Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results
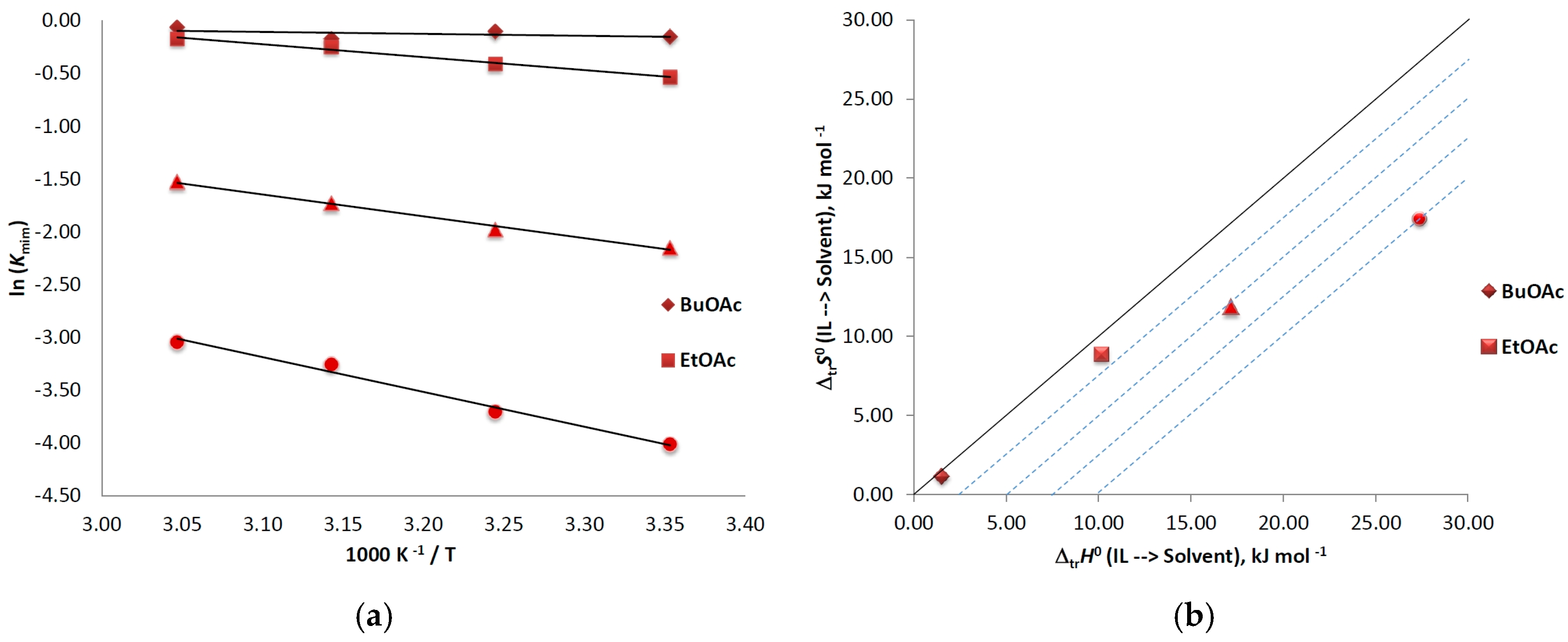
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G. Ionic Liquids as Alternative Solvents for Extraction of Natural Products. In Alternative Solvents for Natural Products Extraction, Green Chemistry and Sustainable Technology; Chemat, F., VianAbert, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- Hijo, A.A.C.T.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of ionic liquids in the food and bioproducts industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; eSilva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Raj, J.J.; Wilfred, C.D.; Shah, S.N.; Pranesh, M.; Mutalib, M.A.; Lethesh, K.C. Physicochemical and thermodynamic properties of imidazolium ionic liquids with nitrile and ether dual functional groups. J. Mol. Liq. 2017, 225, 281–289. [Google Scholar] [CrossRef]
- Zhao, N.; Jacquemin, J. The development of the UNIFAC-CONDUCT model as a novel approach for the estimation of the conductivity of pure ionic liquids. Fluid PhaseEquilibr. 2017, 449, 60–67. [Google Scholar] [CrossRef]
- Zhao, N.; Menegolla, H.B.; Degirmenci, V.; Wagner, Z.; Bendová, M.; Jacquemin, J. Group contribution method for evaluation of volumetric properties of ionic liquids using experimental data recommended by mathematical gnostics. Ind. Eng. Chem. Res. 2017, 56, 6827–6840. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Zhang, X.; Zhang, S. Quantitative prediction of viscosity of ionic liquids using Sσ-profile molecular descriptors. Phys. Chem. Chem. Phys. 2015, 17, 3761–3767. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.A.P.; Carvalho, P.J.; Oliveira, N.M.C. Predictive methods for the estimation of thermophysical properties of ionic liquids. RSC Adv. 2012, 2, 7322–7346. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Kantlehner, W. Simple prediction of some physical properties of ionic liquids: The residual volume approach. Z. Naturforsch. B J. Chem. Sci. 2009, 64, 215–222. [Google Scholar] [CrossRef]
- Widegren, J.A.; Laesecke, A.; Magee, J.W. The effect of dissolved water on the viscosities of hydrophobic room-temperature ionic liquids. Chem. Commun. 2005, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- McIntosh, A.J.S.; Griffith, J.; Gräsvik, J. Methods of synthesis and purification of ionic liquids. In Application, Purification, and Recovery of Ionic Liquids; Kuzmina, O., Hallett, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 59–99. Available online: https://doi.org/10.1016/B978-0-444-63713-0.00002-X (accessed on 21 September 2017).
- Böwing, A.G.; Jess, A. Kinetics of single- and two-phase synthesis of the ionic liquid 1-butyl-3-methylimidazolium chloride. Green Chem. 2005, 7, 230–235. [Google Scholar] [CrossRef]
- Chiappe, C.; Mezzetta, A.; Pomelli, C.S.; Puccini, M.; Seggiani, M. Product as reaction solvent: An unconventional approach for ionic liquid synthesis. Org. Process Res. Dev. 2016, 20, 2080–2084. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Petkova, D.; Hristeva, S.; Svinyarov, I.; Kantlehner, W. New guanidinium-based room-temperature ionic liquids. Substituent and anion effect on density and solubility in water. Z. Naturforsch. B J. Chem. Sci. 2010, 65, 37–48. [Google Scholar] [CrossRef]
- Clough, C.; Griffith, J.; Sulaiman, M.R.; Corbett, P.; Welton, T. Alkylation of 1-methylimidazole with 1-chlorobutane; the ionic liquid 1-butyl-3-methylimidazolium chloride. SCHEMSPIDER. [CrossRef]
- Nockemann, P.; Binnemans, K.; Driesen, K. Purification of imidazolium ionic liquids for spectroscopic applications. Chem. Phys. Lett. 2005, 415, 131–136. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.S.; Wareing, R. A simple colorimetric method for the quality control of 1-alkyl-3-methylimidazolium ionic liquid precursors. Green Chem. 2001, 3, 33–36. [Google Scholar] [CrossRef]
- Stark, A.; Behrend, P.; Braun, O.; Müller, A.; Ranke, J.; Ondruschka, B.; Jastorff, B. Purity specification methods for ionic liquids. Green Chem. 2008, 10, 1152–1161. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D.; Coutinho, J.A.P. Extraction of vanillin using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2010, 75, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Freire, M.G.; Teles, A.R.R.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Marrucho, I.M.; Coutinho, J.A.P. Partition coefficients of alkaloids in biphasic ionic-liquid-aqueous systems and their dependence on the Hofmeister series. Sep. Sci. Technol. 2012, 47, 284–291. [Google Scholar] [CrossRef]
- Tonova, K.; Svinyarov, I.; Bogdanov, M.G. Hydrophobic 3-alkyl-1-methylimidazolium saccharinates as extractants for L-lactic acid recovery. Sep. Purif. Technol. 2014, 125, 239–246. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Marques, C.F.C.; Boal-Palheiros, I.; Freire, M.G.; Coutinho, J.A.P. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem. 2014, 16, 259–268. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Keremedchieva, R.; Svinyarov, I. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. III. Ionic liquid regeneration and glaucine recovery from ionic liquid-aqueous crude extract of Glauciumflavum Cr. (Papaveraceae). Sep. Purif. Technol. 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Keremedchieva, R.; Svinyarov, I.; Bogdanov, M.G. Ionic liquid-based aqueous biphasic systems—A facile approach for ionic liquid regeneration from crude plant extracts. Processes 2015, 3, 769–778. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Zaalberg, B.; Kersten, S.R.A.; Schuur, B. Extraction of volatile fatty acids from fermented wastewater. Sep. Purif. Technol. 2016, 161, 61–68. [Google Scholar] [CrossRef]
- Lima, Á.S.; Soares, C.M.F.; Paltram, R.; Halbwirth, H.; Bica, K. Extraction and consecutive purification of anthocyanins from grape pomace using ionic liquid solutions. Fluid PhaseEquilibr. 2017. [Google Scholar] [CrossRef]
- Dimitrijević, A.; Ignjatović, L.; Tot, A.; Vraneš, M.; Zec, N.; Gadžurić, S.; Trtić-Petrović, T. Simultaneous extraction of pesticides of different polarity applying aqueous biphasic systems based on ionic liquids. J. Mol. Liq. 2017, 243, 646–653. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Efficient purification of halide-based ionic liquids by means of improved apparatus for continuous liquid-liquid extraction. Sep. Purif. Technol. 2017. [Google Scholar] [CrossRef]
- Ren, S.; Hou, Y.; Wu, W.; Liu, W. Purification of ionic liquids: Sweeping solvents by nitrogen. J. Chem. Eng. Data 2010, 55, 5074–5077. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I.; Keremedchieva, R.; Sidjimov, A. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glauciumflavum Cr. (Papaveraceae). Sep. Purif. Technol. 2012, 97, 221–227. [Google Scholar] [CrossRef]
- Schade, A.; Behme, N.; Spange, S. Dipolarity versus polarizability and acidity versus basicity of ionic liquids as a function of their molecular structures. Chem. Eur. J. 2014, 20, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Perez-Arlandis, J.M.; et al. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Polarity of ionic liquids determined empirically by means of solvatochromicpyridinium N-phenolatebetaine dyes. Green Chem. 2005, 7, 339–351. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Perlovich, G.L. Thermodynamic approaches to the challenges of solubility in drug discovery and development. Mol. Pharm. 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J. On the solid, liquid and solution structural organization of imidazolium ionic liquids. J. Braz. Chem. Soc. 2004, 15, 341–350. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, L.; Kazarian, S.G.; Salter, P.A.; Welton, T. Molecular states of water in room temperature ionic liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef] [Green Version]
- Ficke, L.E.; Brennecke, J.F. Interactions of ionic liquids and water. J. Phys. Chem. B 2010, 114, 10496–10501. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kurnia, K.A.; Mutelet, F.; Pinho, S.P.; Coutinho, J.A.P. Probing the interactions between ionic liquids and water: Experimental and quantum chemical approach. J. Phys. Chem. B 2014, 118, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Taha, M.; Ribeiro-Claro, P.; Pinho, S.P.; Coutinho, J.A.P. Effect of the cation on the interactions between alkyl methylimidazolium chloride ionic liquids and water. J. Phys. Chem. B 2014, 118, 10503–10514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-G.; Wang, N.-N.; Wang, S.-L.; Yu, Z.-W. Hydrogen bonding behaviors of binary systems containing the ionic liquid 1-butyl-3-methylimidazolium trifluoroacetate and water/methanol. J. Phys. Chem. B 2011, 115, 11127–11136. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Ferro, V.R.; Palomar, J.; Ortega, J.; Rodríguez, J.J. Interactions of ionic liquids and acetone: Thermodynamic properties, quantum chemical calculations and NMR analysis. J. Phys. Chem. B 2013, 117, 7388–7398. [Google Scholar] [CrossRef] [PubMed]
- Kurteva, V.; Atanassova, M.; Billard, I. NMR Study on the possible interactions between imidazolium based ionic liquids and extractants widely applied in solvent extraction and separation of f-ions. J. Solut. Chem. 2015, 44, 2416–2430. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, H.; Yang, Q.; Li, Z.; Chen, T.; Bao, Z.; Ren, Q. Biphasic systems that consist of hydrophilic ionic liquid, water, and ethyl acetate: The effects of interactions on the phase behavior. Ind. Eng. Chem. Res. 2014, 53, 10784–10790. [Google Scholar] [CrossRef]
- Friesen, S.; Buchecker, T.; Cognigni, A.; Bica, K.; Buchner, R. Hydration and counterion binding of [C12MIM] micelles. Langmuir 2017. [Google Scholar] [CrossRef] [PubMed]
- Skarmoutsos, I.; Welton, T.; Hunt, P.A. The importance of timescale for hydrogen bonding in imidazolium chloride ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Lin, S.-T. A priori prediction of the octanol−water partition coefficient (Kow) of ionic liquids. Fluid Phase Equilibr. 2014, 63, 233–238. [Google Scholar] [CrossRef]
- Ropel, L.; Belvèze, L.S.; Aki, S.; Stadtherr, M.A.; Brennecke, J.F. Octanol-water partition coefficients of imidazolium-based ionic liquids. Green. Chem. 2005, 7, 83–90. [Google Scholar] [CrossRef]


| Materials (Abbreviation) | ETN | SdP | SA | SB |
|---|---|---|---|---|
| Water | 1.000 | 0.997 | 1.062 | 0.025 |
| N-methylimidazole (mim) | n.a. 2 | 0.959 | 0.069 | 0.658 |
| butyl acetate (BuOAc) | 0.241 | 0.535 | 0 | 0.525 |
| ethyl acetate (EtOAc) | 0.228 | 0.603 | 0 | 0.542 |
| tert-butyl methyl ether (MTBE) | 0.124 | 0.422 | 0 | 0.567 |
| diethyl ether (Et2O) | 0.117 | 0.385 | 0 | 0.562 |
| Cyclohexane | 0.006 | 0 | 0 | 0.073 |
| [C4C1im]Cl | 0.614 | 1.172 | 0.167 | 0.869 |
| [C6C1im]Cl | 0.562 | 1.139 | 0.159 | 0.932 |
| [C8C1im]Cl | 0.549 | 1.096 | 0.159 | 1.001 |
| [C10C1im]Cl | n.a.2 | 1.115 | 0.159 | 0.932 |
| TemperatureK | Kmim | ln(Kmim) | ∆trG0kJ mol−1 | ∆trH0kJ mol−1 | ∆trS0J mol−1 | T∆trS0kJ mol−1 | ζHtr 1% | ζTStr 2% |
|---|---|---|---|---|---|---|---|---|
| Butyl Acetate | ||||||||
| 298.15 | 0.860 | −0.15 | 0.38 | 1.52 | 3.83 | 1.14 | 57 | 43 |
| 308.15 | 0.900 | −0.11 | 0.34 | |||||
| 318.15 | 0.840 | −0.17 | 0.30 | |||||
| 328.15 | 0.940 | −0.06 | 0.26 | |||||
| Correlationequation: ln (Kmim) = 0.46 − 183× 1/T; R2 = 0.243 | ||||||||
| Ethyl Acetate | ||||||||
| 298.15 | 0.586 | −0.53 | 1.32 | 10.18 | 29.69 | 8.85 | 53 | 47 |
| 308.15 | 0.662 | −0.41 | 1.03 | |||||
| 318.15 | 0.780 | −0.25 | 0.73 | |||||
| 328.15 | 0.841 | −0.17 | 0.43 | |||||
| Correlationequation: ln (Kmim) = 3.75 − 1224 × 1/T; R2 = 0.99 | ||||||||
| tert-Butyl Methyl Ether | ||||||||
| 298.15 | 0.117 | −2.15 | 5.38 | 17.18 | 39.59 | 11.80 | 59 | 41 |
| 308.15 | 0.138 | −1.98 | 4.98 | |||||
| 318.15 | 0.177 | −1.73 | 4.59 | |||||
| 328.15 | 0.217 | −1.53 | 4.19 | |||||
| Correlationequation: ln (Kmim) = 4.76 − 2067 × 1/T; R2 = 0.99 | ||||||||
| Cyclohexane | ||||||||
| 298.15 | 0.018 | −4.02 | 9.97 | 27.39 | 58.41 | 17.42 | 61 | 39 |
| 308.15 | 0.025 | −3.71 | 9.39 | |||||
| 318.15 | 0.038 | −3.26 | 8.81 | |||||
| 328.15 | 0.048 | −3.05 | 8.22 | |||||
| Correlationequation: ln (Kmim) = 7.02 − 3295 × 1/T; R2 = 0.99 | ||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanov, M.G.; Svinyarov, I. Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems. Processes 2017, 5, 52. https://doi.org/10.3390/pr5040052
Bogdanov MG, Svinyarov I. Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems. Processes. 2017; 5(4):52. https://doi.org/10.3390/pr5040052
Chicago/Turabian StyleBogdanov, Milen G., and Ivan Svinyarov. 2017. "Distribution of N-Methylimidazole in Ionic Liquids/Organic Solvents Systems" Processes 5, no. 4: 52. https://doi.org/10.3390/pr5040052




