Effect of Alkane Chain Length on Crystallization in Emulsions during Supercooling in Quiescent Systems and under Mechanical Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
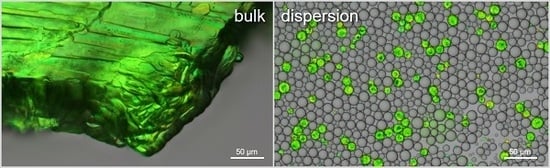
2.3. Droplet Size Distribution Analysis
2.4. Quiescent Crystallization Analysis
2.5. Crystallization under Mechanical Stress
2.6. Statistical Analysis
3. Results and Discussion
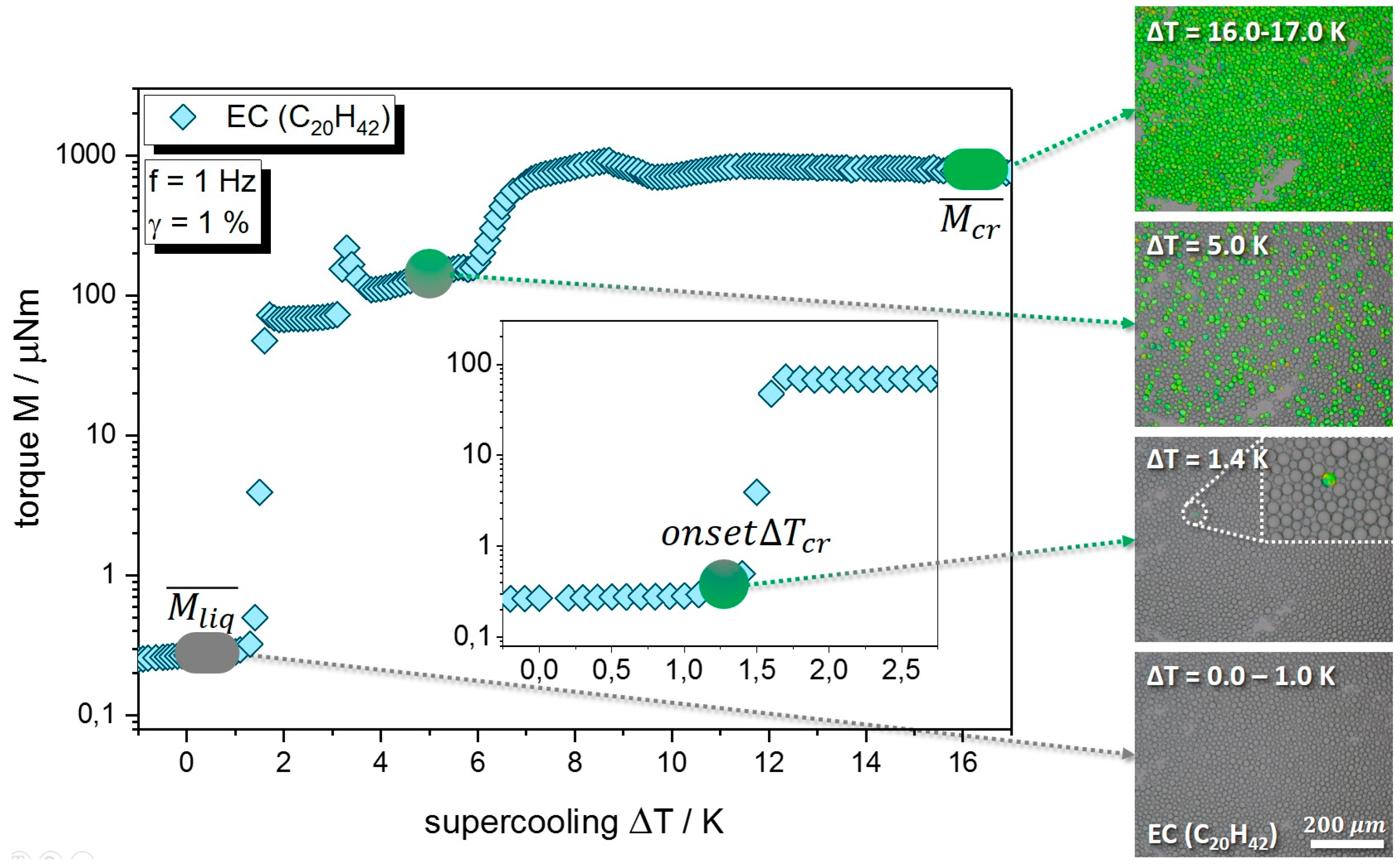
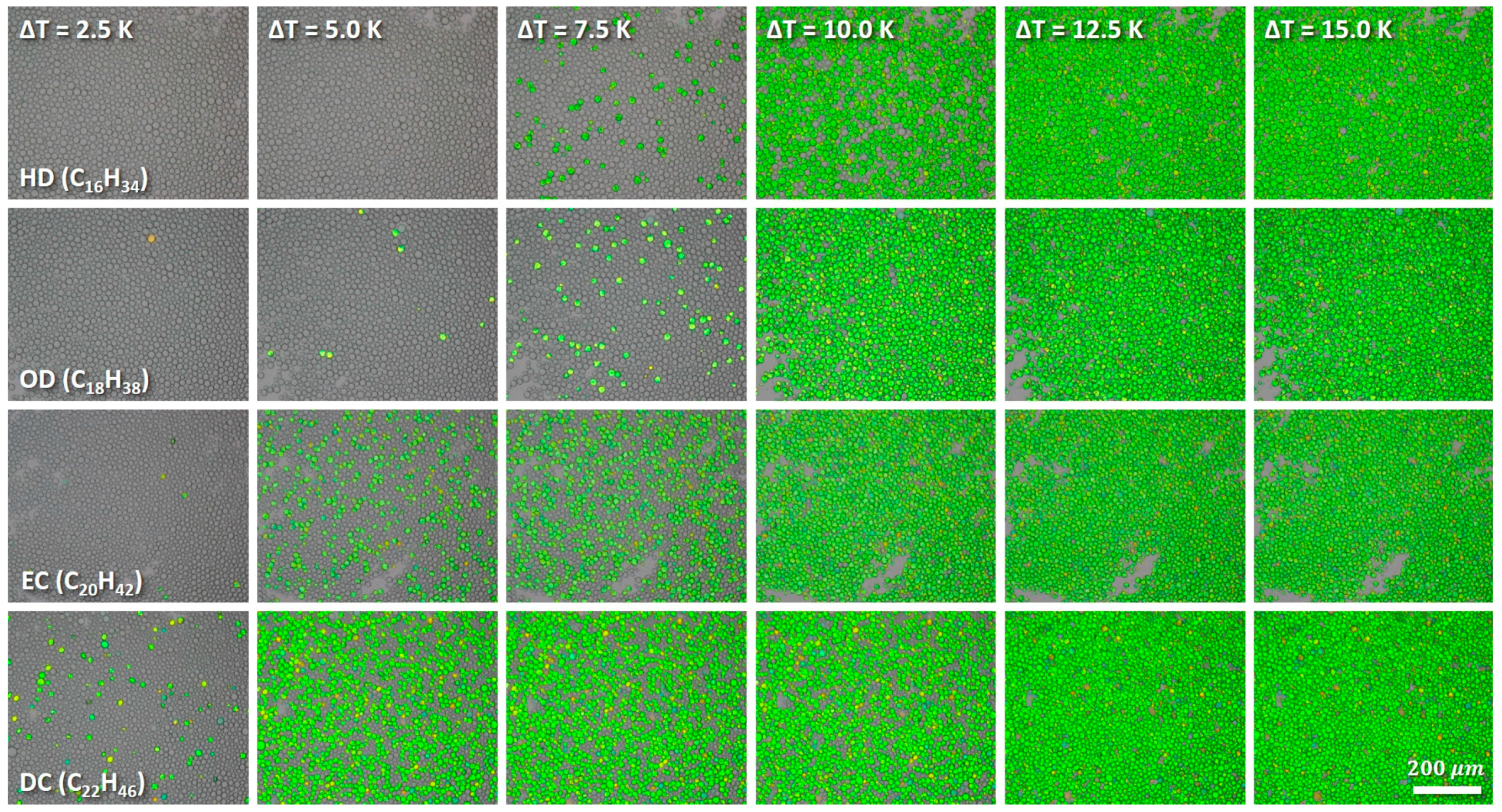
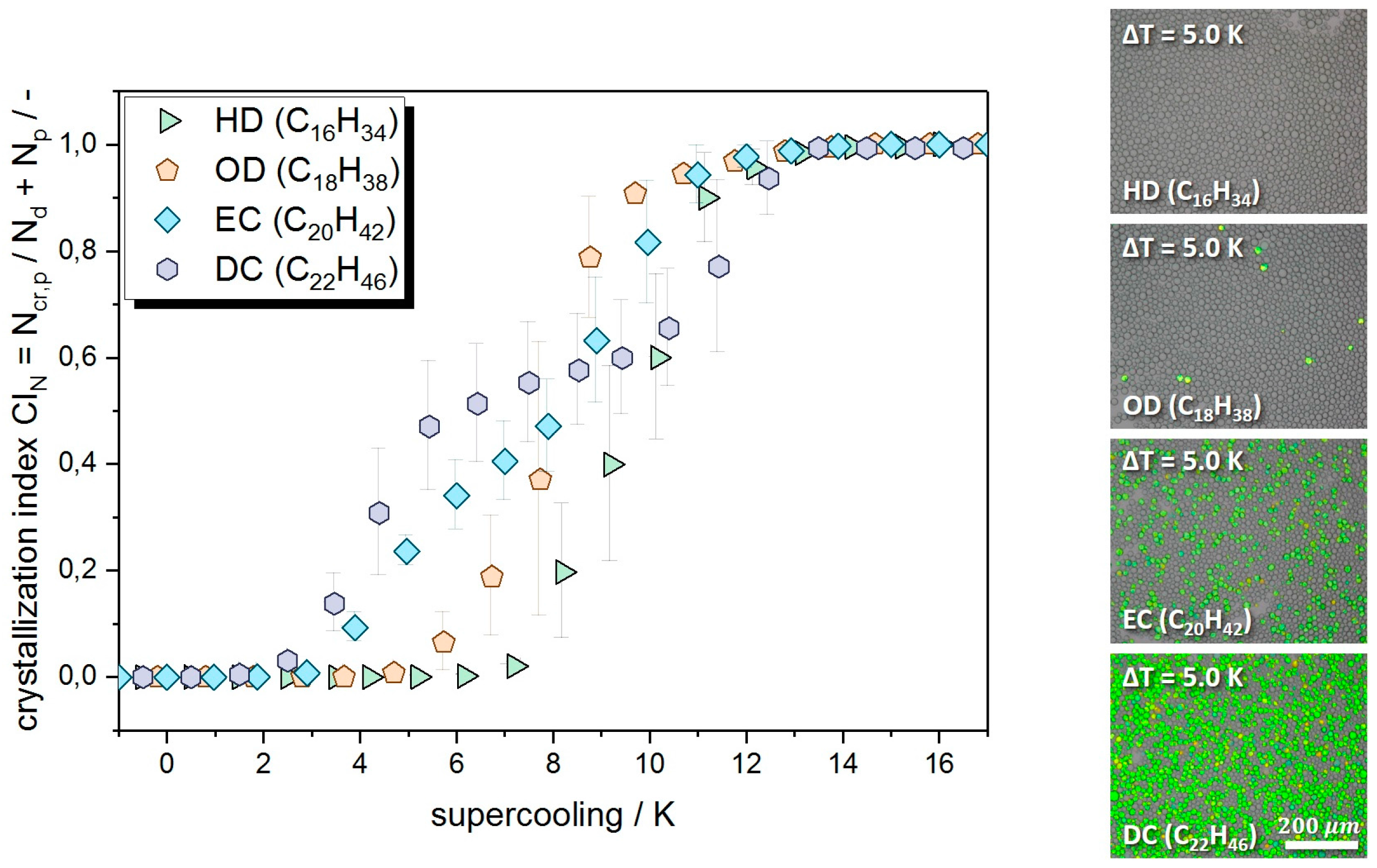
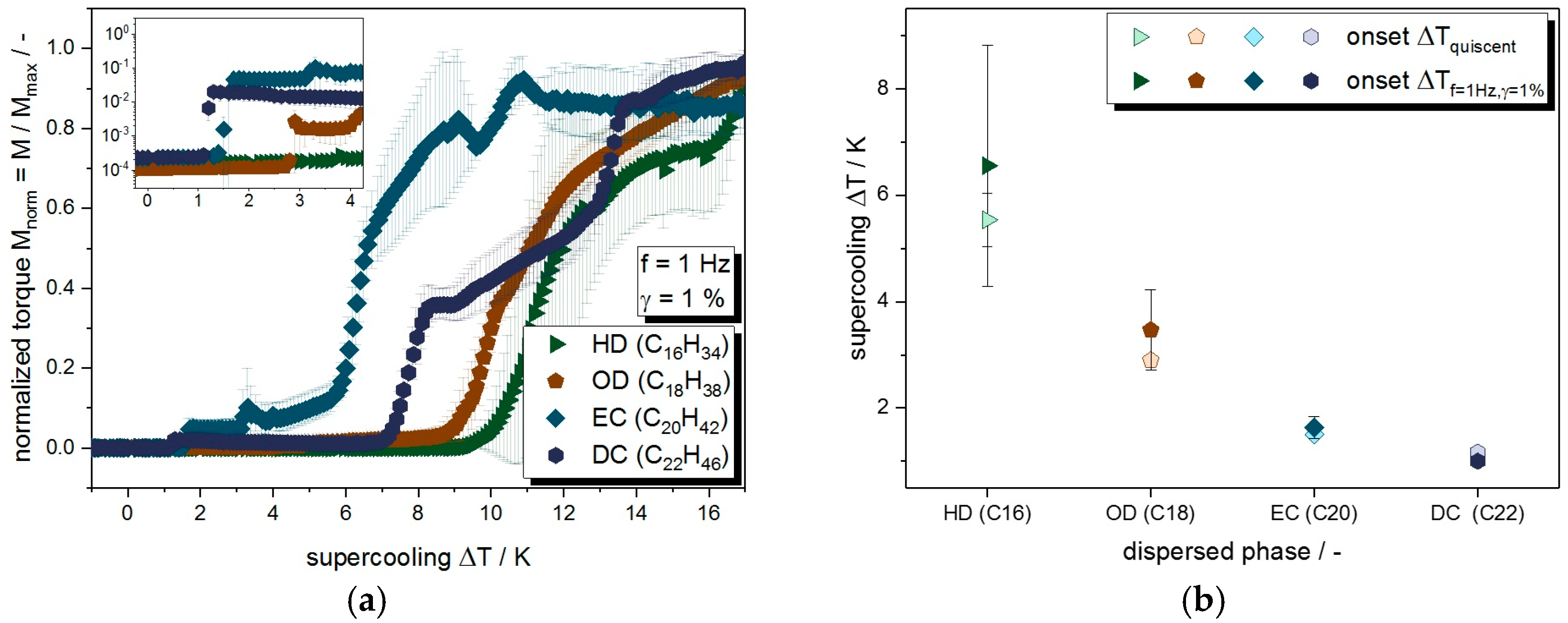
3.1. Quiescent Crystallization
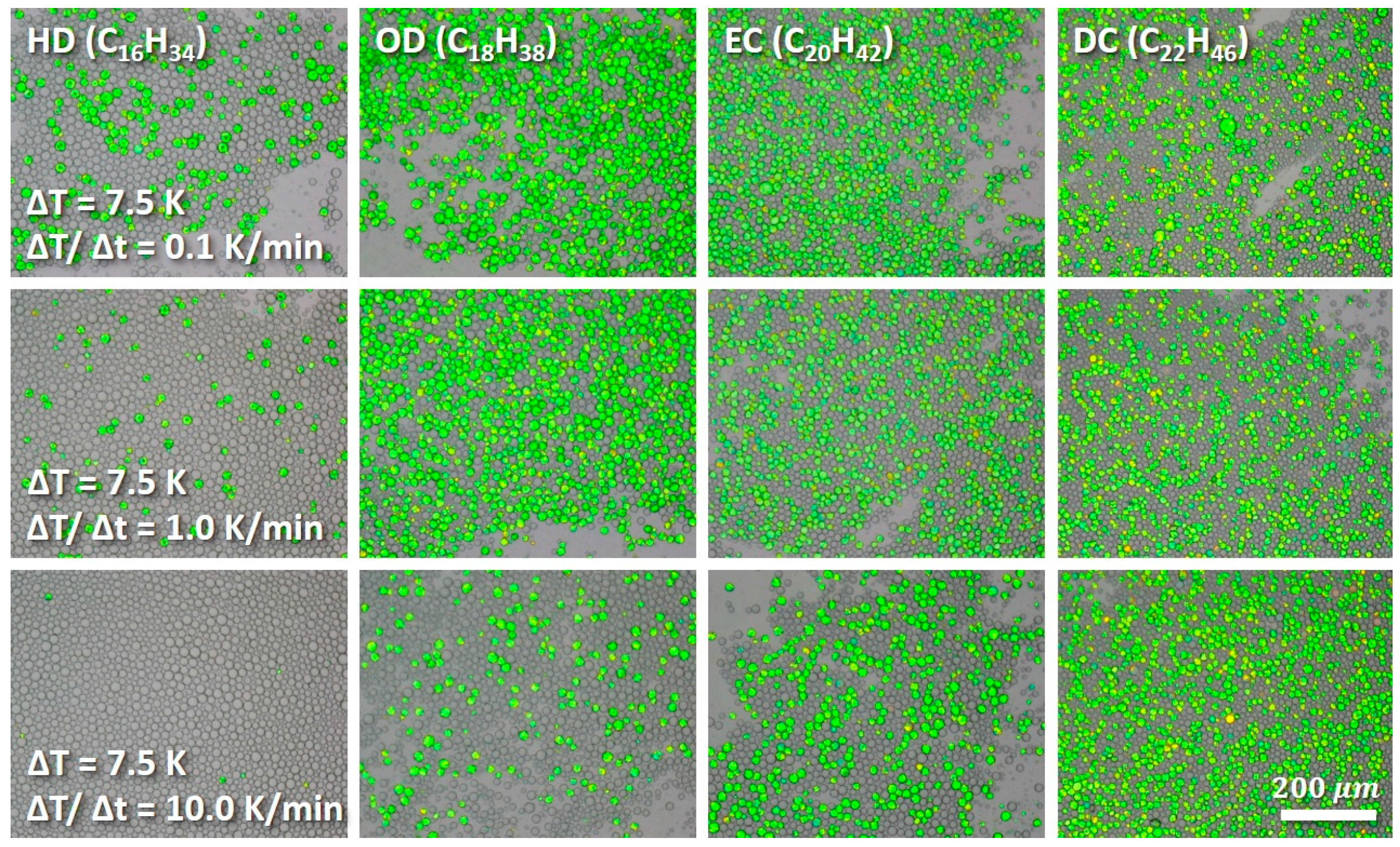
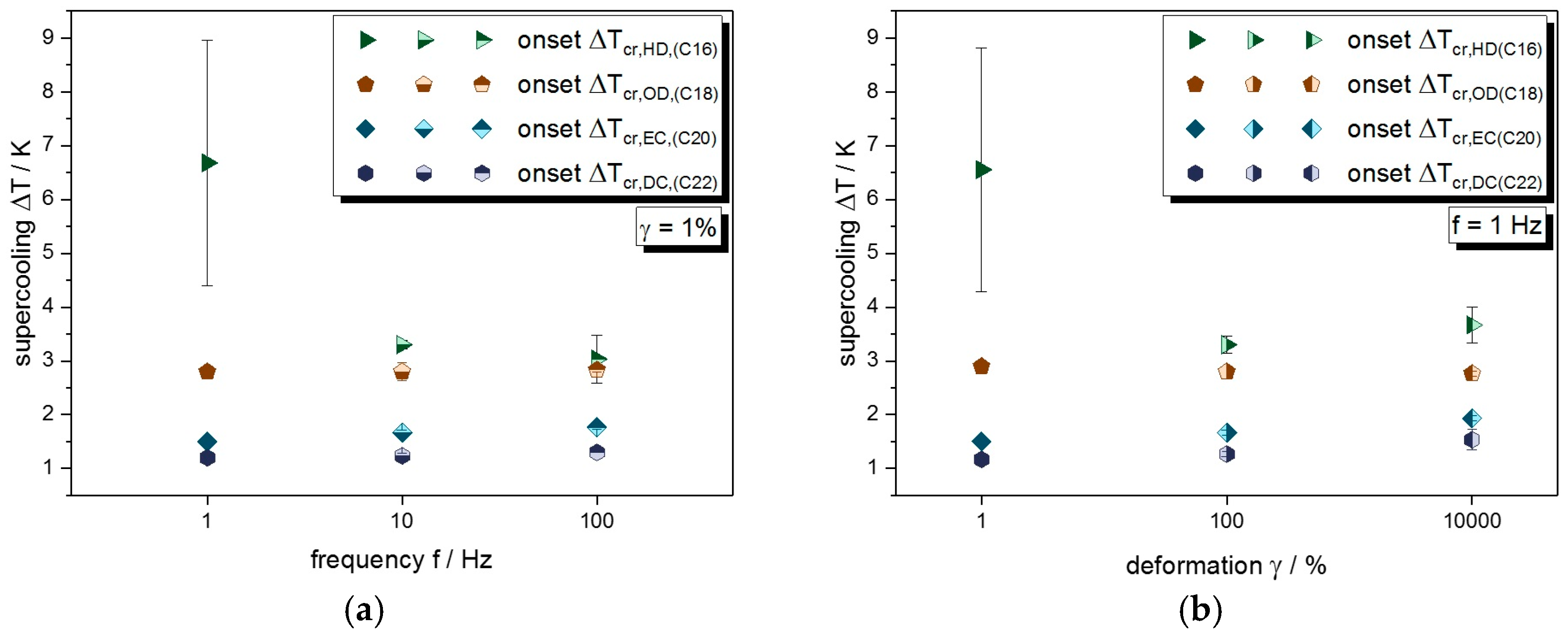
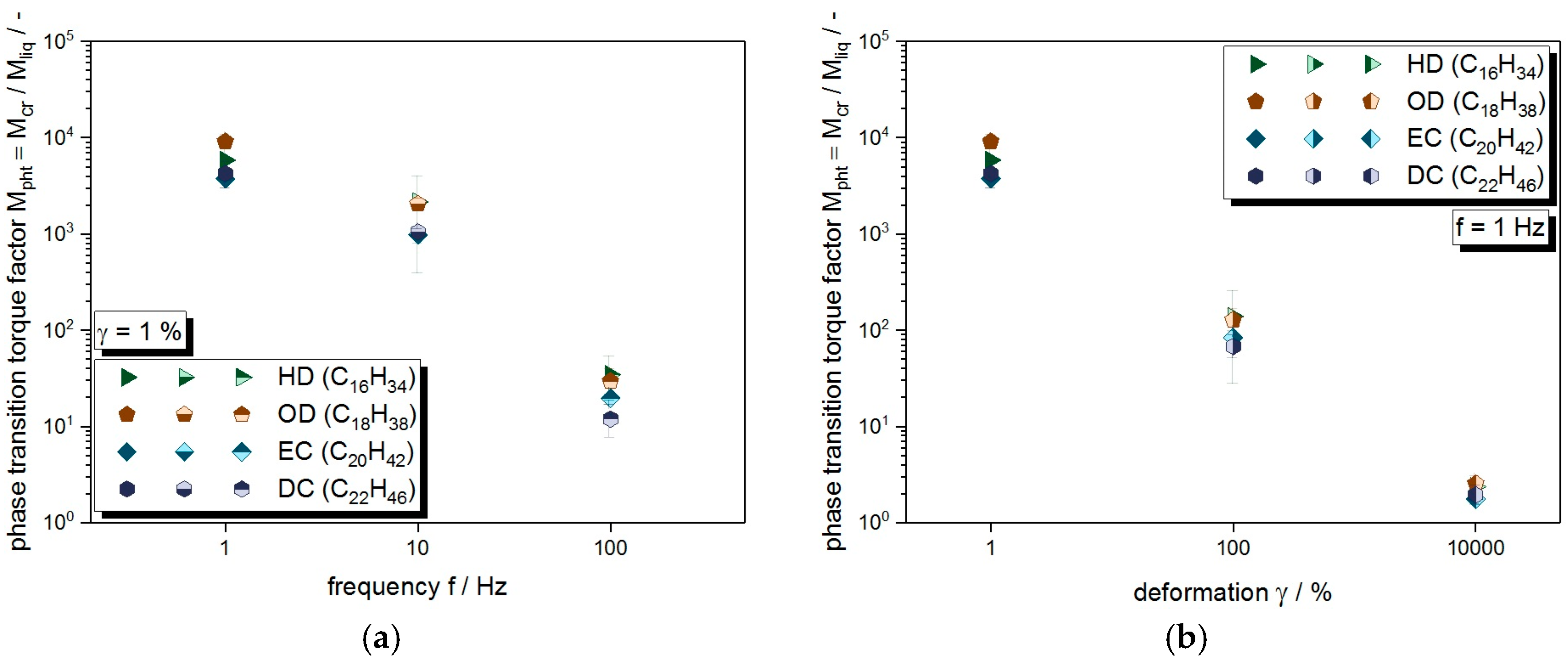
3.2. Crystallization under Mechanical Stress
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Droplet Size Distribution
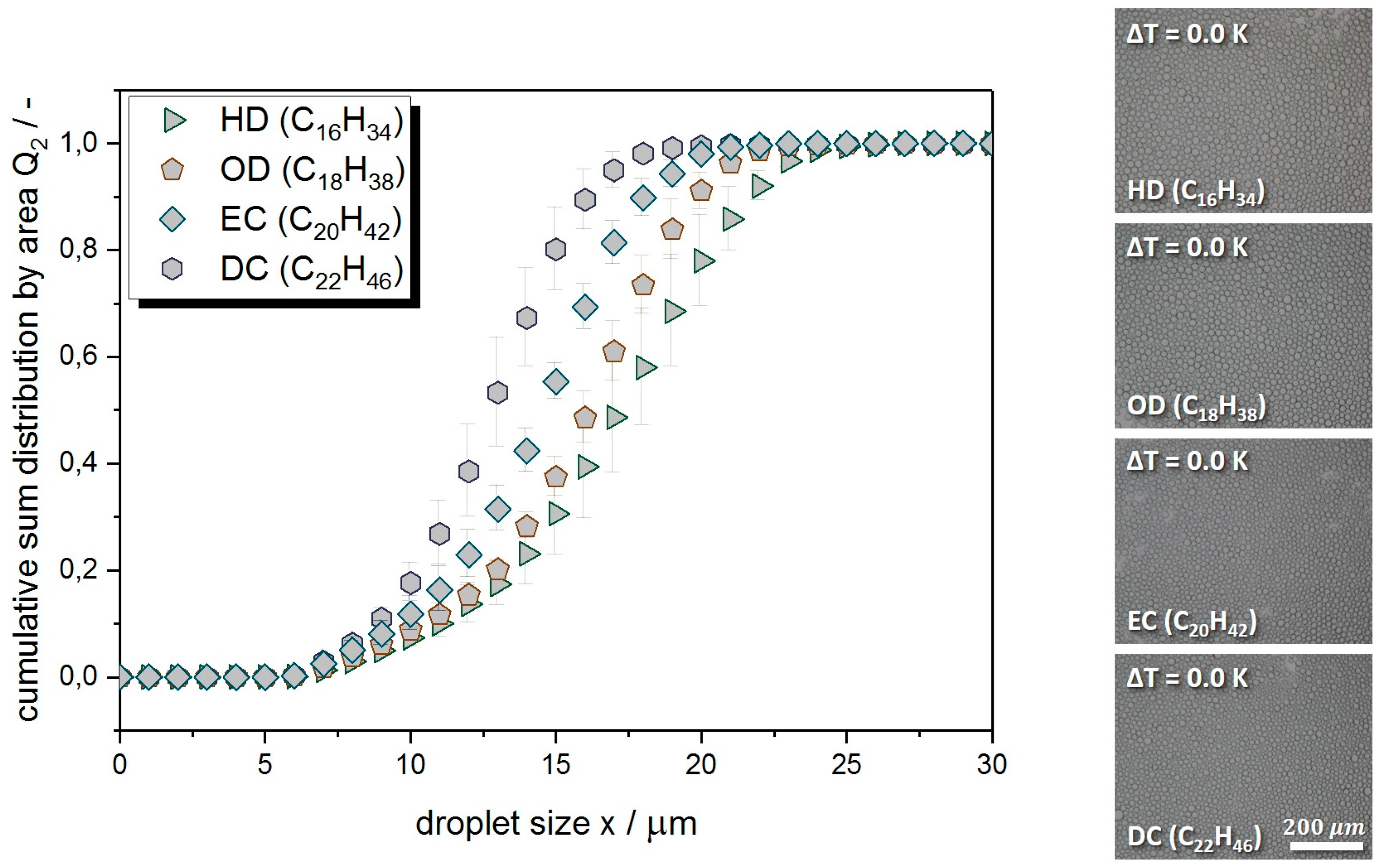
| Alkane (Dispersed Phase) | x50.2 (µm) | N (−) |
|---|---|---|
| C16: hexadecane (HD) | 17.2 ± 1.2 | 1297 ± 306 |
| C18: octadecane (OD) | 16.1 ± 0.4 | 1653 ± 148 |
| C20: eicosane (EC) | 14.6 ± 0.2 | 1774 ± 65 |
| C22: docosane (DC) | 12.8 ± 1.0 | 2506 ± 86 |
Appendix B. Onset Crystallization Temperature and Melting Point
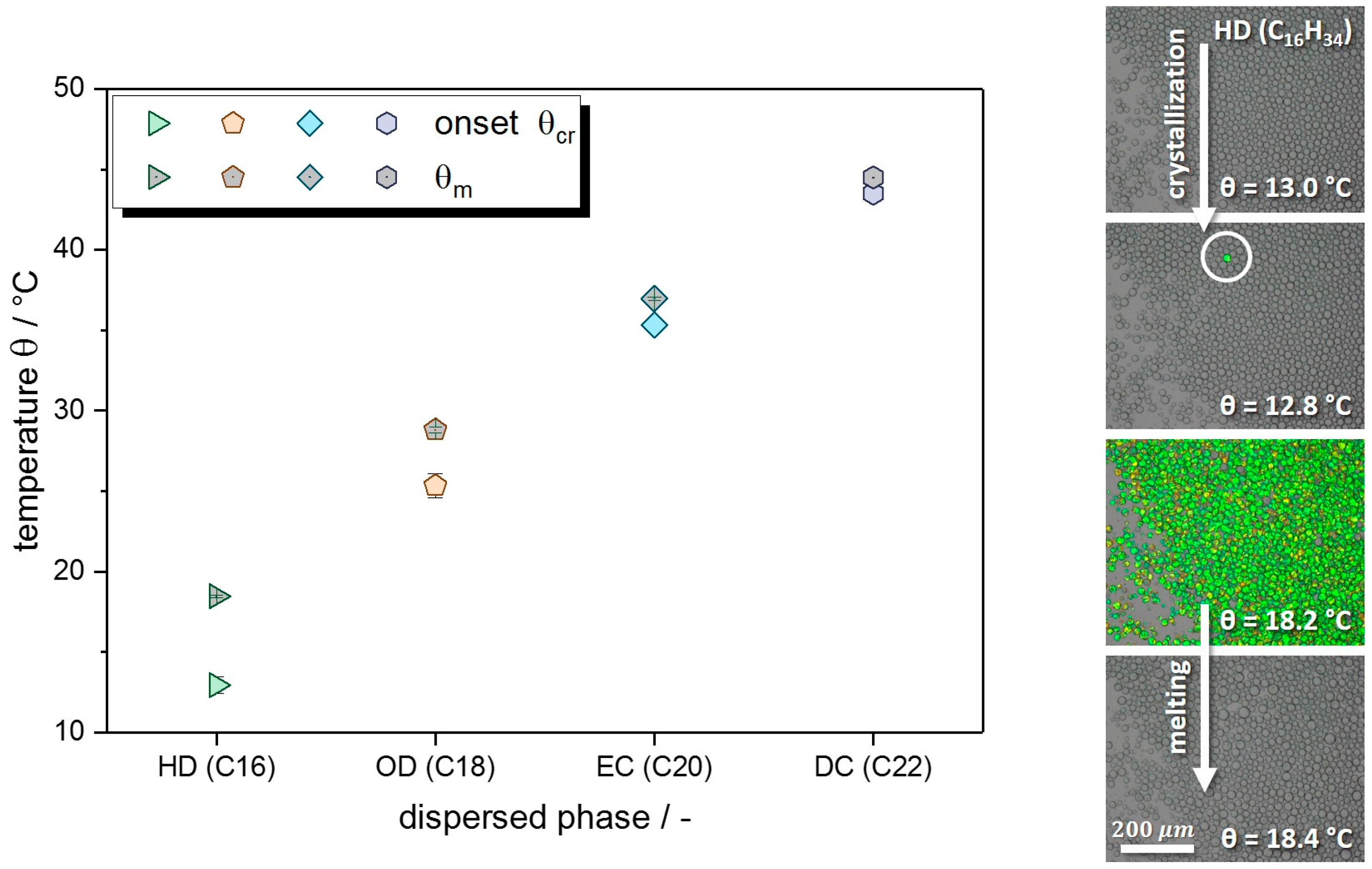
| Alkane (Dispersed Phase) | Onset θcr,dispersion/°C | θm,dispersion/°C | θm,ref,bulk/°C [51] |
|---|---|---|---|
| C16: hexadecane (HD) | 12.9 ± 0.5 | 18.5 ± 0.1 | 18.2 |
| C18: octadecane (OD) | 25.3 ± 0.8 | 28.8 ± 0.2 | 28.2 |
| C20: eicosane (EC) | 35.3 ± 0.2 | 37.0 ± 0.1 | 36.6 |
| C22: docosane (DC) | 43.5 ± 0.1 | 44.5 ± 0.0 | 44.1 |
Appendix C. Time Dependency of Crystallization Process

References
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Müller, R. Solid lipid nanoparticles (SLN) for controlled drug delivery: A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mehnert, W. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Coupland, J.N. Crystallization in emulsions. Curr. Opin. Colloid Interface Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid Lipid Nanoparticles as Delivery Systems for Bioactive Food Components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Behr’s Verlag. Emulgiertechnik. Grundlagen, Verfahren und Anwendungen; Köhler, K., Schuchmann, H.P., Eds.; Behr’s Verlag: Hamburg, Germany, 2012. [Google Scholar]
- Walstra, P. Physical Chemistry of Foods; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Köhler, K.; Hensel, A.; Kraut, M.; Schuchmann, H.P. Melt emulsification—Is there a chance to produce particles without additives? Particuology 2011, 9, 506–509. [Google Scholar] [CrossRef]
- Abramov, S.; Ruppik, P.; Schuchmann, H. Crystallization in Emulsions: A Thermo-Optical Method to Determine Single Crystallization Events in Droplet Clusters. Processes 2016, 4, 25. [Google Scholar] [CrossRef]
- Abramov, S.; Berndt, A.; Georgieva, K.; Ruppik, P.; Schuchmann, H.P. Investigation of the influence of mean droplet size and shear rate on crystallization behavior of hexadecane-in-water dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 513–522. [Google Scholar] [CrossRef]
- Tippetts, M.; Martini, S. Effect of cooling rate on lipid crystallization in oil-in-water emulsions. Food Res. Int. 2009, 42, 847–855. [Google Scholar] [CrossRef]
- Bolzinger, M.A.; Cogne, C.; Lafferrere, L.; Salvatori, F.; Ardaud, P.; Zanetti, M.; Puel, F. Effects of surfactants on crystallization of ethylene glycol distearate in oil-in-water emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 93–100. [Google Scholar] [CrossRef]
- Ghosh, S.; Cramp, G.L.; Coupland, J.N. Effect of aqueous composition on the freeze-thaw stability of emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 82–88. [Google Scholar] [CrossRef]
- Samtlebe, M.; Yucel, U.; Weiss, J.; Coupland, J.N. Stability of Solid Lipid Nanoparticles in the Presence of Liquid Oil Emulsions. J. Am. Oil Chem. Soc. 2012, 89, 609–617. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Palanuwech, J.; Coupland, J.N. Stability of emulsions to dispersed phase crystallization: Effect of oil type, dispersed phase volume fraction, and cooling rate. Colloids Surf. A Physicochem. Eng. Asp. 2002, 204, 227–237. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Palanuwech, J.; Coupland, J.N. Influence of Fat Crystallization on the Stability of Flocculated Emulsions. J. Agric. Food Chem. 2002, 50, 5224–5228. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rousseau, D. Fat crystals and water-in-oil emulsion stability. Curr. Opin. Colloid Interface Sci. 2011, 16, 421–431. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Influence of Polymorphic Transformations on Gelation of Tripalmitin Solid Lipid Nanoparticle Suspensions. J. Am. Oil Chem. Soc. 2008, 85, 501–511. [Google Scholar] [CrossRef]
- Yang, D.; Hrymak, A.N. Crystal Morphology of Hydrogenated Castor Oil in the Crystallization of Oil-in-Water Emulsions: Part I. Effect of Temperature. Ind. Eng. Chem. Res. 2011, 50, 11585–11593. [Google Scholar] [CrossRef]
- Rousseau, D. Fat crystals and emulsion stability—A review. Food Res. Int. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Hindle, S.; Povey, M.J.; Smith, K. Kinetics of Crystallization in n-Hexadecane and Cocoa Butter Oil-in-Water Emulsions Accounting for Droplet Collision-Mediated Nucleation. J. Colloid Interface Sci. 2000, 232, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Ma, J.; Povey, M.J. Crystallization kinetics in oil-in-water emulsions containing a mixture of solid and liquid droplets. Faraday Trans. 1996, 92, 1213. [Google Scholar] [CrossRef]
- Petersen, S.; Ulrich, J. Role of Emulsifiers in Emulsion Technology and Emulsion Crystallization. Chem. Eng. Technol. 2013, 36, 398–402. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ulrich, J. Prediction of degree of deformation and crystallization time of molten droplets in pastillation process. Int. J. Pharm. 2003, 257, 205–215. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Dungan, S.R.; German, J.B.; Simoneau, C.; Kinsella, J.E. Droplet Size and Emulsifier Type Affect Crystallization and Melting of Hydrocarbon-in-Water Emulsions. J. Food Sci. 1993, 58, 1148–1151. [Google Scholar] [CrossRef]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, P.W. Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Org. Process Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Alexander, A.J.; Camp, P.J. Single Pulse, Single Crystal Laser-Induced Nucleation of Potassium Chloride. Cryst. Growth Des. 2009, 9, 958–963. [Google Scholar] [CrossRef]
- Javid, N.; Kendall, T.; Burns, I.S.; Sefcik, J. Filtration Suppresses Laser-Induced Nucleation of Glycine in Aqueous Solutions. Cryst. Growth Des. 2016, 16, 4196–4202. [Google Scholar] [CrossRef] [Green Version]
- Da Pieve, S.; Calligaris, S.; Co, E.; Nicoli, M.C.; Marangoni, A.G. Shear Nanostructuring of Monoglyceride Organogels. Food Biophys. 2010, 5, 211–217. [Google Scholar] [CrossRef]
- Garbolino, C.; Ziegler, G.R.; Coupland, J.N. Ultrasonic determination of the effect of shear on lipid crystallization. J. Am. Oil Chem. Soc. 2000, 77, 157–162. [Google Scholar] [CrossRef]
- Saggin, R.; Coupland, J.N. Shear and longitudinal ultrasonic measurements of solid fat dispersions. J. Am. Oil Chem. Soc. 2004, 81, 27–32. [Google Scholar] [CrossRef]
- Sonwai, S.; Mackley, M.R. The effect of shear on the crystallization of cocoa butter. J. Am. Oil Chem. Soc. 2006, 83, 583–596. [Google Scholar] [CrossRef]
- Tarabukina, E.; Jego, F.; Haudin, J.-M.; Navard, P.; Peuvrel-Disdier, E. Effect of shear on the rheology and crystallization of palm oil. J. Food Sci. 2009, 74, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanapalli, S.A.; Coupland, J.N. Emulsions under shear—The formation and properties of partially coalesced lipid structures. Food Hydrocoll. 2001, 15, 507–512. [Google Scholar] [CrossRef]
- Barfod, N.M.; Krog, N. Destabilization and fat crystallization of whippable emulsions (toppings) studied by pulsed NMR. J. Am. Oil Chem. Soc. 1987, 64, 112–119. [Google Scholar] [CrossRef]
- Goff, H.D. Instability and Partial Coalescence in Whippable Dairy Emulsions. J. Dairy Sci. 1997, 80, 2620–2630. [Google Scholar] [CrossRef]
- Stapley, A.G.F.; Tewkesbury, H.; Fryer, P.J. The effects of shear and temperature history on the crystallization of chocolate. J. Am. Oil Chem. Soc. 1999, 76, 677–685. [Google Scholar] [CrossRef]
- Hindle, S.A.; Povey, M.J.; Smith, K.W. Characterizing cocoa butter seed crystals by the oil-in-water emulsion crystallization method. J. Am. Oil Chem. Soc. 2002, 79, 993–1002. [Google Scholar] [CrossRef]
- Thomson, W. On the equilibrium of vapor at a curved surface of liquid. Philos. Mag. 1871, 448–452. [Google Scholar]
- Povey, M.J.W. Crystal nucleation in food colloids. Food Hydrocoll. 2014, 42, 118–129. [Google Scholar] [CrossRef]
- Pound, G.M.; La Mer, V.K. Kinetics of Crystalline Nucleus Formation in Supercooled Liquid Tin 1,2. J. Am. Chem. Soc. 1952, 74, 2323–2332. [Google Scholar] [CrossRef]
- Rodriguez-Spong, B.; Price, C.P.; Jayasankar, A.; Matzger, A.J.; Rodriguez-Hornedo, N. General principles of pharmaceutical solid polymorphism: A supramolecular perspective. Adv. Drug Deliv. Rev. 2004, 56, 241–274. [Google Scholar] [CrossRef] [PubMed]
- Domalski, E.S.; Hearing, E.D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III. J. Phys. Chem. Ref. Data 1996, 25, 1–525. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramov, S.; Shah, K.; Weißenstein, L.; Karbstein, H.P. Effect of Alkane Chain Length on Crystallization in Emulsions during Supercooling in Quiescent Systems and under Mechanical Stress. Processes 2018, 6, 6. https://doi.org/10.3390/pr6010006
Abramov S, Shah K, Weißenstein L, Karbstein HP. Effect of Alkane Chain Length on Crystallization in Emulsions during Supercooling in Quiescent Systems and under Mechanical Stress. Processes. 2018; 6(1):6. https://doi.org/10.3390/pr6010006
Chicago/Turabian StyleAbramov, Serghei, Kinza Shah, Lydia Weißenstein, and Heike Petra Karbstein. 2018. "Effect of Alkane Chain Length on Crystallization in Emulsions during Supercooling in Quiescent Systems and under Mechanical Stress" Processes 6, no. 1: 6. https://doi.org/10.3390/pr6010006