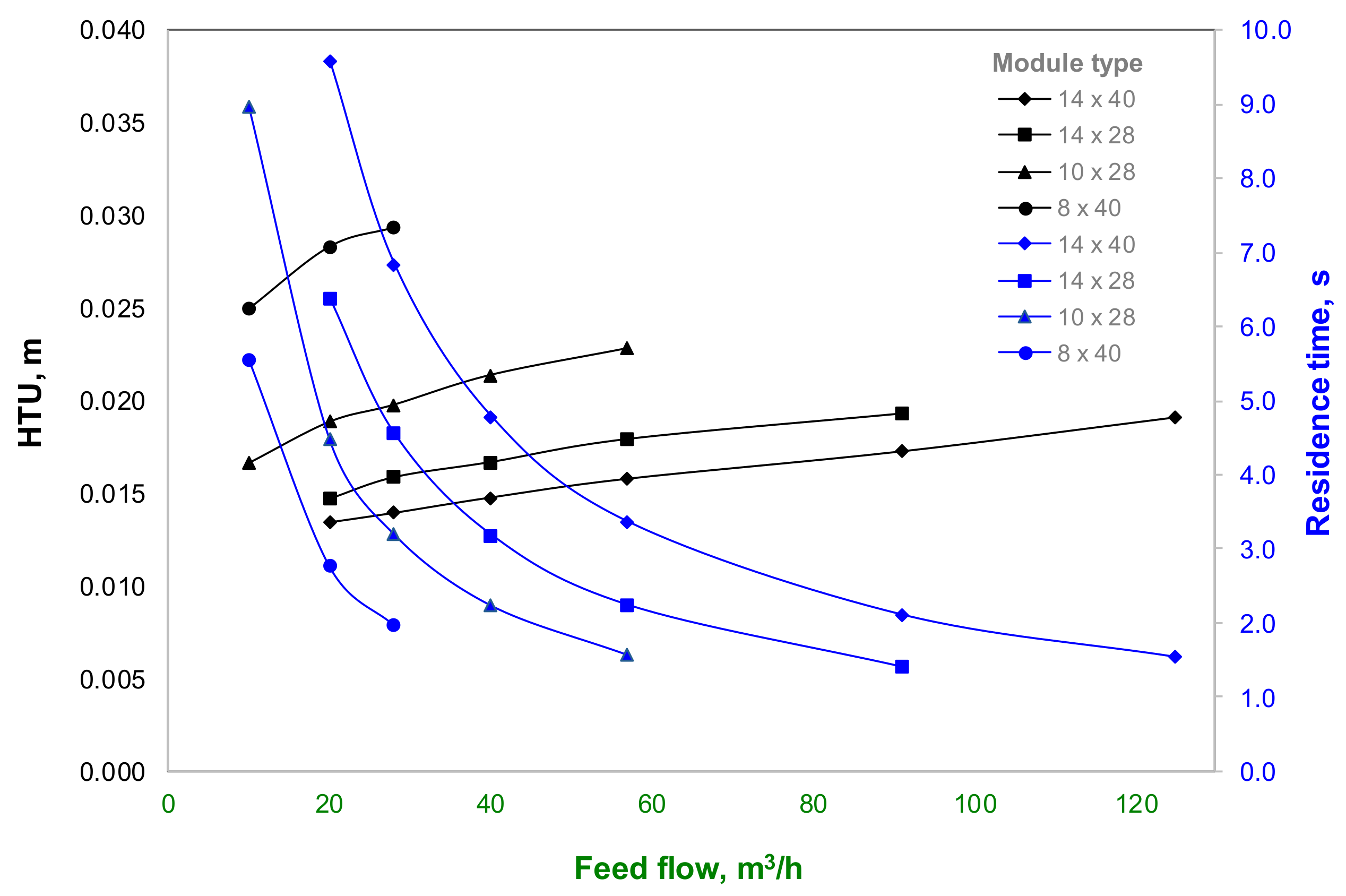
Figure 7 shows the results of
HTU values for the industrial Extra-Flow modules assessed as a function of the feed-flow rate. Here, the 14 × 40 Extra-Flow module presents the lowest
HTU values and the
HTU value at maximum capacity for each membrane module is different, so that it supports the earlier explanation of cyanide-recovery results from
Figure 6. Hence, for a high requirement of cyanide solution treatment, the best performance represented by the highest cyanide recovery with minimum contact surface area could be achieved with the 14 × 40 Extra-Flow module at 125 m
3/h. Nevertheless, when the required treatment capacity is lower and a membrane module with less capacity is selected for economic reasons, the kinetics of cyanide transfer will be slower, increasing the required contact surface area for mass transfer. Likewise, the same cyanide-recovery values having different
HTU values for each membrane module are explained by different
NTU values, i.e., when the
HTU value increases, the
NTU value decreases. This is promoted by the residence time reached for each membrane module at different feed-flow rates.
Figure 7 shows the residence time values for each membrane module where the smallest modules present lower residence-time values with respect to the bigger ones. This fact promotes a decrease of cyanide transfer, yet the mass-transfer coefficient is higher in these modules with respect to the larger ones. Thus, the main issue of scaling-up a membrane module based on the flow rate capacity is the increase of surface area available for mass transfer, which should increase with additional membrane modules to reach a cyanide recovery higher than 90%, particularly for a plant with capacities below 57 m
3/h.
3.3. Determination of the Optimal Operational Condition
The energy losses promoted by the pressure drop in the membrane modules are the main cost intrinsically associated with a membrane contactor, and is related to the investment cost for each module. For the cyanide-recovery process, the acid consumption has to be considered in an economic evaluation; however, this cost is constant for each case because the initial mass of cyanide to be extracted is the same and equal to 3000 kg. Therefore, it has not been considered in this evaluation.
Figure 8 presents the results of total cash flow for a 5-year time period, considering the cyanide recovery reached at 5 min and 10 min for each feed-flow rate and for each membrane module. These results show a minimum effect of pressure drop in the membrane module selection, due to the high cyanide-recovery value. In fact, the expected curve for each case should have a parabolic behavior, since the energy consumption increases when feed-flow rate does as well. However, in this case the increase in energy consumption is not enough to decrease the estimated cash flow. Furthermore, these results show a continuous behavior of each curve from each membrane module, generating a single cash flow curve for 5 min and 10 min. This result can be explained by the results of cyanide recovery reported in
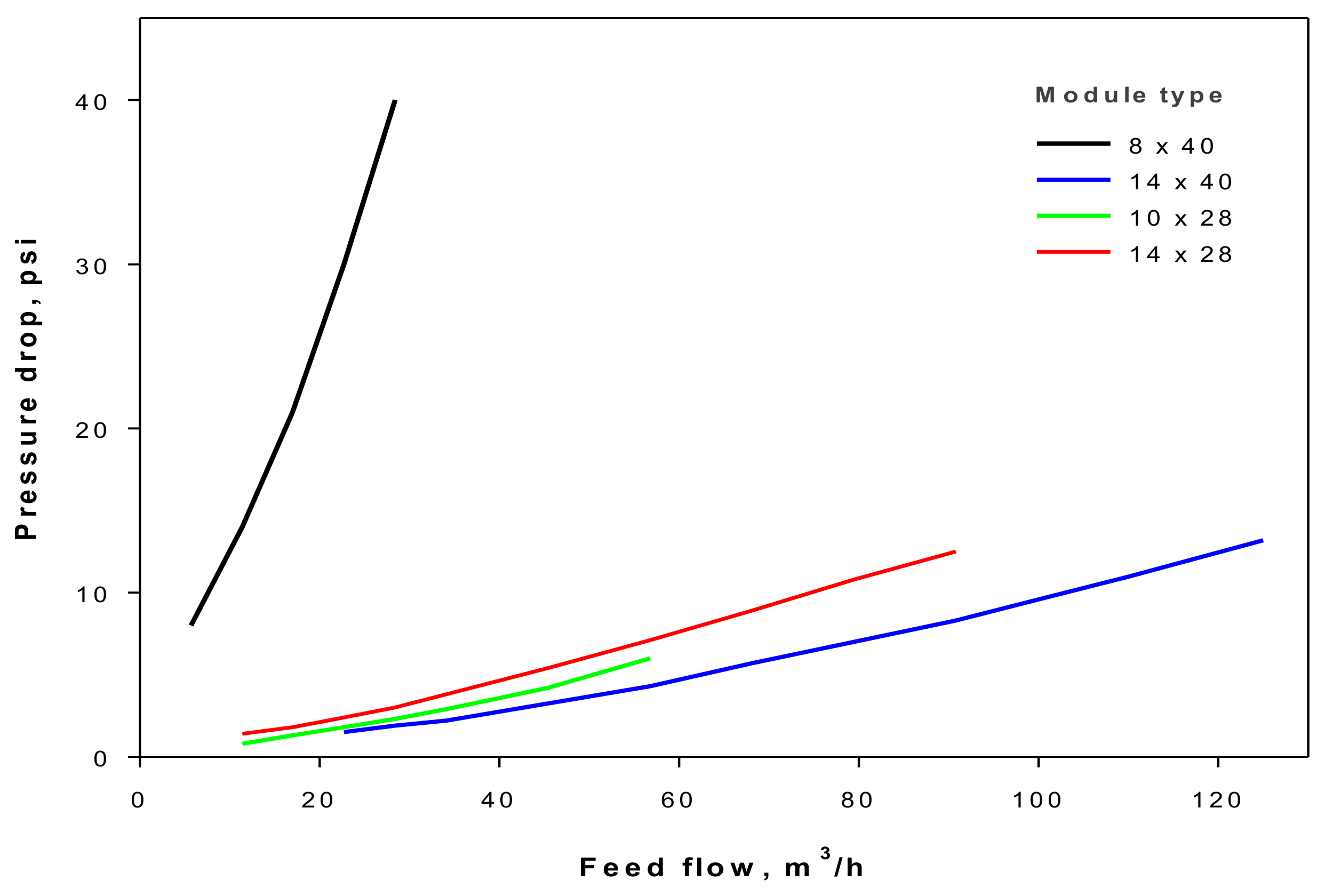
Figure 6 where the cyanide recovery determines the incomes, and is equal for each module at the same feed-flow rate of the treated solution. Moreover, the low impact of energy consumption does not allow a clear difference between the curves of cash flow for each module to be observed. The optimal operation for each module will, thus, always be observed at maximum flow rate, as explained by the increase of the mass-transfer coefficient, and consequently, by the cyanide recovery. Likewise, there is no difference in economic terms related to the selection of the best membrane module at a specific feed-flow rate of treated solution. These results could change for another element with a lower sale price, although this methodology should be performed to select the optimum feed-flow rate and membrane module. In this framework, the pressure drop information reported in
Figure 4 is useful to predict the most efficient module in terms of energy, where the 14 × 40 HFMC shows the lowest curve of pressure drop.
Finally, the results obtained for 5 min of treatment are better than those estimated at 10 min, regardless of the higher cyanide recovery obtained at 10 min. In this case, the energy consumption is doubled, increasing the operational costs and, therefore, decreasing the related cash flow.
3.4. Design of GFMA Plant
The phenomenological model that considers Equations (1)–(3) as its main expressions has been solved and applied to estimate the surface contact area, and the required number of membrane modules for the cyanide recovery through the GFMA process.
Table 6 shows the results of a case of study, which considers the treatment of a cyanide solution flow rate equal to 250 m
3/h, using the different Extra-Flow modules tested in there. According to the results reported in
Section 3.3, the best performance of each module is achieved at the maximum flow rate capacity; thereby, this analysis has been performed using the maximum feed-flow rate specified from the supplier for each membrane module. In this framework, the 14 × 28 Extra-Flow module shows the highest values of the overall mass-transfer coefficient, even better than the 14 × 40 Extra-Flow module that shows a high
HTU value (
Figure 7). This is because of its large contact surface area, higher than the value of the 14 × 28 Extra-Flow module. The higher mass-transfer coefficient determines, then, a lower area required per module with respect to the 14 × 40 module, and also the lowest area required in comparison with the other modules tested in this study. However, the higher transfer area of 14 × 40 module involves the lowest number of required modules (equal to 2), due to its high treatment capacity. Even though the use of the 14 × 40 module involves the lowest number of required modules and the 14 × 28 one includes the lowest contact surface area for mass transfer, all modules show a high overall mass-transfer coefficient. In fact, they associate the requirement of modules with the total flow rate capacity (total flow rate capacity/module capacity). This fact can be verified by the high performance that the Extra-Flow Liqui-Cel modules show with a design that maximizes the overall transfer coefficient. A similar result has been obtained by the study case that considers a cyanide solution flow rate equal to 57 m
3/h (
Table 7), where the number of required modules is proportional to the total capacity of treatment. Furthermore, the highest overall mass-transfer coefficient has been obtained for the 10 × 28 module operating at maximum capacity in this case, although the total area is lower than those of the 14 × 40 and 14 × 28 modules, determining a lower
HTU value. Thus, the results of the overall mass-transfer coefficient from
Table 6 and
Table 7 are consistent with the results reported in
Section 3.2 and
Section 3.3, and there are no significant performance differences between the modules operating at the same flow rate because of their
K ×
A relationship. In this way, a safe selection of membrane module for this application could be the 14 × 40 module, even taking into account its lower pressure drop and irrelevant difference of capital cost when compared with other modules. These results justify, as a design recommendation, the change in the scaling-up criteria: from keeping capacity to keeping the
HTU value at different scales. Thus, when the treatment capacity is lower than 50–60 m
3/h, a smaller membrane module will be more attractive.
The importance of the mass-transfer coefficient value can be verified from the results summarized in
Table 8, which compares the estimations of this work with the data reported in a previous work [
4]. Here, the 14 × 40 module was assessed in a parallel flow mode, using both the correlation proposed by Basu and collaborators [
8,
9] with the 14 × 40 module operating in cross-flow mode with a center baffle (Extra-Flow module), and also the correlations reported by Schoner and coworkers [
24], and Shen and coworkers [
28]. This last correlation was evaluated since it estimates the lowest mass-transfer coefficient value for the Extra-Flow modules (
Table 5). The operational conditions of these results were fitted to the previous work. The results of
Table 8 show the highest impact of the overall mass-transfer coefficient value in terms of design purposes, including the selection of the mass-transfer correlation and the type of module. The results obtained from the correlation proposed by Shen and coworkers increase the total number of modules from 4 to 28 with respect to the result using the correlation reported by Schoner and collaborators. As mentioned in
Section 3.1, the correlation by Shen and coworkers is based on different systems and compiles a great variety of different results and systems in order to obtain a common correlation. Therefore, it is expected that the results are closer to those obtained from the correlation made by Schoner and collaborators. Nevertheless, it is recommended these results are verified with further experimental studies.
Finally, the results obtained in our previous study, where a cylindrical HFMC module operating in parallel flow mode was used and simulated, show the performance difference between the Extra-Flow module and a module in a typical parallel flow mode, increasing the number of required modules from 4 to 140 (156 according to the previous work [
4], adding stand-by units). This last result is relevant not only for the obvious difference in the quantity of membrane modules, but also for the problems generated due to the high number of modules in-series. In addition, it is important to consider intermedia pumping systems because of the maximum tolerable feed pressure of modules as well as the increase of sensors, tanks and pumps associated with this requirement. This aspect was discussed in the previous work [
4]. In this framework, an update of the economic evaluation using units operating at cross-flow mode with center baffle can be highly recommended in further research.