1. Introduction
With the rapid development of the world’s society and economy, especially in developing countries, the growing needs for energy supply are becoming devastating. Global energy demand is expected to double by 2050 [
1]. More than 80% of global energy demand is still satisfied by fossil fuels due to their abundance and availability [
2]. However, the world fossil fuels are depleting and produce different environmental problems, suggesting that alternative solutions are needed.
For these reasons, renewable energies (RES) are suggested as an alternative to traditional fossil fuels, and significant progress has been achieved. The need to find clean and sustainable energy is a critical factor for society [
3].
In this context, power-to-liquid (PtL) systems seem to represent an efficient solution for future energy scenarios: a PtL technology concerns a process that is able to absorb energy (overproduction of RES on the grid), converting and storing that energy into liquid fuels through the use of carbon dioxide as a reagent. This system is an effective way to moderate the intermittency of renewable energy in order to stabilize the electrical grid. In addition, the conversion of carbon dioxide into fuels allows the recycling of carbon dioxide and the reduction of greenhouse gas (GHG) emissions.
Processes based on methanol synthesis have received significant attention over the past decade. Carbon dioxide and hydrogen are used as raw materials according to hydrogenation catalytic reaction. In recent years, several efforts have been made to develop efficient CO
2 utilization technologies, and the more attractive processes are the hydrogenation of CO
2 and the reforming of CO
2 with CH
4 [
4,
5,
6]. Sources of carbon dioxide can be: power plants, air, ammonia plants, anaerobic digestion plants, process industries like cement, iron, and steel, aluminum, pulp and paper, and refineries from which it is produced by way of carbon capture and utilization (CCU) processes [
7]. Waste is then recovered and enhanced to produce chemical compounds. Hydrogen, instead, can be produced by the electrolysis of water using renewable energies such as solar or wind energy. It may be produced through the alkaline or proton exchange membrane electrolysis (PEM), while the solid oxide electrolyser cells (SOECs) are currently less developed [
8]. Hydrogen can also be obtained by coke oven gas (COG) from steel works, syngas, methane steam reforming, chlorine alkali, and petrochemical plants [
9]. An innovative technology allows the production of hydrogen by photocatalytic water splitting [
10]. A catalytic reaction producing methanol takes place in the ranges of temperature and pressure equal to 423–523 K and 50–100 bar, respectively, on CuO/ZnO/Al
2O
3 as catalyst [
11,
12].
It is evident that the use of renewable resources to substitute fossil fuels is one of the technological options to mitigate GHG emissions [
13]. Using this in relation to methanol production is then a green process, with lower environmental impacts.
Other routes can be used for the production of methanol, such as the dry reformation of methane and carbon dioxide into syngas that is converted into methanol through the Fischer–Tropsch reaction [
14,
15], the hydrogenation of syngas [
16], the co-electrolysis of carbon dioxide and water [
17,
18], or the photocatalytic reaction of carbon dioxide and water [
19,
20]. Additionally, as an alternative to carbon dioxide, other raw materials can be used to produce methanol as natural gas through a reforming reaction or as coal and biomass via gasification [
21]. Currently, methanol is predominantly produced from natural gas via steam reforming or from coal via gasification on an industrial scale [
22].
Methanol is a key chemical intermediate for numerous commodities: dimethyl ether, methylamines, chloromethanes, solvents, propylene, olefins, formaldehyde, acetic acid, methyl methacrylate, gasoline/fuels [
13,
23,
24]. Methanol-to-olefins (MTO) and methanol-to-propylene (MTP) demand has increased in recent years, rising from 6% of end use demand in 2011 to 22% by 2016, especially in China [
25]. Other uses of methanol include wastewater de-nitrification, the hydrogen carrier for fuel cells and the transesterification of vegetable oils for biodiesel production and electricity generation [
26,
27]. Methanol can then be used from energy uses to chemical uses [
28,
29]. With a high-octane number, methanol ensures good antiknock performance, in addition to high volatility, denser fuel–air charge, and excellent lean burn properties [
30]. The current annual consumption of methanol is over 60 million metric tons globally, and it will grow [
31]. In 2030, meeting the European yearly demand will require 41–76 MtCO
2/year, meaning that 16–31 MtCO
2/year of CO
2 will not be emitted, because through the use of CCU systems 28–52 Mt MeOH/year will be produced [
32].
The production of methanol via the hydrogenation of carbon dioxide and hydrogen is a mature technology, and the literature mainly covers the economic and technical aspects of its process. Pilot plants that use renewable hydrogen are in operation in Japan, Iceland (with a capacity of 4000 ton/year, constructed by Carbon Recycling International and operating since 2011), and Osaka (with a capacity of 100 ton/year) [
11]. The catalytic hydrogenation of carbon dioxide has become technically competitive with the industrial production of methanol from syngas [
11,
33,
34], and shows great potential for large-scale applications and carbon dioxide consumption [
35].
Bellotti et al. [
22] analyzed a process for the production of methanol by capturing carbon dioxide from power plants and producing hydrogen from PEM electrolyzers. The power to process methanol is made possible by water electrolyzers and carbon capture systems, using amine solution and a methanol reactor. An economic feasibility study of the plant was carried out, taking into consideration three different sizes (4000, 10,000, 50,000 ton/year of CH
3OH produced), using W-ECoMP software. A sensitivity analysis was also developed, varying methanol selling price, oxygen selling options for industrial applications, and the capital cost of PEM electrolyzers. Results show that the PEM electrolyzer affects more than 75% of total capital investment and a larger plant leads to higher capital investment but allows for a slower payback period.
Atsonios et al. [
36] analyzed a process to produce methanol using carbon dioxide obtained by power plants or other intensive carbon emission industries (cement plant, steel industry) through mono-ethanolamine (MEA) absorption, while hydrogen is obtained by water electrolysis with alkaline electrolyzers. Different electric sources are considered: grids, thermal power plants, wind energies. An economic analysis of an H
2 production scheme reveals that each of three main parameters for the determination of H
2 cost (electrolyzer capital cost, electricity cost, and storage cost) can play a key role in the feasibility of the plant. Then, hydrogen production cost is the most significant factor influencing the economy of this methanol process. The total specific power consumption of the methanol plant was equal to 113.4 kWhe/ton
MeOH.
Rivera-Tinoco et al. [
37] found a methanol cost of 891 €/ton for the PEM/methanol process and 5459 €/ton for the SOEC/methanol process. Promising reduction can be obtained by improving the electrolyzed system: its energy efficiency is a key parameter to decrease costs. On the other hand, research activities for high-temperature CO
2 electrolysis are accelerating [
38].
Perez-Fortes et al. [
7] evaluated a techno-economic and environmental analysis for a process producing methanol from H
2 and CO
2 captured by power plants. In particular, the net reduction of CO
2 emissions and production cost were evaluated in comparison with the conventional synthesis processes of MeOH in Europe. The evaluated plant produces 440 kton/year of methanol, and its configuration is the result of a heat integration process. A simulation of the system was developed in ChemCad 6.3
® software (Chemstation, Huston, Texas, USA). Results show that in order to have an economically feasible plant, the price of methanol should be around 800 €/ton, H
2 costs should be around 1236 €/ton, or CO
2 should have a value of around 222 €/t. Additionally, compared to traditional plants, the process has a lower capital cost but higher variable costs. Regarding an environmental analysis, the system allows the emission of 2 tons of carbon dioxide to be avoided for one ton of produced methanol.
Kiss et al. [
9] developed a new process to produce methanol by the hydrogenation of carbon dioxide, using wet hydrogen available as a by-product in chlor-alkali production. The key feature of this novel process is the use of a stripping unit, where wet hydrogen (saturated with water) flows in counter-current with a condensed mixture of methanol–water resulting from flash separation after reaction. In this way, CO
x is removed by methanol–water mixture, allowing a complete recycling of CO
2, also removing water from wet hydrogen (initially saturated with water) and thus avoiding a negative impact on reaction equilibrium conversion.
Atsonios et al. [
39] developed a new membrane reactor with high selectivity either in methanol permeation or in water permeation. Methanol was produced according to the hydrogenation of carbon dioxide obtained by power plants while hydrogen was obtained by the electrolysis of water in alkaline cells. Results showed that the membrane did not influence thermal efficiency, but increased methanol yield. This allows the reduction of the recycling gas flow rate, leading to reduced reactor dimensions and investment costs.
Al-Kalbani et al. [
31] compared two different processes to produce methanol using Aspen Hysys software: the hydrogenation of carbon dioxide for the first scheme and the co-electrolysis of water and carbon dioxide for the second scheme. Results show that a higher co-electrolysis temperature determines a lower energy demand compared to hydrogenation systems. The energy efficiency of co-electrolysis is equal to 41%—almost double that of the hydrogenation processes. A heat integration is suggested by recovering heat from methanol reaction in order to produce electricity through a Rankine cycle.
Harp et al. [
40] developed a process by integrating a methanol plant in a steelworks plant with a section for the capture of CO
2 by power plant flue gas, a pressure swing adsorption (PSA) section for hydrogen separation from coke oven gas, and the use of the residual gas within the works gas grid, a water electrolysis section, a methanol synthesis section, and its distillation. Oxygen from water electrolysis replaced oxygen required for the blast furnace. In addition to PSA processes to recover hydrogen from COG, membranes are under development. In China, the first plant according to this scheme has been realized: with a purity above 99%, a recovery rate of up to 90% is possible [
41].
An integration between the methanol and biogas plant was developed by Pedersen and Schultz [
42]: the biogas plant provided carbon dioxide for a hydrogenation reaction producing methanol. Hydrogen was obtained by water electrolysis. Additionally, methane recovered by the upgrading of biogas can be used with carbon dioxide to produce methanol through steam reforming reaction. In Matzen et al. [
43], carbon dioxide was obtained by fermentation of ethanol, while wind energy was used for the electrolysis of water producing hydrogen. The energy efficiency of the process is comparable with others based on syngas.
A comparison of different processes to produce methanol was developed by Gai et al. [
44], where co-electrolysis of CO
2 and H
2O with a F-T reactor, a photocatalytic reactor for CO
2 and H
2O, and an electro-catalytic reactor for CO
2 and H
2O are analyzed. The overall NZCE (near-zero-carbon-emission) power plant using CO
2–CH
3OH circulation as the working medium was simulated and analyzed using Aspen Plus software. Results suggest that the energy consumption of methanol synthesis from CO
2 and H
2O through photo-catalysis is lower than other routes. The NZCE power plant can reduce CO
2 emission of 3.8 × 10
6 ton/year, while supplying the electricity of 5.87 × 10
6 MWh/year.
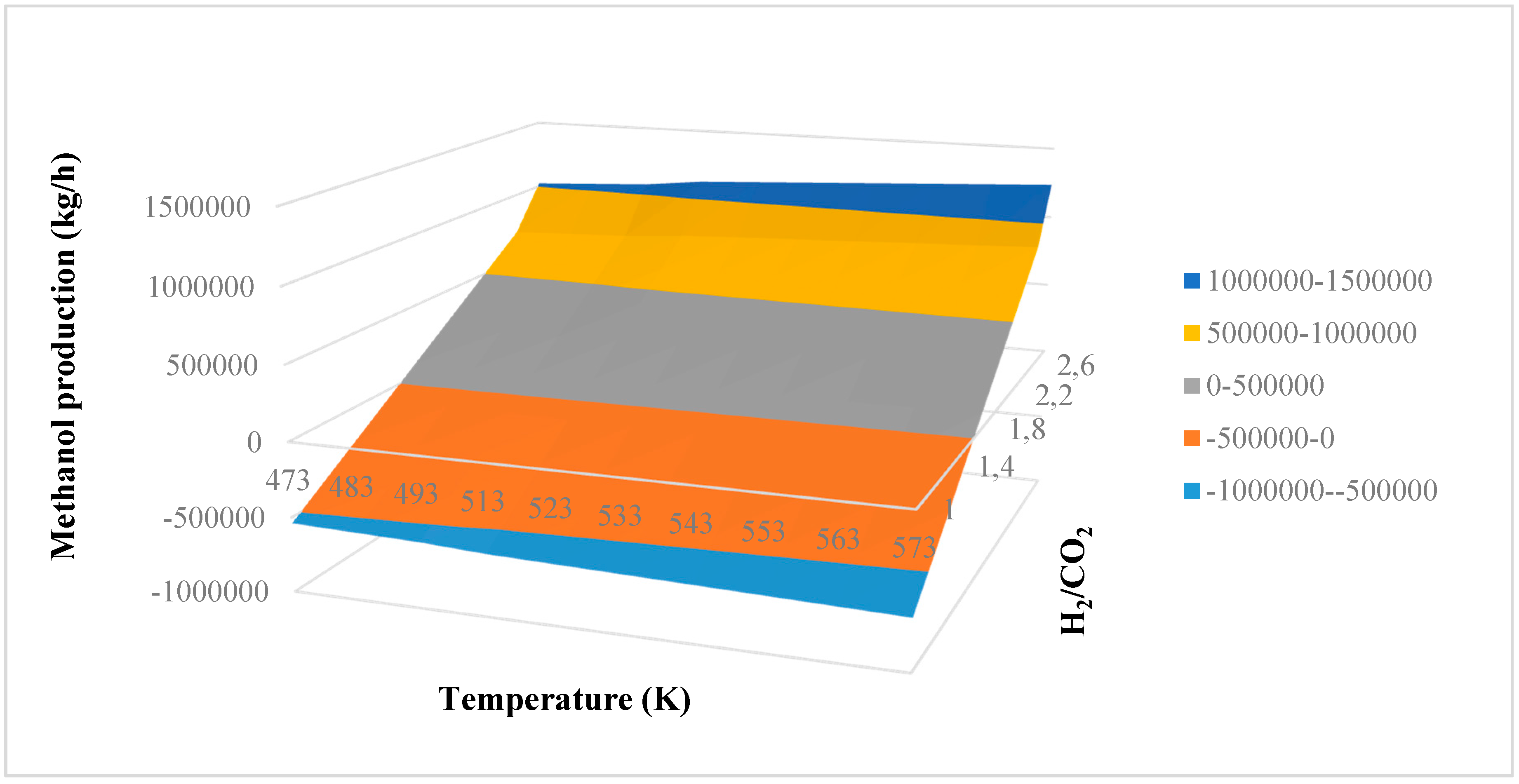
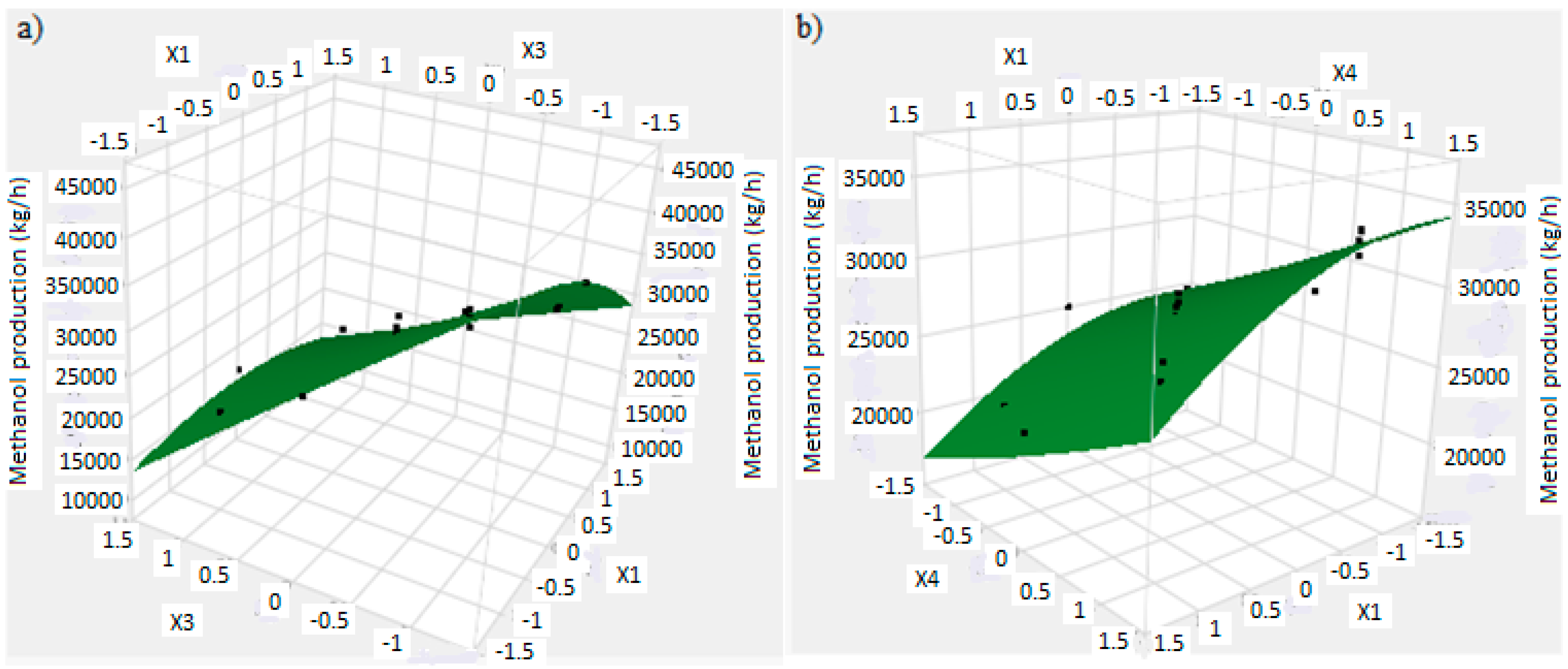
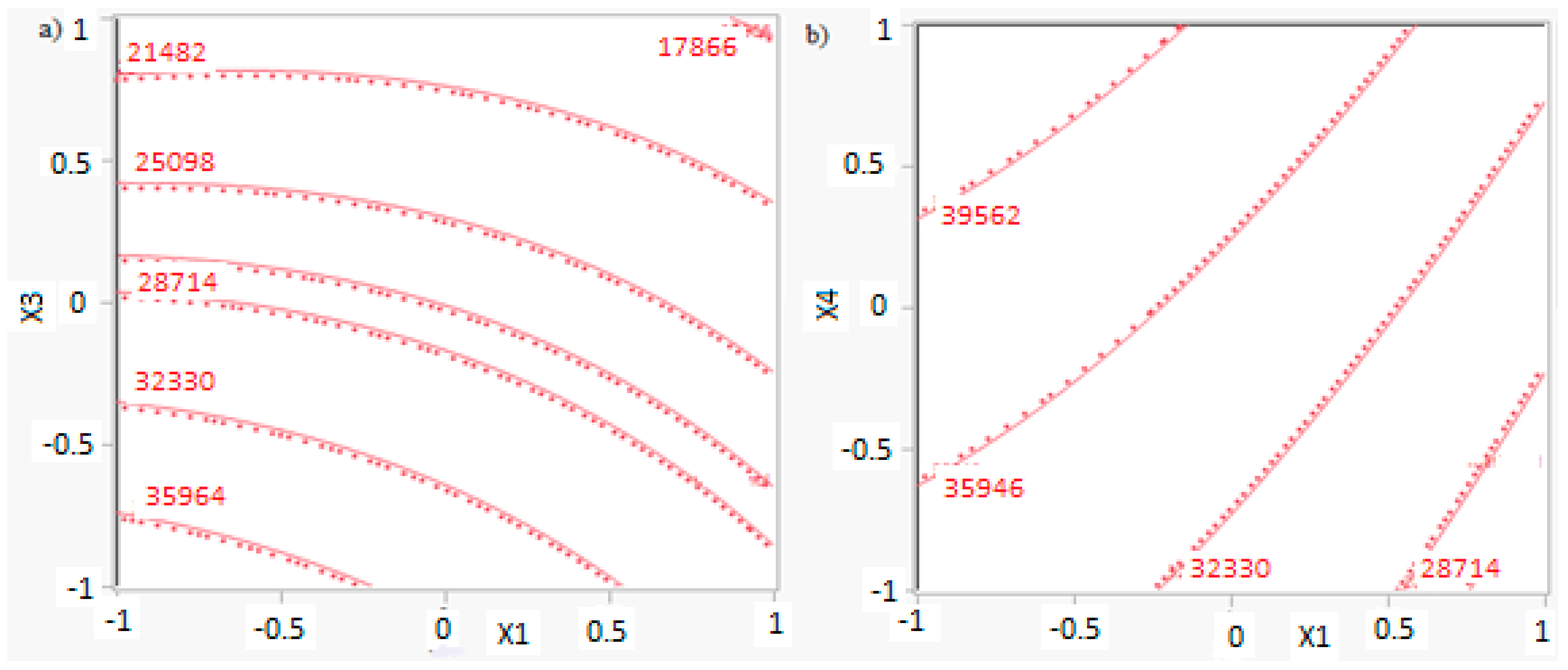
In this research, an ANOVA analysis was developed for the reactor producing methanol by the hydrogenation of carbon dioxide. Hydrogen was obtained by water electrolysis using solar energy, while carbon dioxide was captured by fuel gas. Compared to other production methods, the water electrolysis has the advantages of wide availability, flexibility, and high product purity [
31]. A similar work to those shown above is not present in literature regarding the design and economic analysis of plants that produce methanol. Reaction temperature, reaction pressure, H
2/CO
2 ratio, and the recycle of produced stream are the chosen factors in the factorial design. The methanol production and the reactor volume are the analyzed responses. In this way, it is possible to determine how to improve the methanol production reducing the reactor volume, as well as factors that are significant in the chosen responses. A central composite design (CCD) was also developed in order to find the optimal operating conditions of the process and a response surface plot for the methanol production, with a respective mathematical model. Modeling data for the two analyses were obtained from the simulation of the reactor in ChemCad 6.3
® software. The reactor was modelled according to the kinetics of Graaf et al. [
45] using Cu/ZnO/Al
2O
3 as catalyst. It is evident that the production of MeOH is especially attractive in emerging economies as a liquid fuel to replace conventional sources of energy. Additionally, CCU appears as a developing alternative, with an important potential to motivate carbon capture.
4. Conclusions
Carbon capture and utilization (CCU) is presented as a medium-term alternative to mitigate climate change. Carbon dioxide can be used to produce many chemical compounds, such as methanol, methane, syngas, formic acid, and dimethyl ether through hydrogenation reactions.
Methanol is one of the most valuable chemicals, with a series of uses either as fuel or as building block for the synthesis of other chemicals. Methanol synthesis by the hydrogenation of carbon dioxide is a feasible and efficient process, in an exothermic reaction. Other different raw materials and routes can be used to produce methanol. However, the use of carbon dioxide and hydrogen by renewable energy allows a green process with a lower environmental impact.
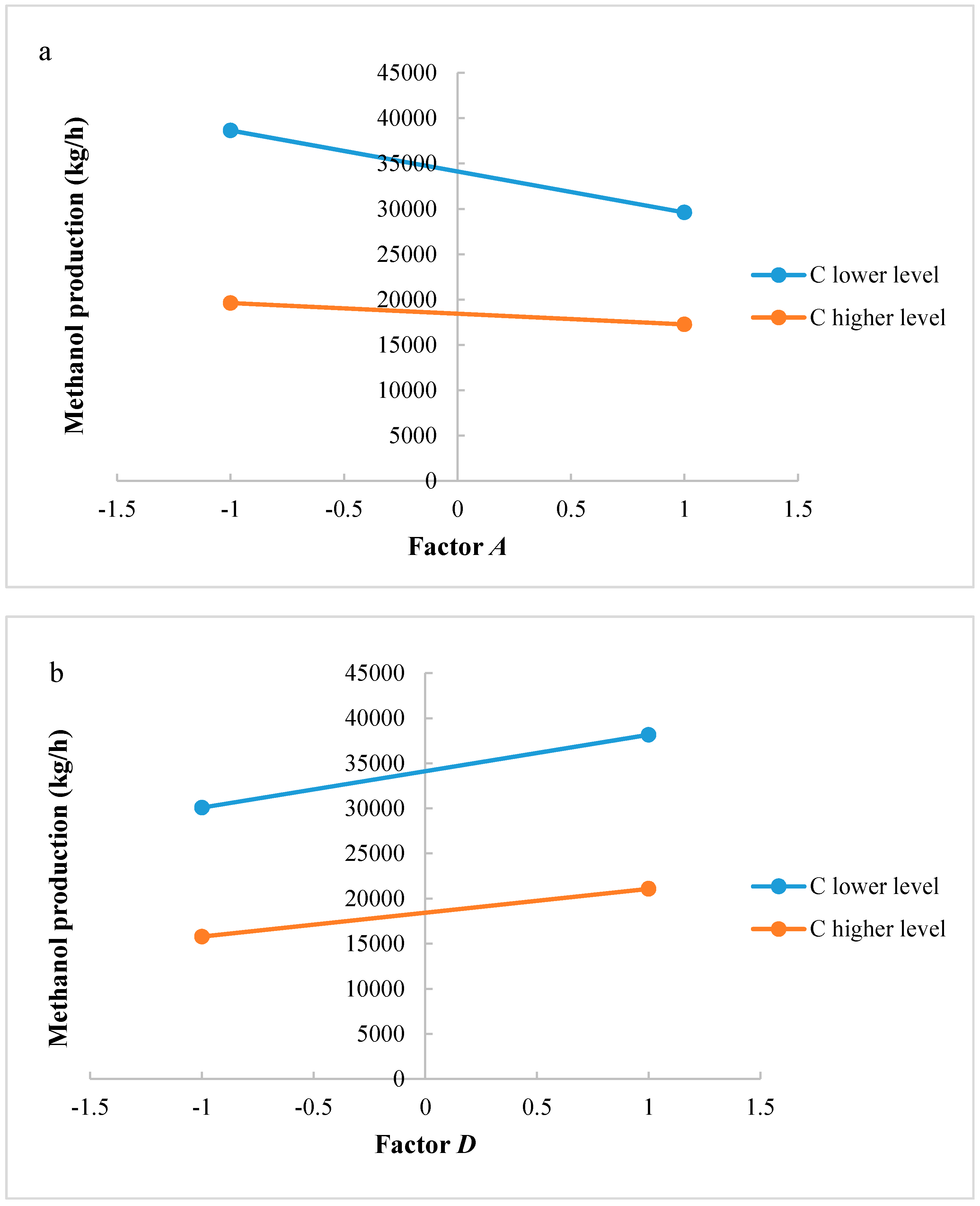
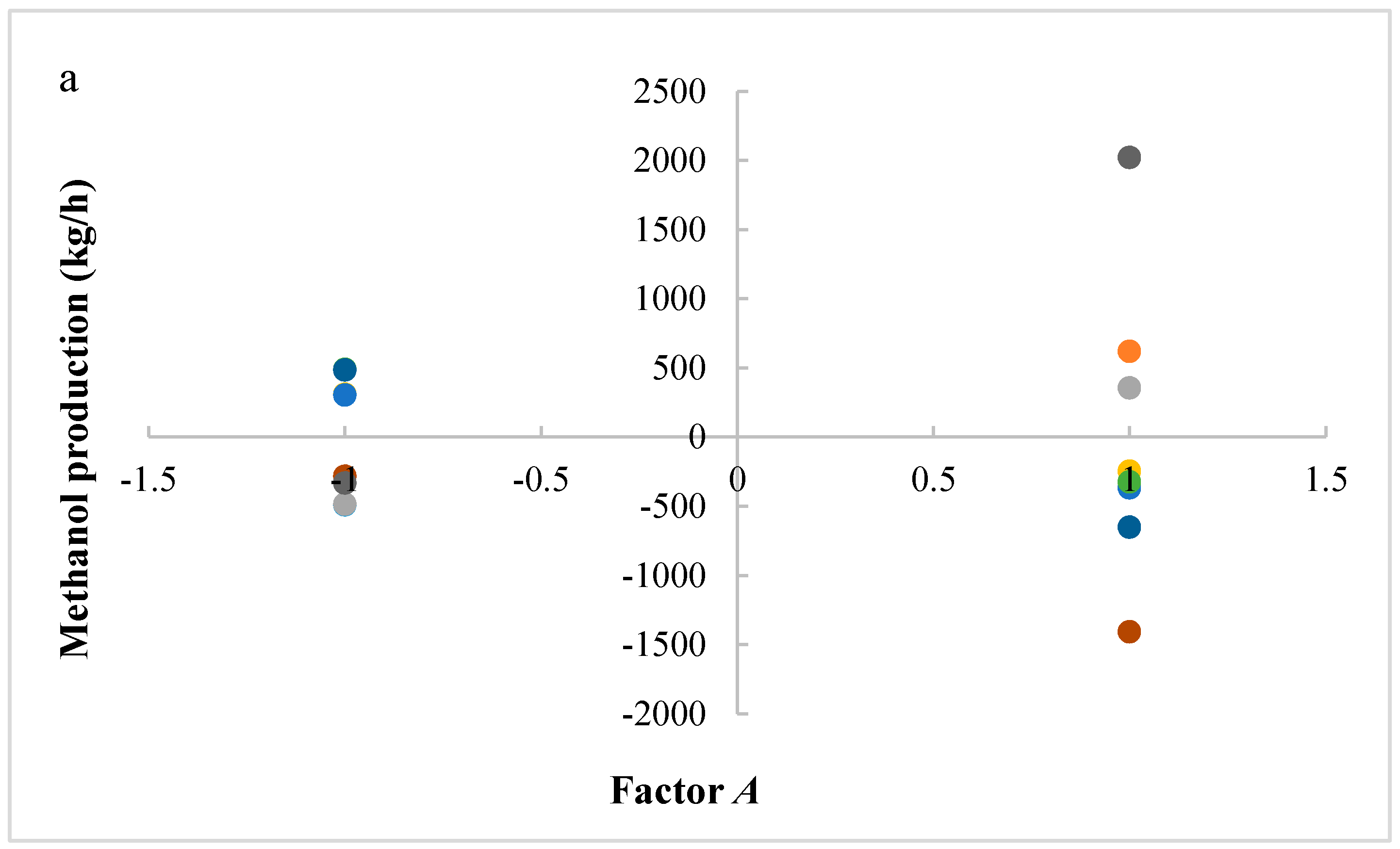
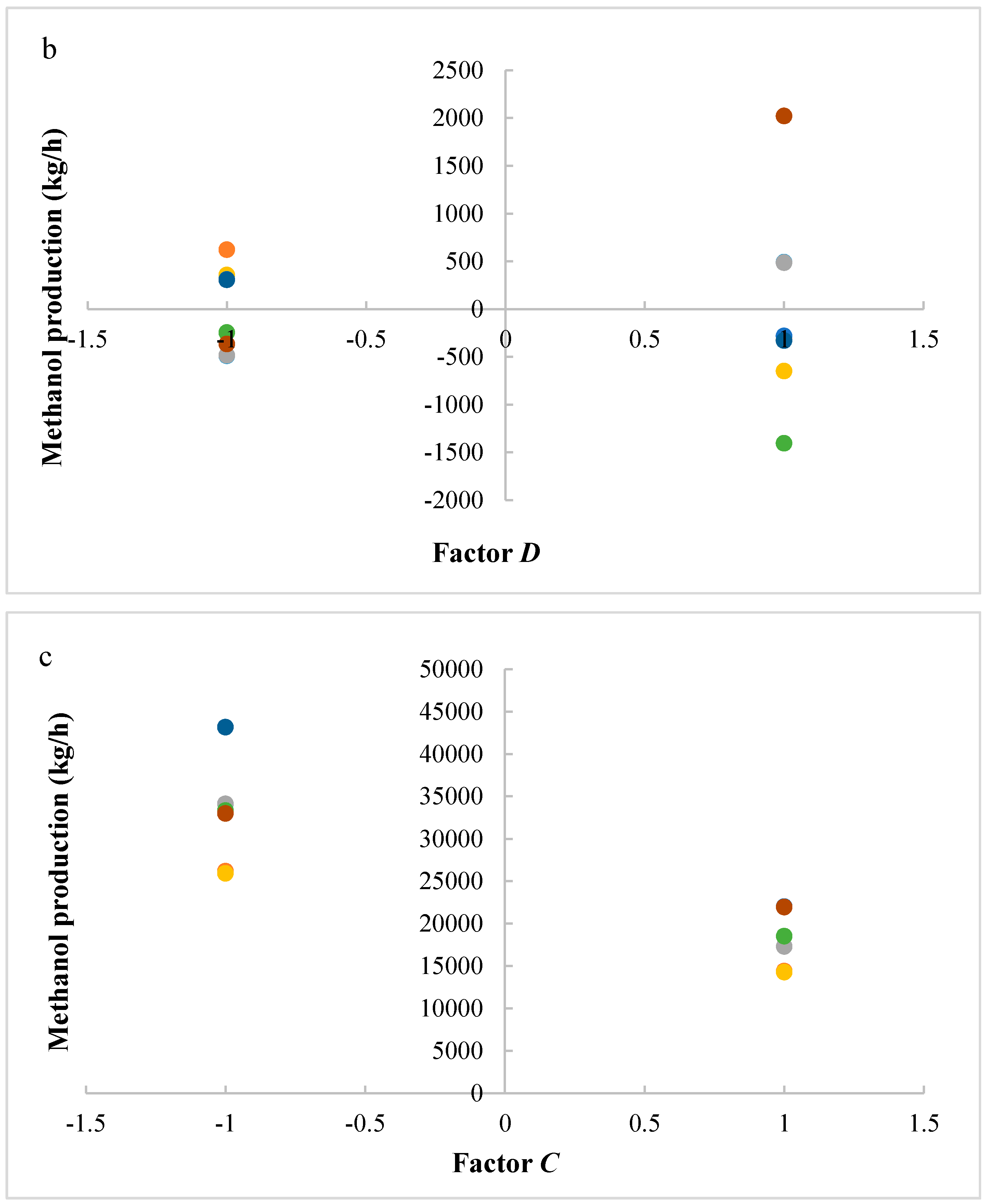
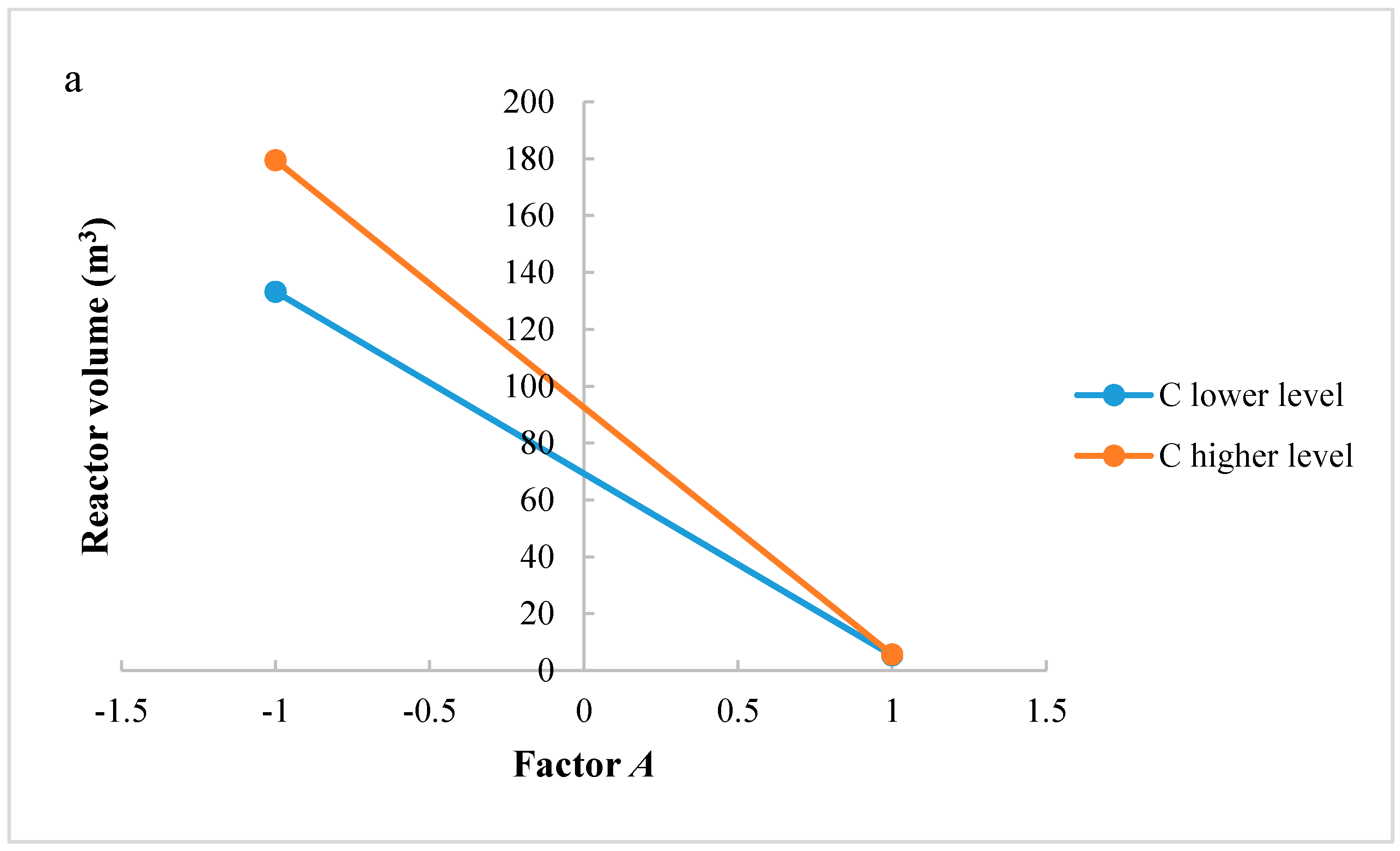
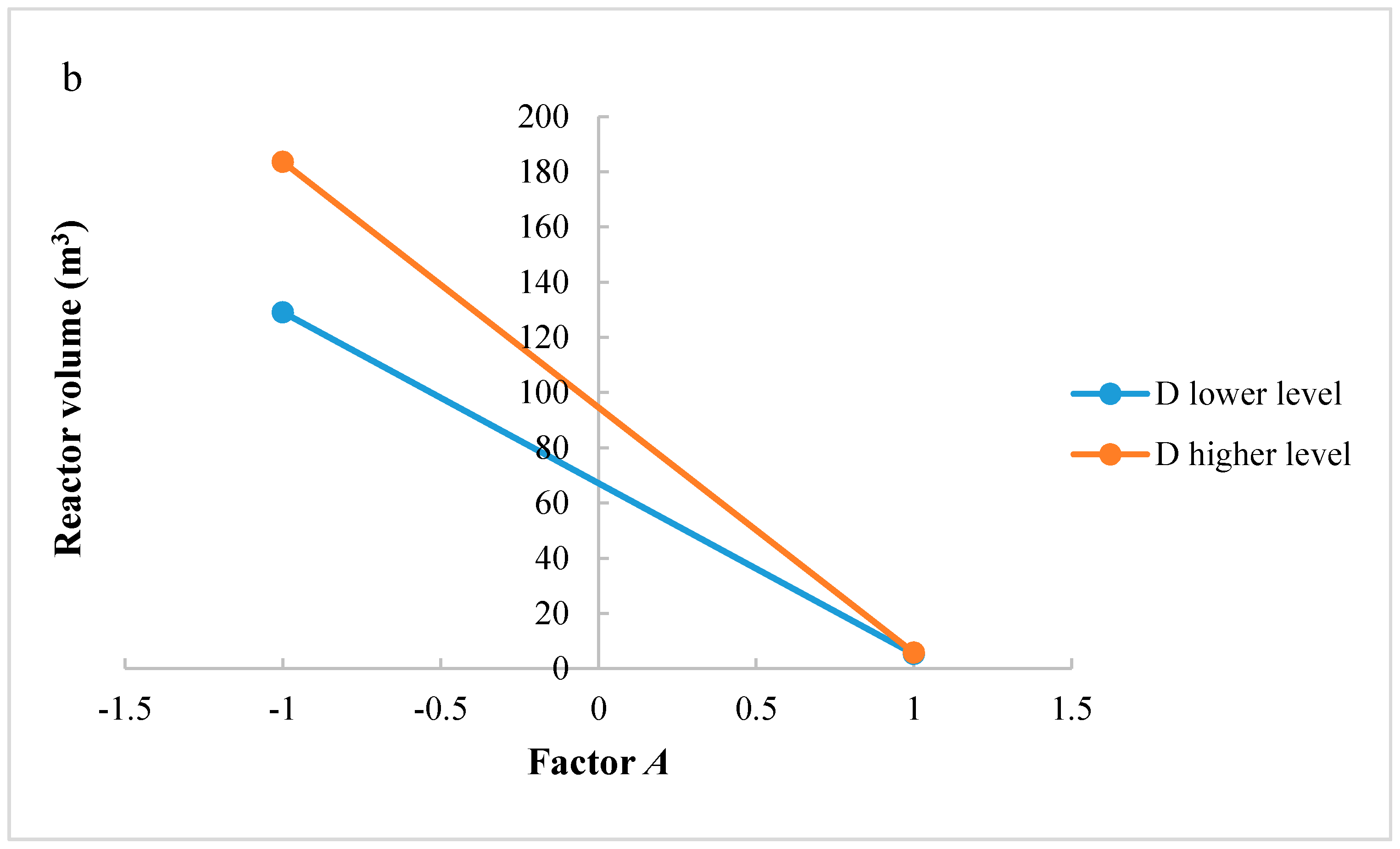
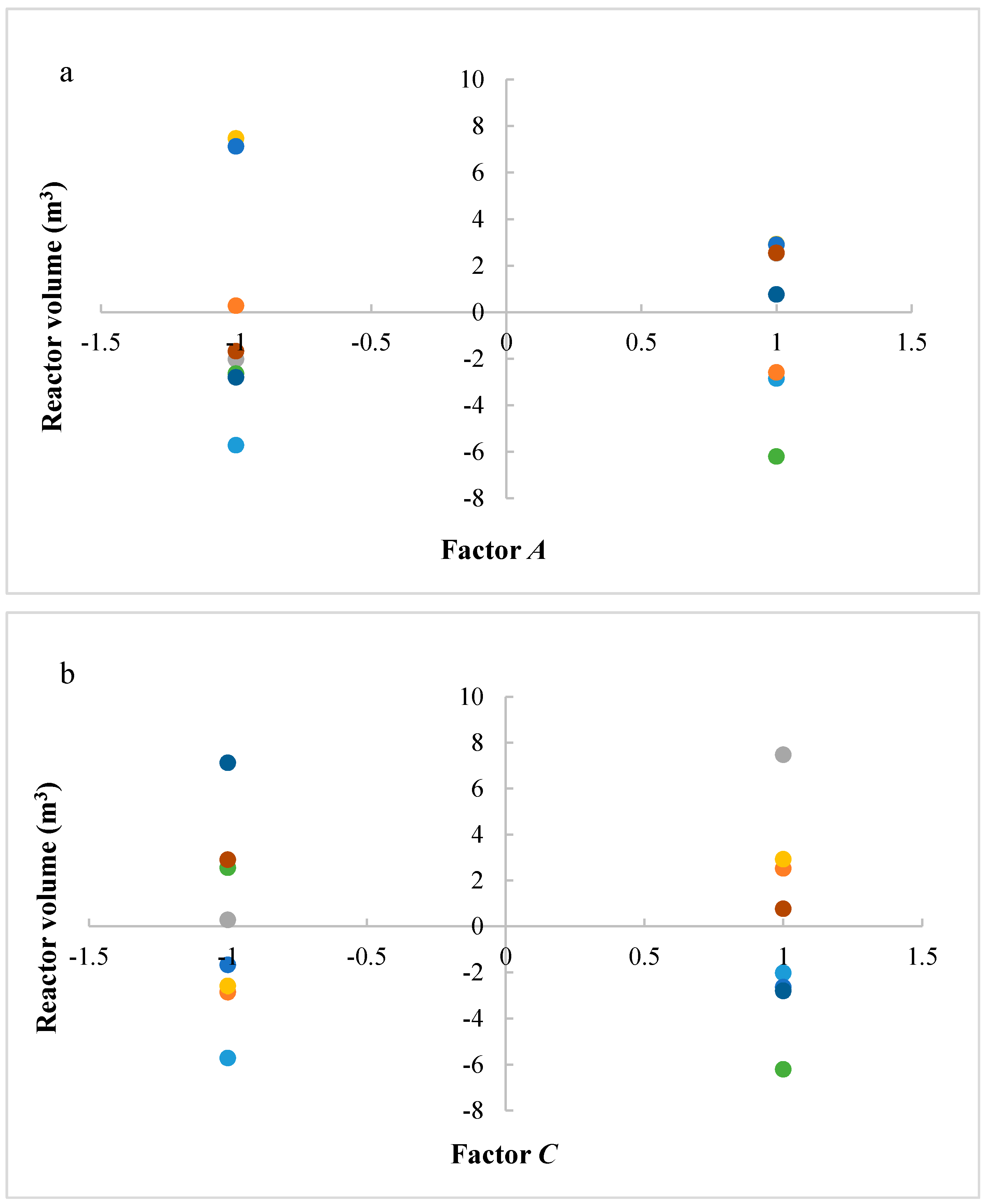
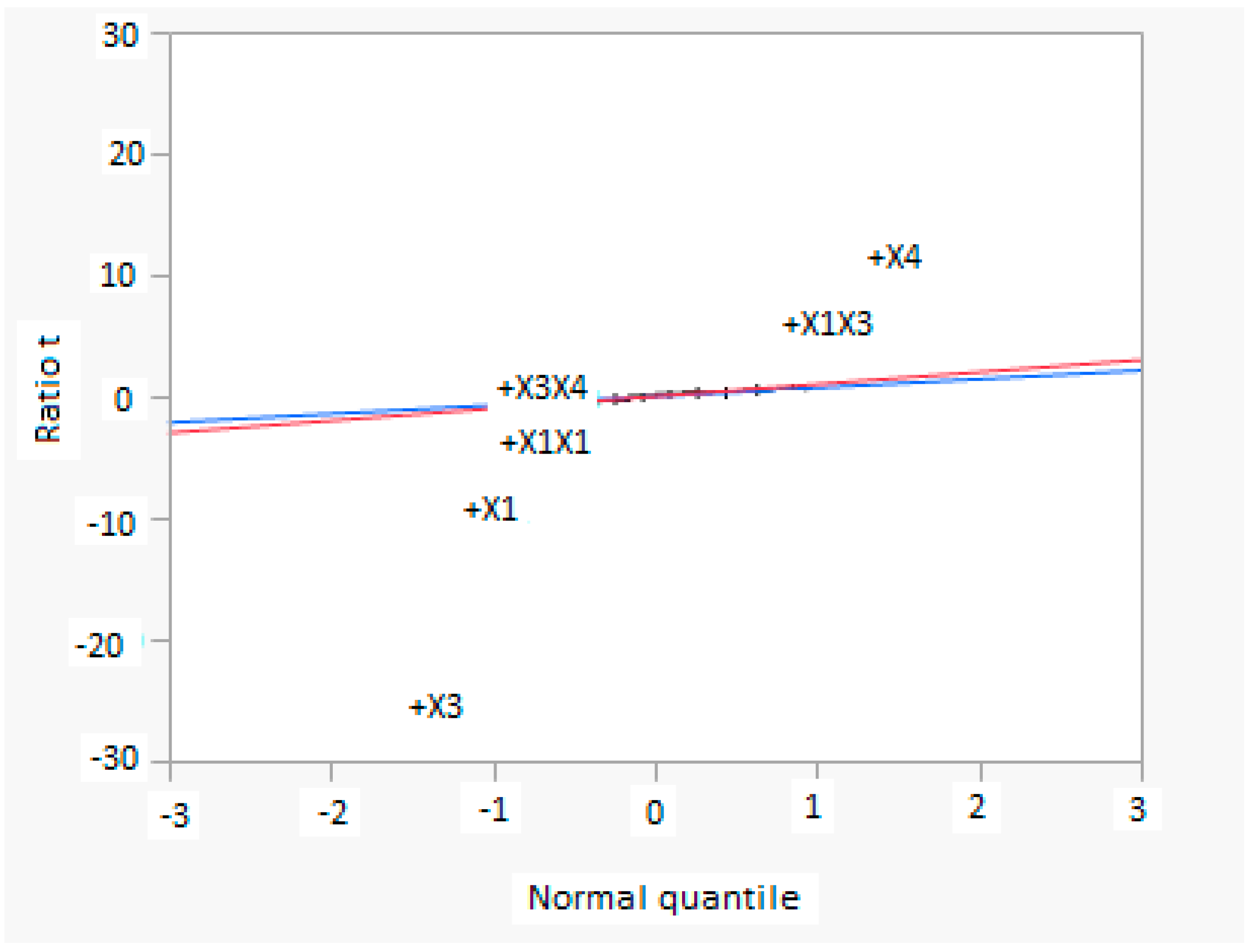
In this research, an ANOVA analysis and a central composite design were developed for a reactor producing methanol by the hydrogenation of carbon dioxide. The reactor was modelled in ChemCad 6.3® software and simulation results were used for two analyses. The aim of this study was to find the conditions that allow a higher methanol production and a lower reactor volume to be obtained. The kinetics of Graaf were used to describe the methanol production, while Cu/ZnO/Al2O3 was used as catalyst. The kinetic model contains the hydrogenation of CO and CO2. Major kinetic studies for methanol synthesis were conducted as early as 1977, and even recently researchers have been trying to model the kinetic process. In ANOVA analysis, reaction temperature (factor A), reaction pressure (factor B), H2/CO2 ratio (factor C), and the recycle of produced stream (factor D) were the chosen factors, while the methanol production and the reactor volume were the analyzed responses. Two different mathematical models for these responses were obtained with significant factors and interactions. Results show that AC is a common interaction, and it allows a higher methanol production to be obtained with a lower reactor volume. In particular, it is preferable to work with factor A at higher level and factor C at lower level. An analysis of residues and a test of the two levels for the significant interactions were also carried out, for a better comprehension of the process. A central composite design was developed in order to find the response surface plot of methanol production: an analysis of four factors with five levels was developed. Optimal conditions were also found: factors A, C, D must be at their higher, lower, and higher levels respectively. In these conditions, the optimal methanol production is equal to 33,540 kg/h, while the reactor volume is 6 m3. The operative conditions of a catalytic reactor that produces methanol by the hydrogenation of carbon dioxide were then obtained in this research, maximizing the productivity and reducing the costs. A similar work is not present in literature, so the novelty of the study is evident. Future researchers should consider a detailed economic analysis in order to have lower costs. Important considerations were obtained by the developed analysis that can be used for the design of methanol reactors or for the optimization of existing reactors.