Enantioselective Extraction of Phenylalanine Enantiomers Using Environmentally Friendly Aqueous Two-Phase Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of ChAAILs and DESs
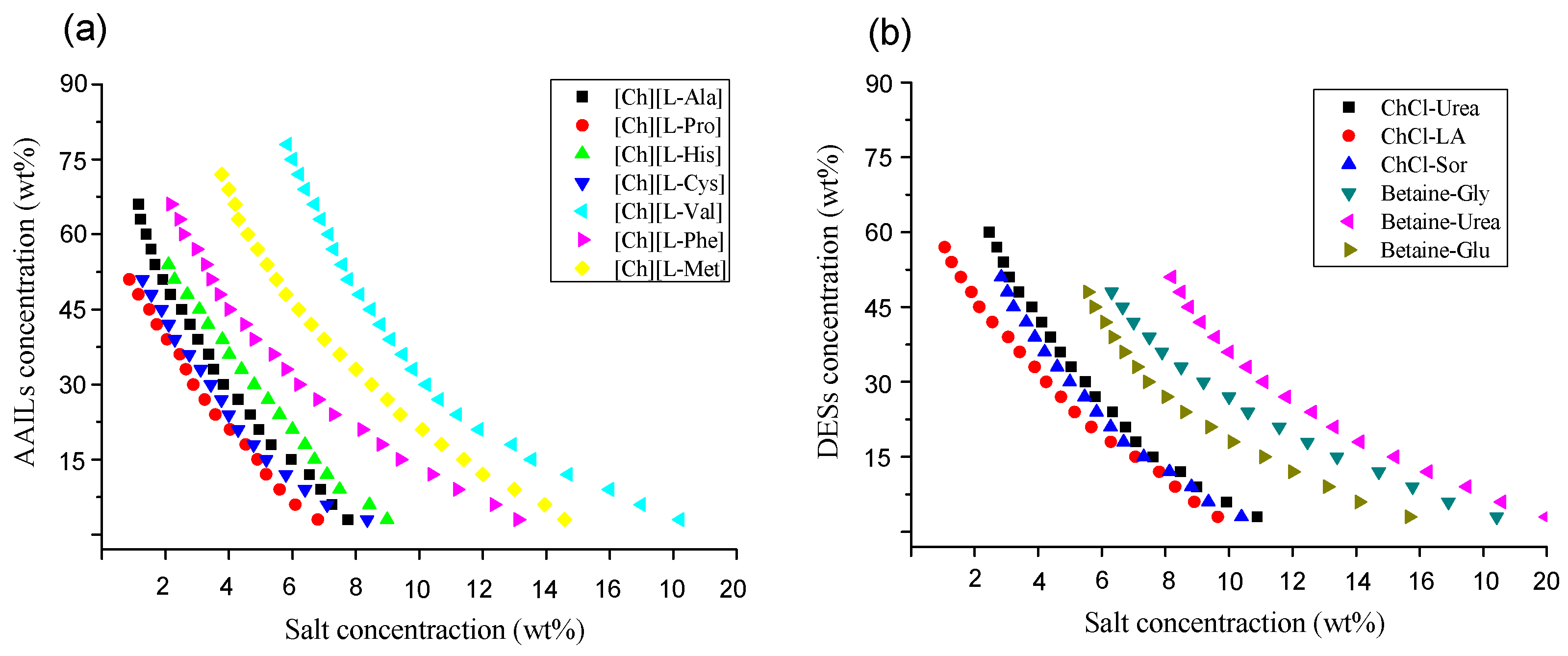
2.3. Phase Diagrams
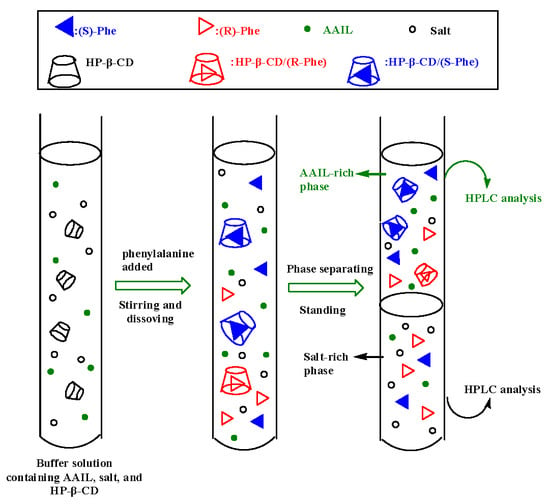
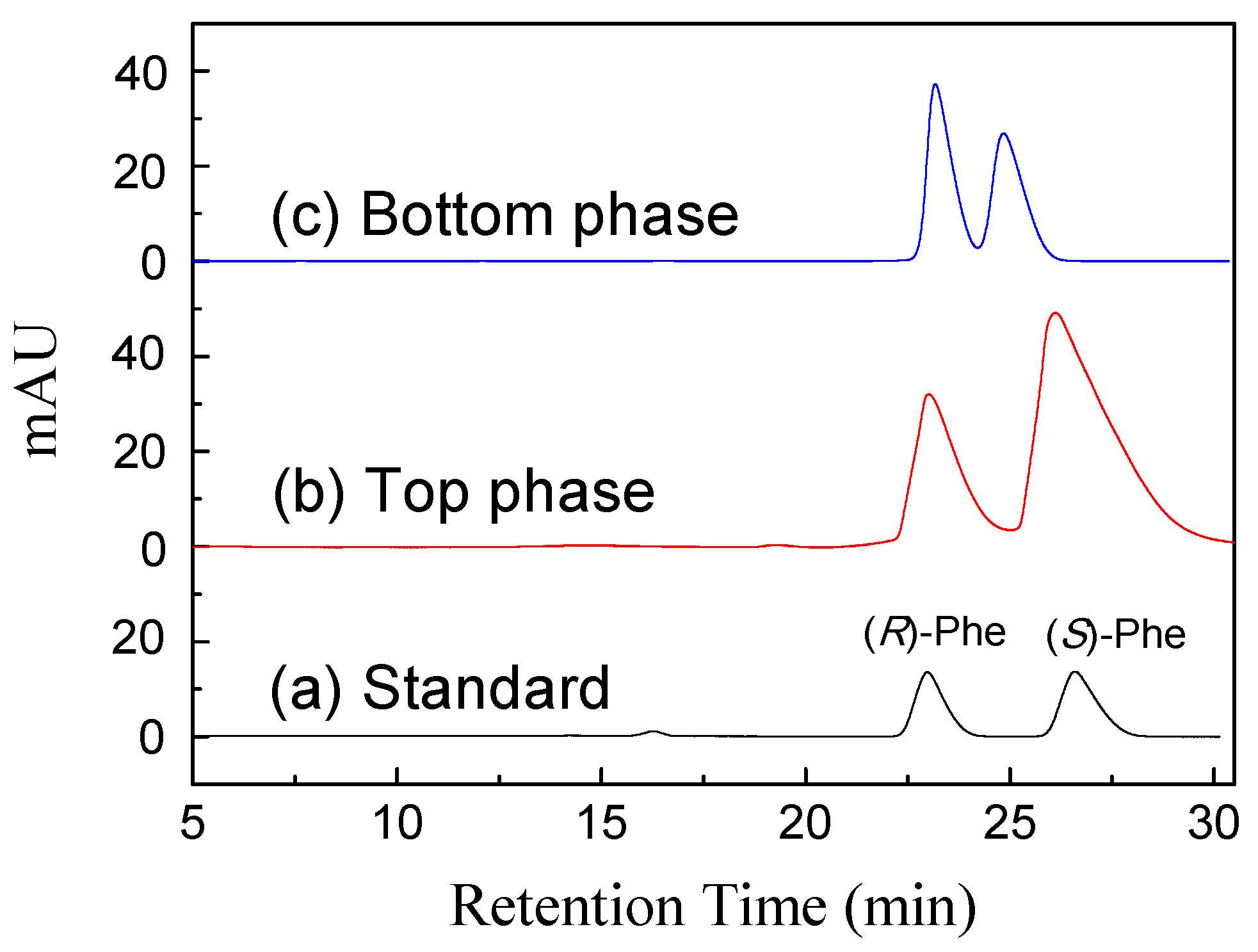
2.4. Chiral Extraction of Phenylalanine Enantiomers in ATPS
2.5. Derivatization of Phenylalanine
2.6. HPLC Conditions
3. Results and Discussion
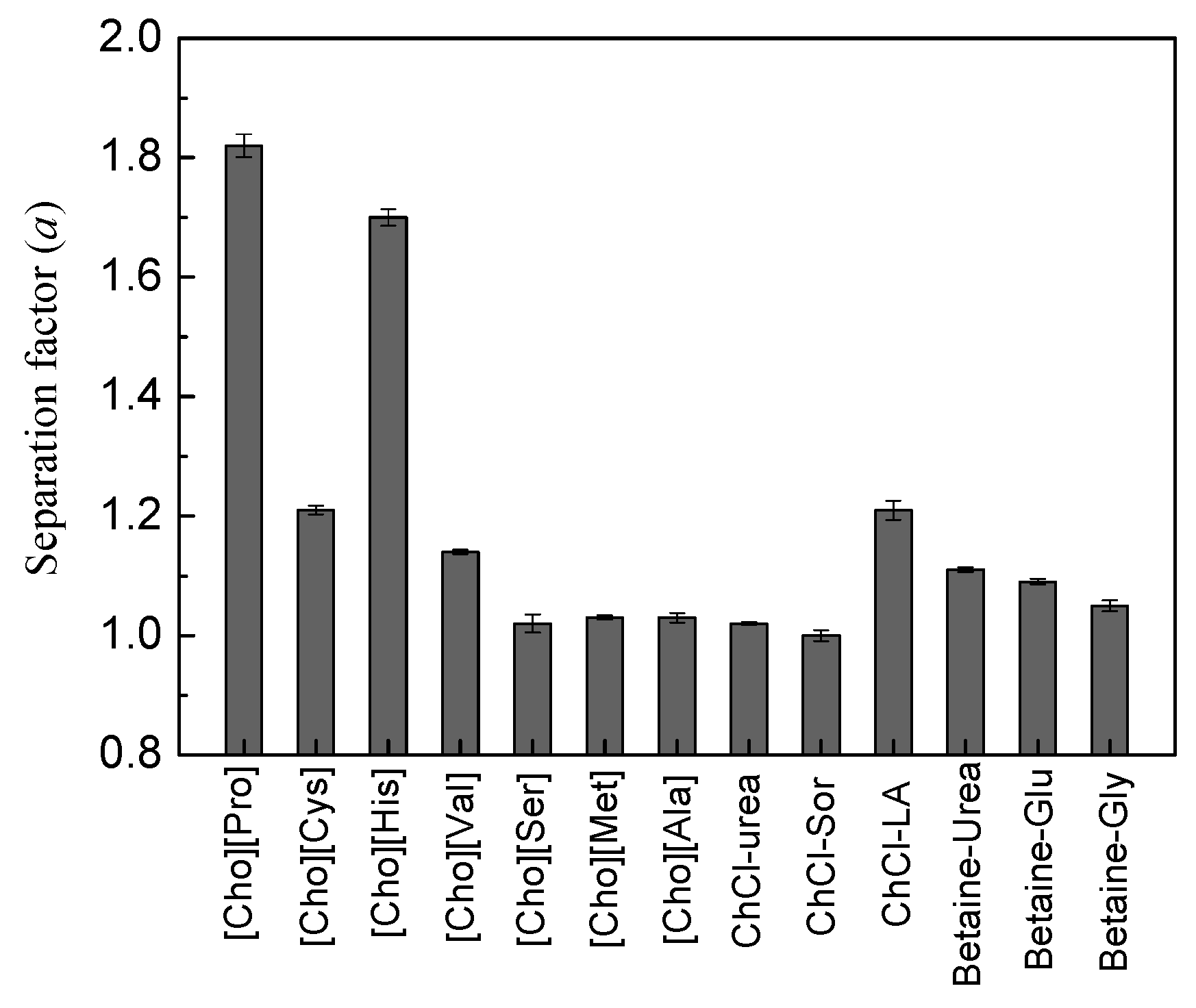
3.1. Screening the Optimal ATPS Used for the Chiral Separation
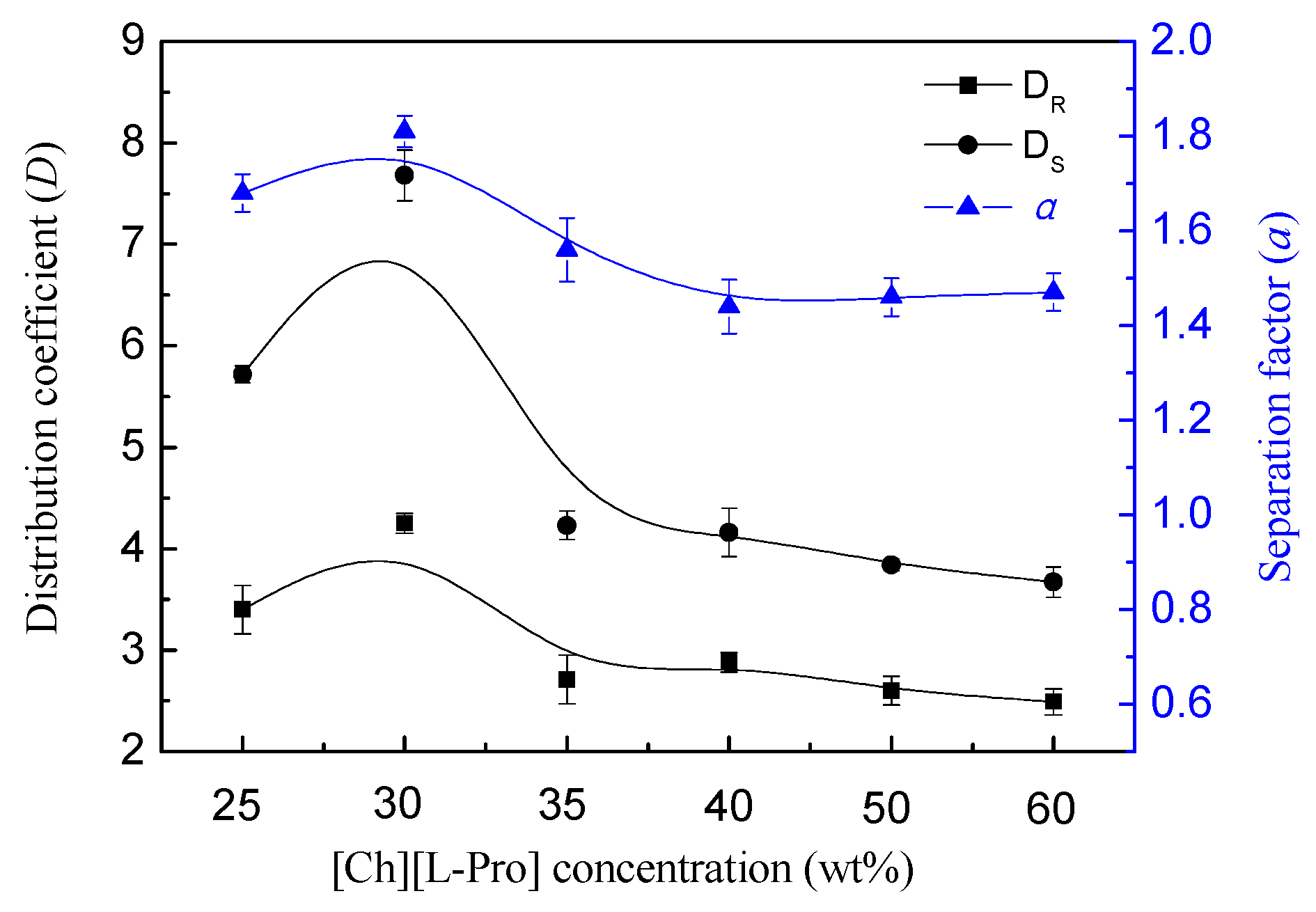
3.2. Effect of ChAAILs Concentration
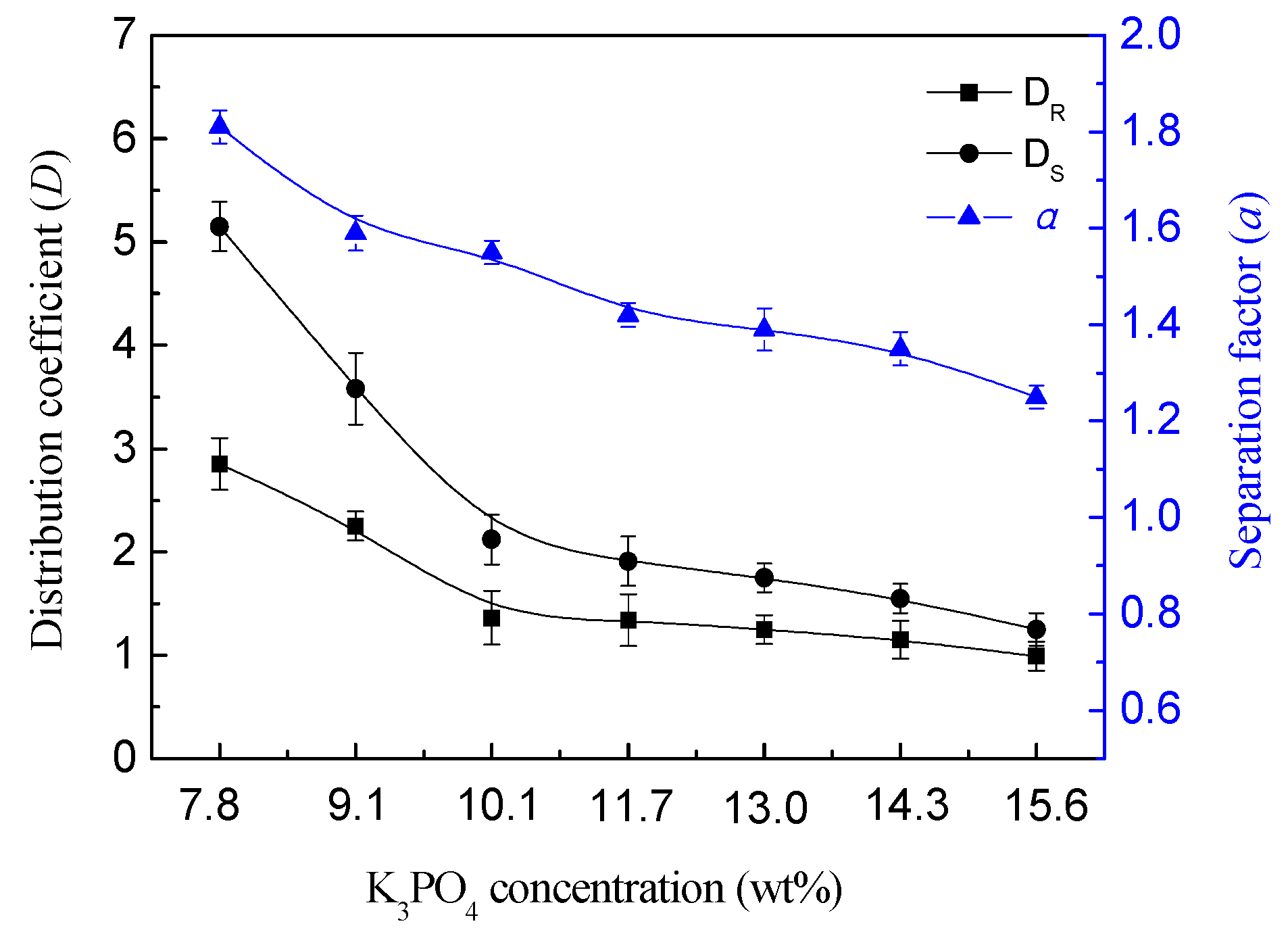
3.3. Effect of Salt Type and Concentration
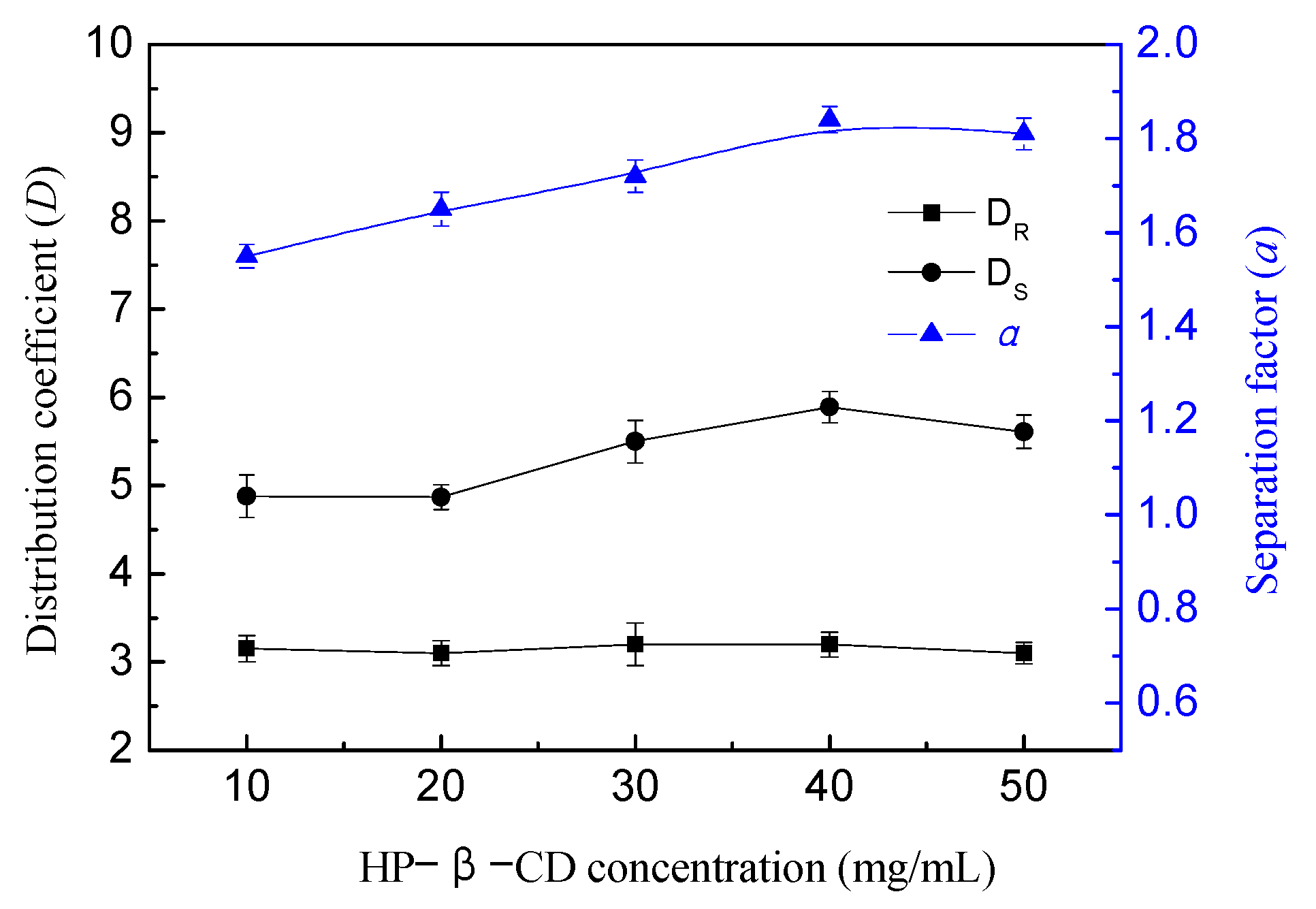
3.4. Effect of HP-β-CD Concentration
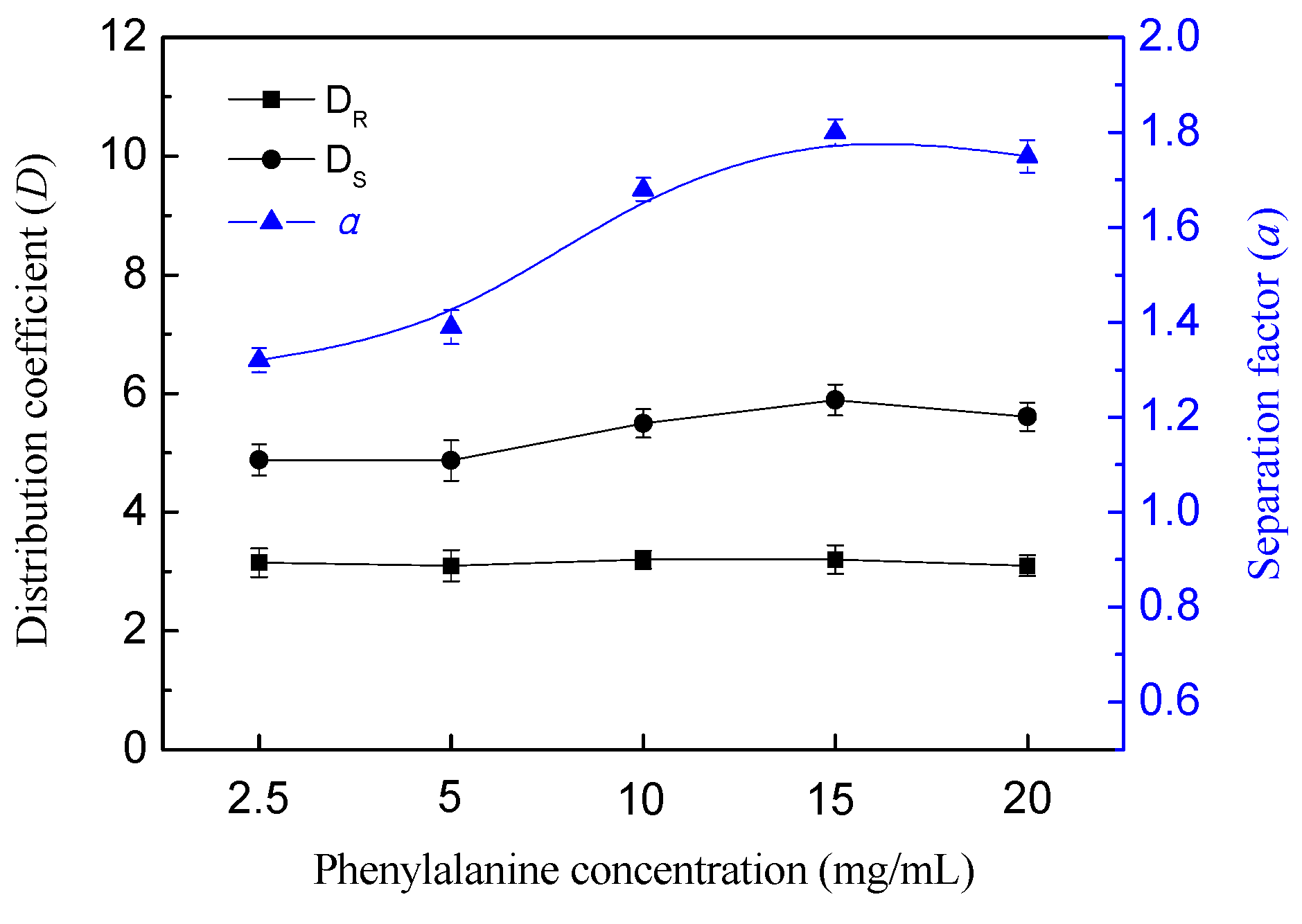
3.5. Effect of Phenylalanine Concentration
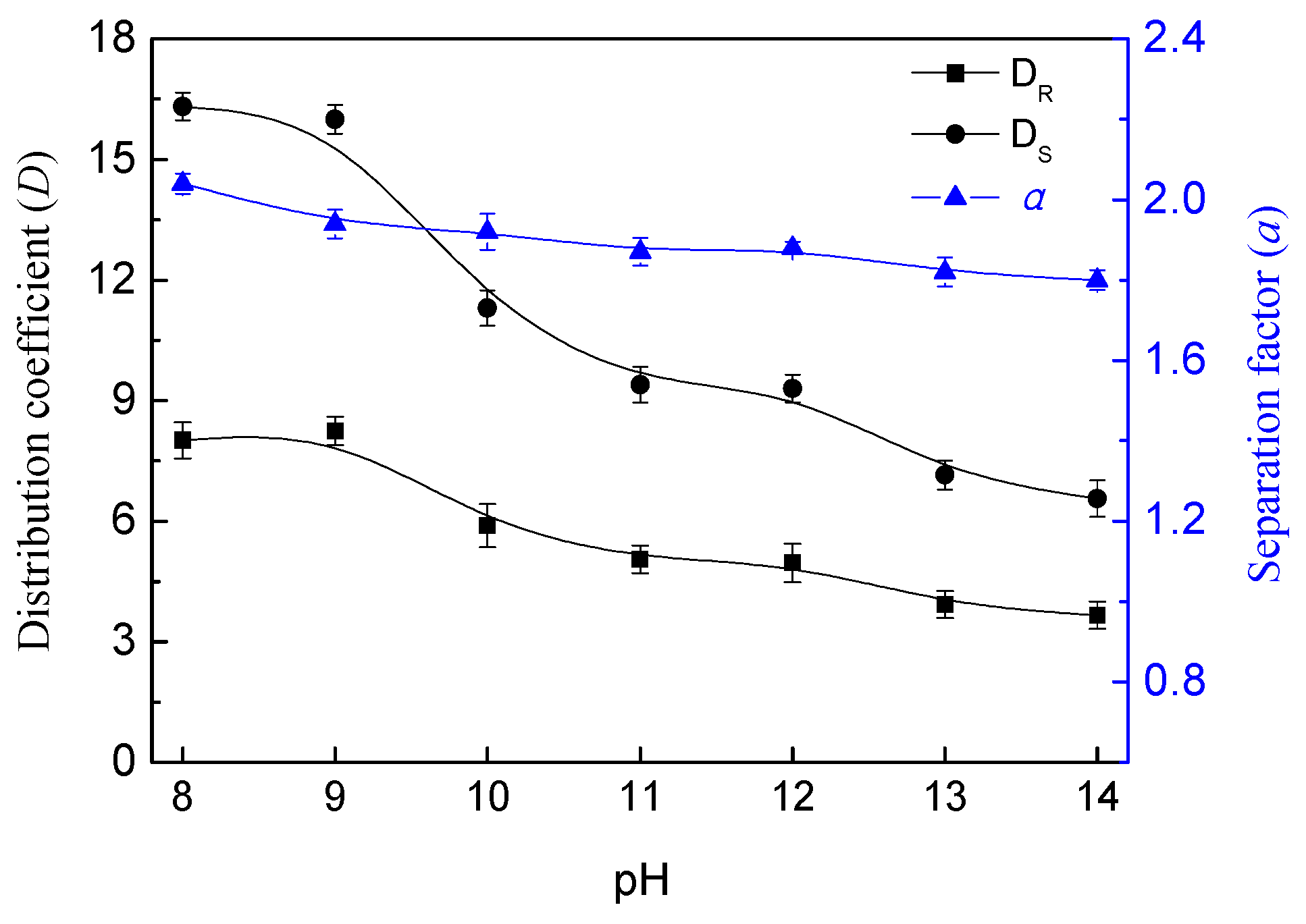
3.6. Effect of System pH
3.7. Effect of Operation Temperature
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, J.H.; Wu, J.Y.; Siuzdak, G.; Finn, M.G. Measurement of enantiomeric excess by kinetic resolution and mass spectrometry. Angew. Chem. Int. Ed. 1999, 38, 1755–1758. [Google Scholar] [CrossRef]
- Martens, J.; Gunther, K.; Schickedanz, M. Resolution of optical isomers by thin-layer chromatography: Enantiomeric purity of methyldopa. Arch. Pharm. 1986, 319, 572–574. [Google Scholar] [CrossRef]
- Bezhitashvili, L.; Bardavelidze, A.; Ordjonikidze, T.; Chankvetadze, L.; Chity, M.; Farkas, T.; Chankvetadze, B. Effect of pore-size optimization on the performance of polysaccharide-based superficially porous chiral stationary phases for the separation of enantiomers in high-performance liquid chromatography. J. Chromatogr. A 2017, 1482, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-M.; Yuan, L.-M. Recent progress of chiral stationary phases for separation of enantiomers in gas chromatography. J. Sep. Sci. 2017, 40, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Sanagi, M.M.; Aboul-Enein, H.Y. Advances in chiral separations by nonaqueous capillary electrophoresis in pharmaceutical and biomedical analysis. Electrophoresis 2014, 35, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, Y.; Choi, Y.K.; Choi, E.; Kim, K.; Park, J.; Kim, M.J. Dynamic kinetic resolution of diarylmethanols with an activated lipoprotein lipase. Acs Catal. 2014, 5, 683–689. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, S.; Shi, Y.; Gao, S.; Zheng, G. Enantioselective resolution of gamma-lactam by a novel thermostable type ii (+)-gamma-lactamase from the hyperthermophilic archaeon aeropyrum pernix. Appl. Biochem. Biotechnol. 2015, 176, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, Y.; Fukushima, C.; Yoshikawa, M. Molecularly imprinted nanofiber membranes from cellulose acetate aimed for chiral separation. J. Membr. Sci. 2010, 357, 90–97. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, H.; Zhang, P. Continuous separation of α-cyclohexyl-mandelic acid enantiomers by enantioselective liquid-liquid extraction in centrifugal contactor separators: Experiments and modeling. Ind. Eng. Chem. Res. 2013, 52, 3893–3902. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Wang, Z.; Luo, Y.; Song, H. High resolution of racemic phenylalanine with dication imidazolium-based chiral ionic liquids in a solid-liquid two-phase system. J. Chromatogr. A 2017, 1479, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Su, P.; Huang, J.; Wang, S.; Yang, Y. Synthesis of teicoplanin-modified hybrid magnetic mesoporous silica nanoparticles and their application in chiral separation of racemic compounds. J. Colloid Interface Sci. 2013, 399, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, J.; Wang, Q.; Huang, B. Chiral speciation and determination of dl-selenomethionine enantiomers on a novel chiral ligand-exchange stationary phase. Anal. Sci. 2005, 21, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Sanchez, J.; Argente-Garcia, A.I.; Moliner-Martinez, Y.; Roca-Sanjuan, D.; Antypov, D.; Campins-Falco, P.; Rosseinsky, M.J.; Marti-Gastaldo, C. Peptide metal-organic frameworks for enantioselective separation of chiral drugs. J. Am. Chem. Soc. 2017, 139, 4294–4297. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Feng, X.; Zhang, P.; Xu, W. Experimental and model study on separation of α-cyclopentylmandelic acid enantiomers by liquid-liquid extraction. J. Chem. Eng. Data 2016, 61, 3090–3097. [Google Scholar] [CrossRef]
- Tan, Z.; Li, F.; Zhao, C.; Teng, Y.; Liu, Y. Chiral separation of mandelic acid enantiomers using an aqueous two-phase system based on a thermo-sensitive polymer and dextran. Sep. Purif. Technol. 2017, 172, 382–387. [Google Scholar] [CrossRef]
- Albertsson, P.A. Partition of Cell Particles and Macromolecules; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Liu, C.L.; Nikas, Y.J.; Blankschtein, D. Novel bioseparations using two-phase aqueous micellar systems. Biotechnol. Bioeng. 1996, 52, 185–192. [Google Scholar] [CrossRef]
- Bertrand, B.; Mayolo-Deloisa, K.; Gonzalez-Gonzalez, M.; Tinoco-Valencia, R.; Serrano-Carreon, L.; Martinez-Morales, F.; Trejo-Hernandez, M.R.; Rito-Palomares, M. Pleurotus ostreatus laccase recovery from residual compost using aqueous two-phase systems. J. Chem. Technol. Biotechnol. 2016, 91, 2235–2242. [Google Scholar] [CrossRef]
- Liu, J.; Cao, X. Preparation of novel alkaline ph-responsive copolymers for the formation of recyclable aqueous two-phase systems and their application in the extraction of lincomycin. J. Sep. Sci. 2016, 39, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.W.; Tey, B.T.; Hii, S.L.; Mazlina, S.; Kamal, M.; Lan, J.C.W.; Ariff, A.; Ling, T.C. Purification of lipase derived from burkholderia pseudomallei with alcohol/salt-based aqueous two-phase systems. Process Biochem. 2009, 44, 1083–1087. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Farias, F.O.; Passos, H.; Sanglard, M.G.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Designer solvent ability of alcohols in aqueous biphasic systems composed of deep eutectic solvents and potassium phosphate. Sep. Purif. Technol. 2018, 200, 84–93. [Google Scholar] [CrossRef]
- Grilo, A.L.; Raquel Aires-Barros, M.; Azevedo, A.M. Partitioning in aqueous two-phase systems: Fundamentals, applications and trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]
- Tan, L.; Long, Y.; Jiao, F.; Chen, X. Enantioselective extraction of mandelic acid enantiomers by aqueous two-phase systems of polyethylene glycol and ammonium sulfate containing β-cyclodextrin as chiral selector. J. Iran Chem. Soc. 2011, 8, 889–896. [Google Scholar] [CrossRef]
- Ni, Y.; Zhou, J.; Sun, Z. Production of a key chiral intermediate of betahistine with a newly isolated kluyveromyces sp. In an aqueous two-phase system. Process Biochem. 2012, 47, 1042–1048. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Jiao, F.; Wang, Z. Extraction of phenylalanine enantiomers by aqueous two phase systems containing combinatorial chiral selector. Chin. J. Chem. 2012, 30, 965–969. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Dong, Q.-L.; Yu, J.-G.; Jiao, F.-P. Extraction of tryptophan enantiomers by aqueous two-phase systems of ethanol and (nh4)2so4. J. Chem. Technol. Biotechnol. 2013, 88, 1545–1550. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, L.; Fan, H.; Wan, Q.; Wu, X.; Tang, X.; Tang, J.Z. Enantioseparation of racemic flurbiprofen by aqueous two-phase extraction with binary chiral selectors of l-dioctyl tartrate and L-tryptophan. Chirality 2015, 27, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, Y.; Cai, P.; Shen, S.; Pan, Y. Specific cooperative effect for the enantiomeric separation of amino acids using aqueous two-phase systems with task-specific ionic liquids. J. Chromatogr. A 2015, 1395, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yao, S.; Qian, G.; Yao, T.; Song, H. A resolution approach of racemic phenylalanine with aqueous two-phase systems of chiral tropine ionic liquids. J. Chromatogr. A 2015, 1418, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.M.; Kulpa, J.C.F. Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem. 2005, 7, 185–189. [Google Scholar] [CrossRef]
- Thuy Pham, T.P.; Cho, C.-W.; Yun, Y.-S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zeng, L.; Wang, C.; Yang, Y.; Zhou, W.; Li, F.; Tan, Z. Assessment of the cytotoxicity of ionic liquids on spodoptera frugiperda 9 (sf-9) cell lines via in vitro assays. J. Hazard. Mater. 2018, 348, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-D.; Smith, T.J.; Li, N.; Zong, M.-H. Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Radosevic, K.; Zeleznjak, J.; Cvjetko Bubalo, M.; Radojcic Redovnikovic, I.; Slivac, I.; Gaurina Srcek, V. Comparative in vitro study of cholinium-based ionic liquids and deep eutectic solvents toward fish cell line. Ecotoxicol. Environ. Saf. 2016, 131, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (dess) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible deep eutectic solvents based on choline chloride: Characterization and application to the extraction of rutin from sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Fu, N.; Lv, R.; Guo, Z.; Guo, Y.; You, X.; Tang, B.; Han, D.; Yan, H.; Row, K.H. Environmentally friendly and non-polluting solvent pretreatment of palm samples for polyphenol analysis using choline chloride deep eutectic solvents. J. Chromatogr. A 2017, 1492, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araujo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chang, Y.; Tan, Z.; Li, F. Applications of choline amino acid ionic liquid in extraction and separation of flavonoids and pectin from ponkan peels. Sep. Sci. Technol. 2016, 51, 1093–1102. [Google Scholar] [CrossRef]
- De, S.S.; Masci, G.; Casciotta, F.; Caminiti, R.; Scarpellini, E.; Campetella, M.; Gontrani, L. Cholinium-amino acid based ionic liquids: A new method of synthesis and physico-chemical characterization. Phys. Chem. Chem. Phys. 2015, 17, 20687. [Google Scholar]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Tan, Z.; Li, F.; Xu, X. Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two-phase system. Sep. Purif. Technol. 2012, 98, 150–157. [Google Scholar] [CrossRef]
- Forsman, U. Enantiomeric resolution of an optically active guanine derivative by high-performance liquid chromatography with phenylalanine-cu(ii) in the mobile phase. J. Chromatogr. A 1984, 303, 217–221. [Google Scholar] [CrossRef]
- Altun, O.; Bilcen, S. Spectroscopic characterization of Cu(ii) complex of L-phenylalanine and D,L-tryptophan. Spectrochim. Acta A 2010, 75, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.T.; Blaise, A.; Larroque, M. Rapid automated high performance liquid chromatography method for simultaneous determination of amino acids and biogenic amines in wine, fruit and honey. J. Chromatogr. A 2010, 1217, 7385–7392. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chang, Y.; Tan, Z.; Li, F. Phase behavior of aqueous biphasic systems composed of novel choline amino acid ionic liquids and salts. J. Mol. Liq. 2016, 222, 836–844. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Wu, S.; Tan, Z. Salting-out extraction of allicin from garlic (Allium sativum L.) based on ethanol/ammonium sulfate in laboratory and pilot scale. Food Chem. 2017, 217, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Liu, Q.; Wang, J.; Zeng, H.; Yang, H.; Chen, X. Enantioseparation of (rs)-ibuprofen by closed recycling high-speed counter-current chromatography using hydroxypropyl-β-cyclodextrin as chiral selector. Tetrahedron 2016, 27, 301–306. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, G.; Tang, K.; Yang, W.; Sui, G.; Zhou, C. Enantiomeric separation of oxybutynin by recycling high-speed counter-current chromatography with hydroxypropyl-β-cyclodextrin as chiral selector. J. Sep. Sci. 2014, 37, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Li, F.-F. Chiral separation of alpha-cyclohexyl-mandelic-acid by aqueous two phase system combined with cu-2-beta-cyclodextrin complex. Chem. Eng. J. 2012, 211, 240–245. [Google Scholar] [CrossRef]
- Li, F.-F.; Tan, Z.-J.; Guo, Z.-F. Enantioseparation of mandelic acid and alpha-cyclohexylmandelic acid using an alcohol/salt-based aqueous two-phase system. Chem. Pap. 2014, 68, 1539–1545. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Yao, S.; Lin, M.; Song, H. A new and recyclable system based on tropin ionic liquids for resolution of several racemic amino acids. Anal. Chim. Acta 2017, 960, 81–89. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Wang, R.; Li, F.; Liu, L.; Tan, Z. Enantioselective Extraction of Phenylalanine Enantiomers Using Environmentally Friendly Aqueous Two-Phase Systems. Processes 2018, 6, 212. https://doi.org/10.3390/pr6110212
Sun D, Wang R, Li F, Liu L, Tan Z. Enantioselective Extraction of Phenylalanine Enantiomers Using Environmentally Friendly Aqueous Two-Phase Systems. Processes. 2018; 6(11):212. https://doi.org/10.3390/pr6110212
Chicago/Turabian StyleSun, Danyu, Runping Wang, Fenfang Li, Lianglei Liu, and Zhijian Tan. 2018. "Enantioselective Extraction of Phenylalanine Enantiomers Using Environmentally Friendly Aqueous Two-Phase Systems" Processes 6, no. 11: 212. https://doi.org/10.3390/pr6110212