Ionic Liquid-Based Aqueous Biphasic Systems—A Facile Approach for Ionic Liquid Regeneration from Crude Plant Extracts
Abstract
:1. Introduction
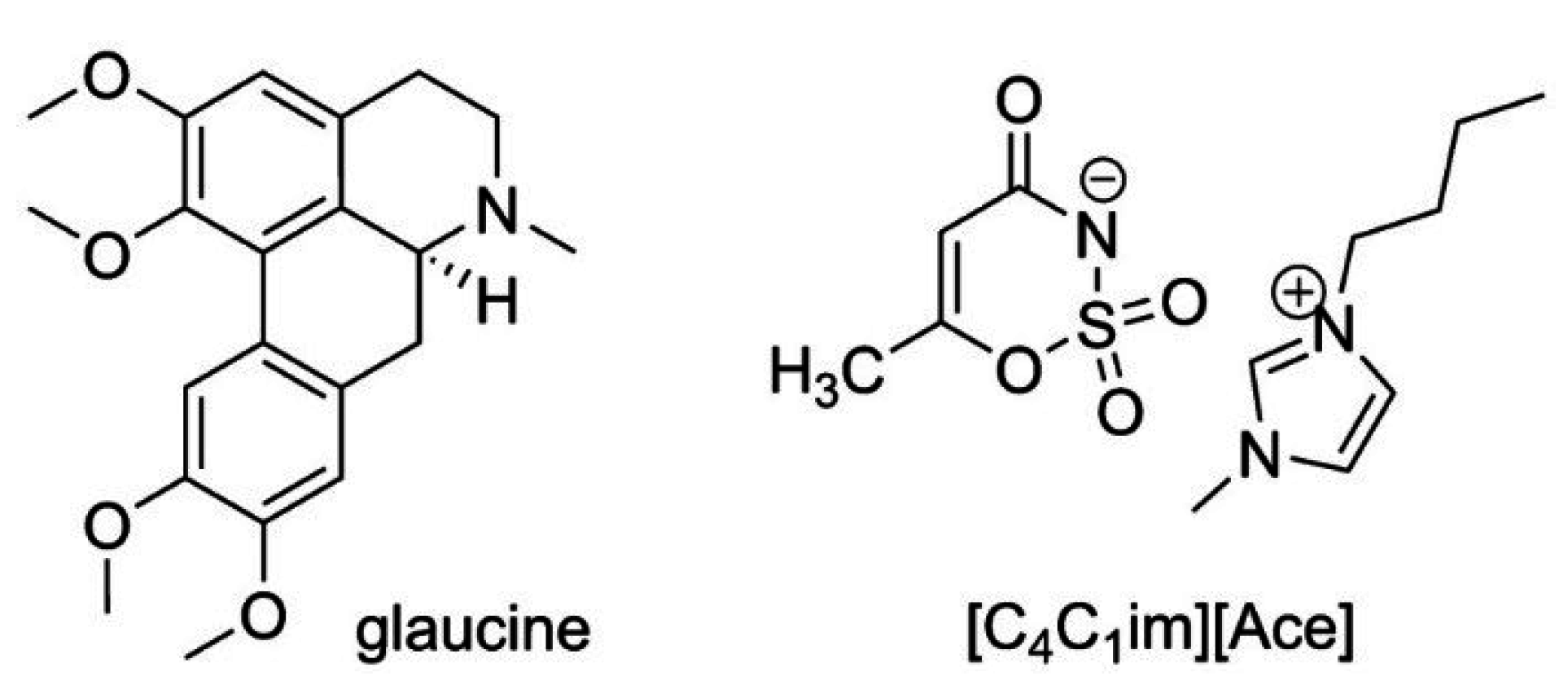
2. Experimental Section
2.1 Chemicals
2.2 Glaucine Quantification
2.3 Partitioning of Glaucine in IL-Based ABS and Calculations
3. Results and Discussion
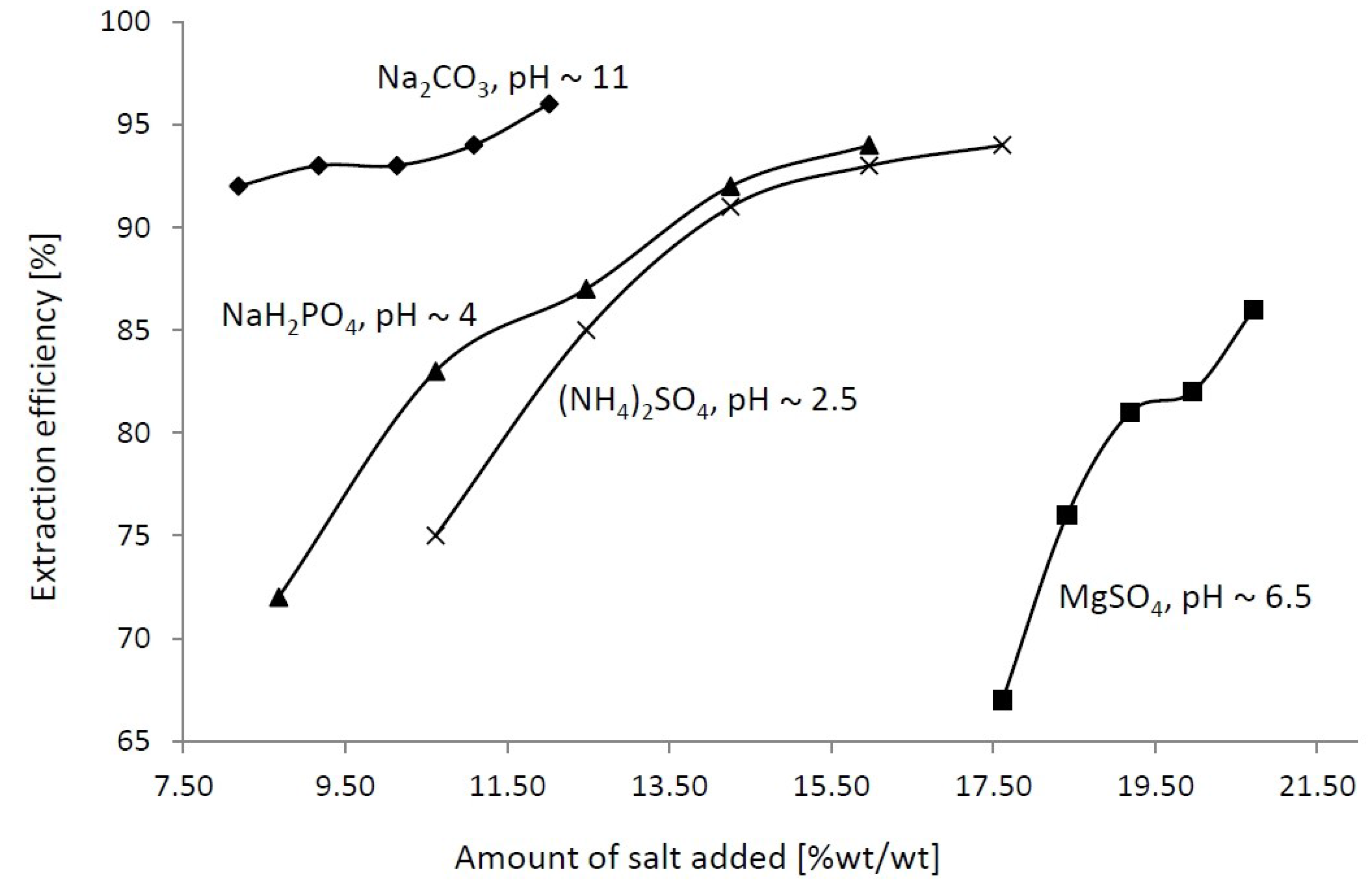
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G. Ionic Liquids as Alternative Solvents for Extraction of Natural Products. In Alternative Solvents for NaturalProducts Extraction, Green Chemistry and Sustainable Technology; Chemat, F., Vian Abert, M., Eds.; Springer-Verlag: Berlin, Germany; Heidelberg, Germany, 2014; pp. 127–166. [Google Scholar]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed]
- Lapkin, A.A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.P.; Rodriguez, O.; Macedo, E.A. Separation of carbohydrates and sugar alcohols from ionic liquids using antisolvents. Sep. Purif. Technol. 2014, 132, 496–504. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Neves, M.C.; Shimizu, K.; Canongia Lopes, J.N.; Freire, M.G.; Coutinho, J.A.P. The magic of aqueous solutions of ionic liquids: Ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.Y.; Fu, Y.J.; Zu, Y.G.; Luo, M.; Zhao, C.J.; Li, C.Y. Microwave-assisted ionic liquids treatment followed by hydro-distillation for the efficient isolation of essential oil from Fructus forsythiae seed. Sep. Purif. Technol. 2013, 107, 228–237. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I.; Keremedchieva, R.; Sidjimov, A. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. I. New HPLC method for quantitative determination of glaucine in Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2012, 97, 221–227. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Keremedchieva, R.; Svinyarov, I. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. III. Ionic liquid regeneration and glaucine recovery from ionic liquid-aqueous crude extract of Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2015, 155, 13–19. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A.P. Enhanced extraction of caffeine from Guaraná seeds using aqueous solutions of ionic liquids. Green Chem. 2013, 15, 2002–2010. [Google Scholar] [CrossRef]
- Ressmann, A.K.; Zirbs, R.; Pressler, M.; Gaertner, P.; Bica, K. Surface-active ionic liquids for micellar extraction of piperine from black pepper. Z. Naturforsch. B J. Chem. Sci. 2013, 68, 1129–1137. [Google Scholar] [CrossRef]
- Tan, Z.J.; Wang, C.Y.; Yang, Z.Z.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Li, F.F. Ionic Liquid-Based Ultrasonic-Assisted Extraction of Secoisolariciresinol Diglucoside from Flaxseed (Linum usitatissimum L.) with Further Purification by an Aqueous Two-Phase System. Molecules 2015, 20, 17929–17943. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Li, F.; Xu, X. Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two-phase system. Sep. Purif. Technol. 2012, 98, 150–157. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Marques, C.F.C.; Boal-Palheiros, I.; Freire, M.G.; Coutinho, J.A.P. Development of back-extraction and recyclability routes for ionic-liquid-based aqueous two-phase systems. Green Chem. 2014, 16, 259–268. [Google Scholar] [CrossRef]
- Zirbs, R.; Strassl, K.; Gaertner, P.; Schröder, C.; Bica, K. Exploring ionic liquid–biomass interactions: Towards the improved isolation of shikimic acid from star anise pods. RSC Adv. 2013, 3, 26010–26016. [Google Scholar] [CrossRef]
- Ma, C.H.; Zu, Y.G.; Yang, L.; Li, J. Two solid-phase recycling method for basic ionic liquid [C4mim]Ac by macroporous resin and ion exchange resin from Schisandra chinensis fruits extract. J. Chromatogr. B 2015, 976–977, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Ma, C.; Fu, Q.; Hu, L.; Lou, Z.; Wang, H.; Tao, G. Application of ionic liquids in an online ultrasonic assisted extraction and solid-phase trapping of rhodiosin and rhodionin from Rhodiola rosea for UPLC. Chromatographia 2013, 76, 195–200. [Google Scholar] [CrossRef]
- Petkov, V.; Stancheva, S. In vitro inhibition of Cyclic 3X, 5X-AMPc phosphodiesterase by a group of structural analogues of glaucine. Acta Physiol. Pharmacol. Bulgaria 1980, 6, 38–46. [Google Scholar]
- Berthe, J.; Remandet, B.; Mazue, G.; Tilson, H.A. Neurobehavioral effects of d-glaucine in rats. Neurobehav. Toxicol. Teratol. 1983, 5, 305–308. [Google Scholar] [PubMed]
- Kasè, Y.; Kawaguchi, M.; Takahama, K.; Miyata, T.; Hirotsu, I.; Hitoshi, T.; Okano, Y. Pharmacological studies on dl-glaucine phosphate as an antitussive. Arzneim.-Forsch. 1983, 33, 936–946. [Google Scholar]
- Zetler, G. Neuroleptic-like, anticonvulsant and antinociceptive effects of aporphine alkaloids: Bulbocapnine, corytuberine, boldine and glaucine. Arch. Int. Pharmacodyn.Ther. 1988, 296, 255–281. [Google Scholar] [PubMed]
- Bogdanov, M.G.; Svinyarov, I. Ionic liquid-supported solid-liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2013, 103, 279–288. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G.; Petkova, D.; Hristeva, S.; Svinyarov, I.; Kantlehner, W. New guanidinium-based room-temperature ionic liquids. Substituent and anion effect on density and solubility in water. Z. Naturforsch. B J. Chem. Sci. 2010, 65, 37–48. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.J.; Li, F.F.; Xu, X.L.; Xing, J.M. Simultaneous extraction and purification of aloe polysaccharides and proteins using ionic liquid based aqueous two-phase system coupled with dialysis membrane. Desalination 2012, 286, 389–393. [Google Scholar] [CrossRef]
- Tonova, K. Separation of poly- and disaccharides by biphasic systems based on ionic liquids. Sep. Purif. Technol. 2012, 89, 57–65. [Google Scholar] [CrossRef]
- Deive, F.J.; Rodríguez, A.; Pereiro, A.B.; Araújo, J.M.M.; Longo, M.A.; Coelho, M.A.Z.; Canongia Lopes, J.N.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Marrucho, I.M. Ionic liquid-based aqueous biphasic system for lipase extraction. Green Chem. 2011, 13, 390–396. [Google Scholar] [CrossRef]
- Marques, C.F.; Mourão, T.; Neves, C.M.; Lima, Á.S.; Boal-Palheiros, I.; Coutinho, J.A.; Freire, M.G. Aqueous biphasic systems composed of ionic liquids and sodium carbonate as enhanced routes for the extraction of tetracycline. Biotechnol. Prog. 2013, 29, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Coutinho, J.A.P. High-performance extraction of alkaloids using aqueous two-phase systems with ionic liquids. Green Chem. 2010, 12, 1715–1718. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Marrucho, I.M.; Coutinho, J.A.P. Partition Coefficients of Alkaloids in Biphasic Ionic-Liquid-Aqueous Systems and their Dependence on the Hofmeister Series. Sep. Sci. Technol. 2012, 47, 284–291. [Google Scholar] [CrossRef]
- Tonova, K.; Svinyarov, I.; Bogdanov, M.G. Hydrophobic 3-alkyl-1-methylimidazolium saccharinates as extractants for l-lactic acid recovery. Sep. Purif. Technol. 2014, 125, 239–246. [Google Scholar] [CrossRef]
- Shahriari, S.; Neves, C.M.; Freire, M.G.; Coutinho, J.A. Role of the Hofmeister Series in the Formation of Ionic-Liquid-Based Aqueous Biphasic Systems. J. Phys. Chem. B 2012, 116, 7252–7258. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, A.; Waksmundzka-Hajnos, M.; Hajnos, M.L. The Effect of Chromatographic Conditions on the Separation of Selected Alkaloids in RP-HPTLC. J. Chromatogr. Sci. 2005, 43, 183–194. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keremedchieva, R.; Svinyarov, I.; Bogdanov, M.G. Ionic Liquid-Based Aqueous Biphasic Systems—A Facile Approach for Ionic Liquid Regeneration from Crude Plant Extracts. Processes 2015, 3, 769-778. https://doi.org/10.3390/pr3040769
Keremedchieva R, Svinyarov I, Bogdanov MG. Ionic Liquid-Based Aqueous Biphasic Systems—A Facile Approach for Ionic Liquid Regeneration from Crude Plant Extracts. Processes. 2015; 3(4):769-778. https://doi.org/10.3390/pr3040769
Chicago/Turabian StyleKeremedchieva, Rozalina, Ivan Svinyarov, and Milen G. Bogdanov. 2015. "Ionic Liquid-Based Aqueous Biphasic Systems—A Facile Approach for Ionic Liquid Regeneration from Crude Plant Extracts" Processes 3, no. 4: 769-778. https://doi.org/10.3390/pr3040769




