TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants
Abstract
:1. Introduction
2. TLC-DB (Thin-Layer Chromatography-Direct Bioautography)
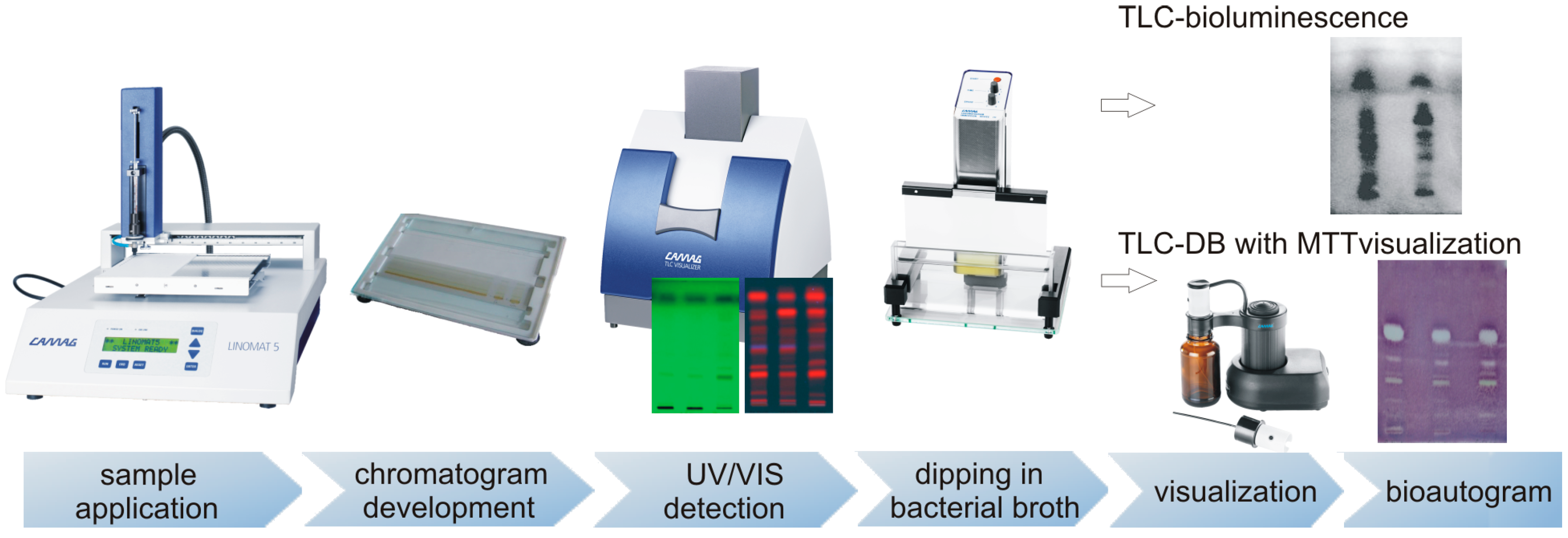
2.1. TLC-DB–“dot-blot” Test

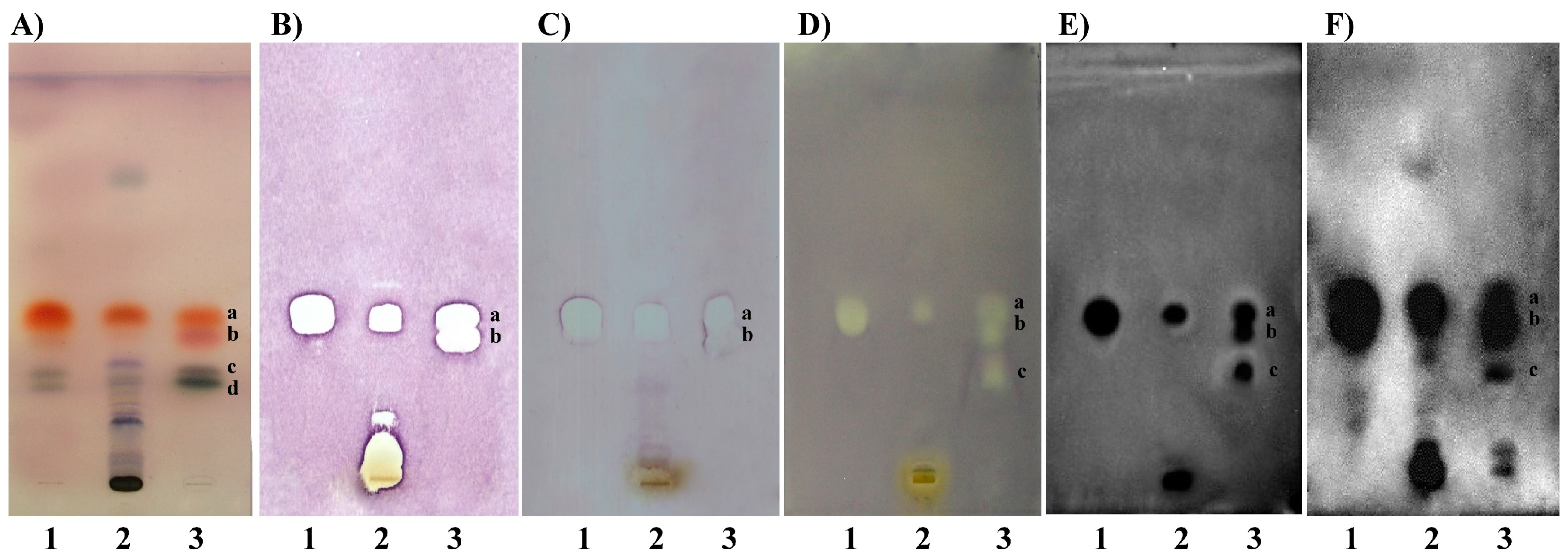
2.2. TLC-DB–Developed Plates
3. Identification of Bioactive Compounds
3.1. Preparative Sample Isolation
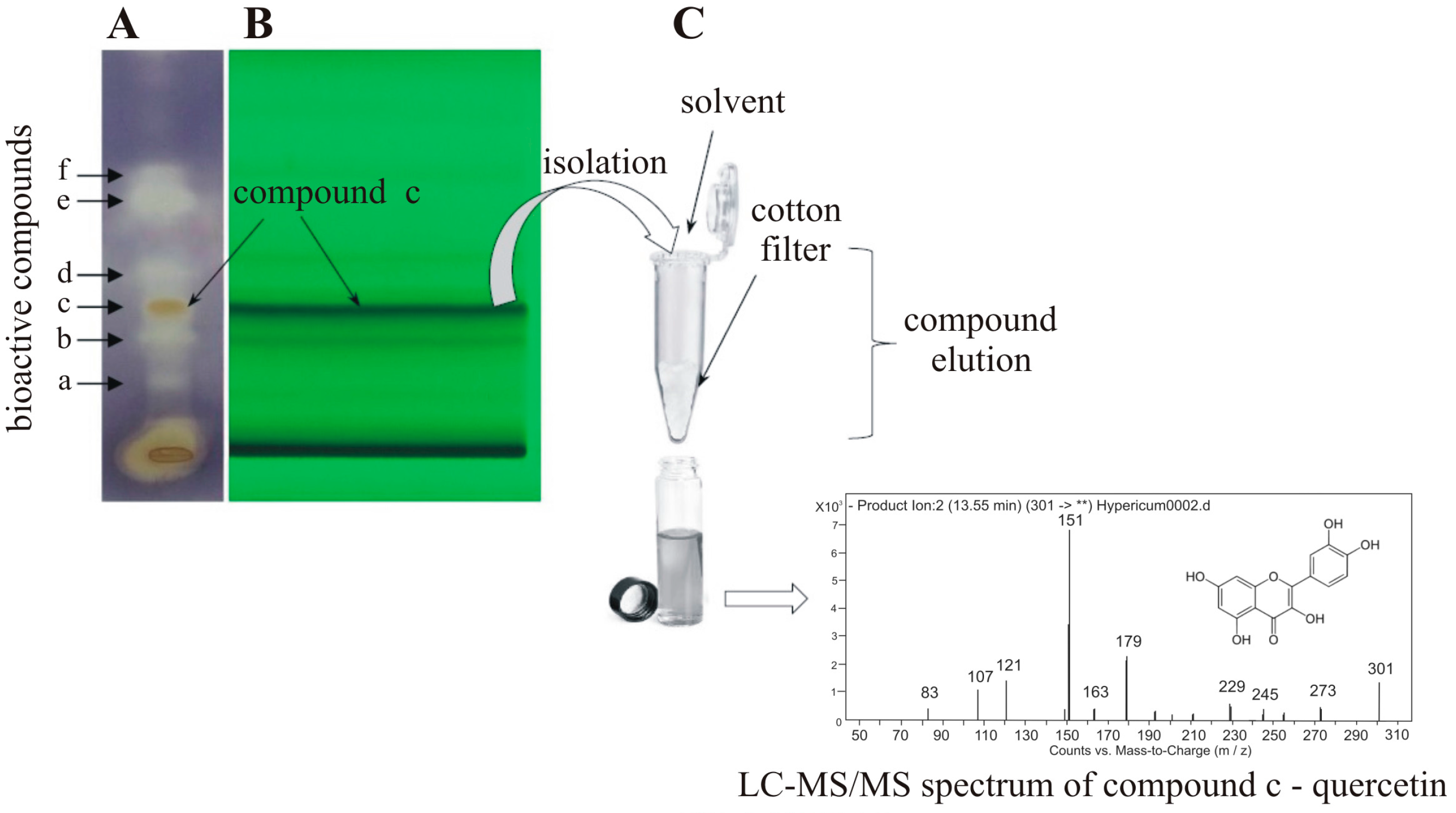
3.2. Coupling TLC to Mass Spectrometry

3.2.1. TLC-MS Interface
3.2.2. The DART System
4. Other TLC-DB Possibilities
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cody, V.; Middleton, E.; Harborne, J.B. Plant Flavonoids in Biology and Medicine-Biochemical, Pharmacological, and Structure-Activity Relationships; Liss, A.R., Ed.; John Wiley & Sons Inc: New York, NY, USA, 1986. [Google Scholar]
- Langseth, L. Oxidants, Antioxidants and Disease Prevention; International Life Sciences Institute Europe Press: Brussels, Belgium, 1995. [Google Scholar]
- The European Parliament and the Council. A second programme of Community action in the field of health (2008-13). Decision No 1350/2007/EC. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32007D1350 (accessed on 21 November 2007).
- The European Commission. Communication From The Commission On A Community Strategy against Antimicrobial Resistance (COM ( (2001) 333 final, volume I). Available online: http://europa.eu/legislation_summaries/public_health/threats_to_health/c11568_en.htm (accessed on 11 May 2015).
- Barrett, J.F.; Davey, P.G.; McEwen, S.A.; O’Brien, T.F.; Levy, S.B.; Boston, M.A. Antibiotic Resistance: Synthesis of Recommendations by Expert Policy Groups; World Health Organization: Boston, MA, USA, 2001. [Google Scholar]
- Choma, I.M.; Jesionek, W. Effects-Directed Biological Detection: Bioautography. In Instrumental Thin-Layer Chromatography; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Brack, W.; Ulrich, N.; Bataineh, M. Separation techniques in effect-directed analysis. In Effect-Directed Analysis of Complex Environmental Contamination; Springer-Verlag: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Brack, W. Effect-directed analysis in ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Hecker, M.; Hollert, H. Effect-directed analysis (EDA) in aquatic ecotoxicology: State of the art and future challenges. Environ. Sci. Pollut. Res. 2009, 16, 607–613. [Google Scholar] [CrossRef]
- Choma, I.; Grzelak, E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Marston, A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A 2011, 1218, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Cretu, G.C.; Morlock, G.E. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem. 2014, 146, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Jesionek, W.; Choma, I.M.; Majer-Dziedzic, B.; Malinowska, I. Screening bacterial and radical scavenging properties of chosen plant extracts using thin-layer chromatography-direct bioautography. J. Liq. Chrom. Relat. Tech. 2014, 37, 2882–2891. [Google Scholar] [CrossRef]
- Jesionek, W.; Grzelak, E.M.; Majer-Dziedzic, B.; Choma, I.M. Thin-layer chromatography-Direct bioautography for the screening of antimicrobial properties of plant extracts. J. Planar Chromatogr. 2013, 26, 109–113. [Google Scholar] [CrossRef]
- Krüger, S.; Urmann, O.; Morlock, G.E. Development of a planar chromatographic method for quantitation of anthocyanes in pomace, feed, juice and wine. J. Chromatogr. A 2013, 1289, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Schwack, W. Hyphenations in planar chromatography. J. Chromatogr. A 2010, 1217, 6600–6609. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Otta, K.H.; Ott, P.G.; Tyihák, E. Separation and detection of aflatoxins using overpressured-layer chromatography and bioautography. J. Planar Chromatogr. 2003, 16, 417–420. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szarka, S.; Ott, P.G.; Héthelyi, É.B.; Szóke, É.; Tyihak, E. Seperation and identification of antibacterial chamomile components using OPLC, bioautography and GC-MS. Med. Chem. 2012, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, E.M.; Majer-Dziedzic, B.; Choma, I.M. Development of a novel direct bioautography-thin-layer chromatography test: optimization of growth condition for Gram-negative bacteria, Escherichia coli. J. AOAC Int. 2011, 94, 1569–1572. [Google Scholar] [CrossRef]
- Grzelak, E.M.; Majer-Dziedzic, B.; Choma, I.M. Development of a novel direct bioautography-thin-layer chromatography test: optimization of growth conditions for gram-positive bacteria, Bacillus subtilis. J. AOAC Int. 2013, 96, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, E.M.; Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Applications of novel direct bioautography tests for analysis of antimicrobials: A review. J. AOAC Int. 2013, 96, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.M.; Grzelak, E.M.; Majer-Dziedzic, B. Comparison of deproteinization methods used before TLC-DB and HPLC analysis of flumequine residues in milk. Med. Chem. 2012, 8, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gałan, A.; Jesionek, W.; Majer-Dziedzic, B.; Lubicki, Ł.; Choma, I.M. Investigation of different extraction methods on the content of main components in Coffea arabica L. extracts. J. Planar Chromatogr. 2015, 28, 178–183. [Google Scholar] [CrossRef]
- Horváth, G.; Jámbor, N.; Végh, A.; Böszörményi, A.; Lemberkovics, É.; Héthelyi, É.; Kovács, K.; Kocsis, B. Antimicrobial activity of essential oils: The possibilities of TLC-bioautography. Flavour Fragr. J. 2010, 25, 178–182. [Google Scholar] [CrossRef]
- Horváth, G.; Szabó, L.G.; Lemberkovics, É.; Botz, L.; Kocsis, B. Characterization and TLC-bioautographic detection of essential oils from some Thymus taxa. Determination of the activity of the oils and their components against plant pathogenic bacteria. J. Planar Chromatogr. 2004, 17, 300–304. [Google Scholar] [CrossRef]
- Shai, L.J.; McGaw, L.J.; Eloff, J.N. Extracts of the leaves and twigs of the threatened tree Curtisia dentata (Cornaceae) are more active against Candida albicans and other microorganisms than the stem bark extract. Afr. J. Bot. 2009, 75, 363–366. [Google Scholar] [CrossRef]
- Suleiman, M.M.; McGaw, L.J.; Naidoo, V.; Eloff, J.N. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr. J. Trad. CAM 2010, 7, 64–71. [Google Scholar]
- Molnár, V.; Billes, F.; Tyihák, E.; Ott, P.G. Bioautographic detection of antimicrobial compounds in the edible shiitake mushroom. J. Planar Chromatogr. 2008, 21, 423–426. [Google Scholar] [CrossRef]
- Horváth, G.; Kocsis, B.; Lemberkovics, É.; Böszörményi, A.; Ott, P.G.; Móricz, Á. Detection of antibacterial activity of essential oil components by TLC-bioautography using luminescent bacteria. J. Planar Chromatogr. 2013, 26, 114–118. [Google Scholar] [CrossRef]
- Verbitski, S.M.; Gourdin, G.T.; Ikenouye, L.M.; McChesney, D.J. Detection of Actaea racemosa adulteration by thin-layer chromatography and combined thin-layer chromatography-bioluminescence. J. AOAC Int. 2008, 91, 268–275. [Google Scholar] [PubMed]
- Baumgartner, V.; Schwack, W. Enhanced quantitative evaluation of the HPTLC-bioluminescence detection. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 980–995. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Szarka, S.; Ott, P.G.; Kertesy, D.; Héthelyi, É.; Szőke, É.; Tyihák, E. Application of direct bioautography and SPME-GC-MS for the study of antibacterial Chamomile ingredients. J. Planar Chromatogr. 2012, 25, 220–224. [Google Scholar] [CrossRef]
- Verbitski, S.M.; Hickey, S.; Gourdin, G.T.; Ikenouye, L.M. Bioluminex: An effective yet simple tool for screening mixtures. CBS CAMAG Bibliogr. Serv. 2007, 99, 11–13. [Google Scholar]
- Horváth, G.; Acs, K.; Kocsis, B. TLC-direct bioautography for determination of antibacterial activity of Artemisia adamsii essential oil. J. AOAC Int. 2013, 96, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.M.; Kowalski, C.; Lodkowski, R.; Burmańczuk, A.; Komaniecka, I. TLC-DB as an alternative to the HPLC method in the determination of cefacetril residues in cow’s milk. J. Liq. Chrom. Relat. Tech. 2008, 31, 1903–1912. [Google Scholar] [CrossRef]
- Choma, I.M.; Choma, A.; Komaniecka, I.; Pilorz, K.; Staszczuk, K. Semiquantitative estimation of enrofloxacin and ciprofloxacin by thin-layer chromatography-direct bioautography. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2071–2085. [Google Scholar] [CrossRef]
- Tyihák, E.; Móricz, Á.M.; Ott, P.G. Use of the BioArena system for indirect detection of endogenous ozone in spots after TLC or OPLC separation. J. Planar Chromatogr. 2008, 21, 77–82. [Google Scholar] [CrossRef]
- Tyihák, E.G.; Móricz, Á.M.; Ott, P.G.; Kátay, G.; Király-Véghely, Z. The potential of BioArena in the study of the formaldehydome. J. Planar Chromatogr. 2005, 18, 67–72. [Google Scholar] [CrossRef]
- Homans, A.L.; Fusch, A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J. Chromatogr. A 1970, 51, 327–329. [Google Scholar] [CrossRef]
- Rodriguez, S.; Wolfender, J.-L.; Hakizamungu, E.; Hostettmann, K. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina. Planta Med. 1995, 61, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.F.; Ioset, J.-R.; Ndjoko, K.; Guntern, A.; Foggin, C.M.; Hostettmann, K. On-line identification of the bioactive compounds from Blumea gariepina by HPLC-UV-MS and HPLC-UV-NMR, combined with HPLC-micro-fractionation. Phytochem. Anal. 2005, 16, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.F.; Wolfender, J.-L.; Atindehou, K.K.; Traore, D.; Hostettmann, K. On-line identification of the antifungal constituents of Erythrina vogelii by liquid chromatography with tandem mass spectrometry, ultraviolet absorbance detection and nuclear magnetic resonance spectrometry combined with liquid chromatographic micro-fractionation. J. Chromatogr. A 2002, 974, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Wolfender, J.-L.; Nianga, M.; Stoeckli-Evans, H.; Hostetmann, K. Antifungal and antibacterial naphthoquinones from Newbouldia laevis roots. Phytochemistry 1996, 42, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Wolfender, J.-L.; Ma, W.G.; Zhang, L.X.; Hostetmann, K. Secoiridoids and antifungal aromatic acids from Gentiana algida. Phytochemistry 1996, 41, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Wolfender, J.-L.; Zhang, L.X.; Ma, W.G.; Fuzzati, N.; Marston, A.; Hostettmann, K. Acyl secoiridoids and antifungal constituents from Gentiana macrophylla. Phytochemistry 1996, 42, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wolfender, J.-L.; Hostettmann, K.; Xu, R.; Qin, G. Antifungal alkaloids and limonoids derivatives from Dictamnus dasycarpus. Phytochemistry 1998, 47, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Meazza, G.; Dayan, F.E.; Wedge, D.E. Activity o quinones on Colletotrichum species. J. Agric. Food Chem. 2003, 51, 3824–3828. [Google Scholar] [CrossRef] [PubMed]
- Altintas, A.; Tabanca, N.; Tyihák, E.; Ott, P.G.; Móricz, A.M.; Mincsovics, E.; Wedge, D.E. Characterization of volatile constituents from Origanum onites and their antifungal and antibacterial activity. J. AOAC Int. 2013, 96, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Demirci, B.; Baser, K.H.C.; Tabanca, N.; Wedge, D.E. Characterization of volatile constituents of Haplopappus greenei and studies on the antifungal activity against phytopathogens. J. Agric. Food Chem. 2006, 54, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Baser, K.H.C.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Navickiene, H.M.D.; Alécio, A.C.; Kato, M.J.; Cavalheiro, A.J.; Furlan, M. Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry 2000, 55, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Móricz, Á.M.; Tyihák, E.; Ott, P.G. Usefulness of transgenic luminescent bacteria in direct bioautographic investigation of Chamomile extracts. J. Planar Chromatogr. 2010, 23, 180–183. [Google Scholar] [CrossRef]
- Jesionek, W.; Móricz, Á.M.; Alberti, Á.; Ott, P.G.; Kocsis, B.; Horváth, G.; Choma, I.M. TLC-DB as a bio-assay guided method for investigation of antibacterial compounds in Hypericum perforatum L. J AOAC Int. 2015, in press. [Google Scholar]
- Móricz, Á.M.; Fornal, E.; Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Effect-directed isolation and identification of antibacterial Chelidonium majus L. alkaloids. Chromatographia 2015, 78, 707–716. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Alberti, Á.; Böszörményi, A.; Lemberkovics, É.; Szőke, É.; Kéry, Á.; Mincsovics, E. Applicability of preparative overpressured layer chromatography and direct bioautography in search of antibacterial chamomile compounds. J. AOAC Int. 2013, 96, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Móricz, A.M.; Ott, P.G.; Böszörményi, A.; Lemberkovics, É.; Mincsovics, E.; Tyihák, E. Bioassay-guided isolation and identification of antimicrobial compounds from thyme essential oil by means of overpressured layer chromatography, bioautography and GC-MS. Chromatographia 2012, 75, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Mincovics, E.; Ott, P.G.; Alberti, Á.; Böszörményi, A.; Héthelyi, É.B.; Szőke, É.; Kéry, Á.; Lemberkovics, É.; Móricz, Á. In-situ clean-up and OPLC fractionation of chamomile flower extract to search active components by bioautography. J. Planar Chromatogr. 2013, 26, 172–179. [Google Scholar] [CrossRef]
- Dzido, T.; Lopaciuk, E.; Płochacz, P.W.; Chomicki, A.; Zembrzycka, M.; Frank, H. Equipment and preliminary results for orthogonal pressurized planar electrochromatography. J. Chromatogr. A 2014, 1334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Schwack, W. Coupling of planar chromatography to mass spectrometry. Trends Anal. Chem. 2010, 29, 1157–1171. [Google Scholar] [CrossRef]
- Cooks, R.G.; Ouyang, R.; Takats, Z.; Wiseman, J.M. Ambient Mass Spectrometry. Science 2006, 311, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, G.J.; Ford, M.J.; Deibel, M.A. Thin-layer chromatography and mass spectrometry coupled using desorption electrospray ionization. Anal. Chem. 2005, 77, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Ueda, Y. New coupling of planar chromatography with direct analysis in real time mass spectrometry. J. Chromatogr. A 2007, 1143, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Klöppel, A.; Grasse, W.; Brűmmer, F.; Morlock, G.E. HPTLC coupled with bioluminescence and mass spectrometry for bioactivity-based analysis of secondary metabolites in marine sponges. J. Planar Chromatogr. 2008, 21, 431–436. [Google Scholar] [CrossRef]
- Morlock, G.E.; Schuele, L.; Grashorn, S. Development of a quantitative high-performance thin-layer chtomatographic method for sucralose in sewage effluent, surface water and drinking water. J. Chromatogr. A 2011, 1218, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Naumoska, K.; Simonovska, B.; Albreht, A.; Vovk, I. TLC and TLC–MS screening of ursolic, oleanolic and betulinic acids in plant extracts. J. Planar Chromatogr. 2013, 26, 125–131. [Google Scholar] [CrossRef]
- Naumoska, K.; Vovk, I. Analysis of triterpenoids and phytosterols in vegetables by thin-layer chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1381, 229–238. [Google Scholar] [CrossRef]
- Smrke, S.; Vovk, I. Comprehensive thin-layer chromatography mass spectrometry of flavanols from Juniperus communis L. and Punica granatum L. J. Chromatogr. A 2013, 1289, 119–126. [Google Scholar] [CrossRef]
- Sajewicz, M.; Staszek, D.; Natic, M.; Waksmundzka-Hajnos, M.; Kowalska, T. TLC–MS versus TLC–LC–MS fingerprints of herbal extracts. Part III. Application of the reversed-phase liquid chromatography systems with C18 stationary phase. J. Chromatogr. Sci. 2011, 49, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.B.; Laramée, J.A. Atmospheric Pressure Ion Source. US Patent Number 6,949,741, 27 September 2005. [Google Scholar]
- Morlock, G.E.; Schwack, W. Determination of isopropylthioxanthone (ITX) in milk, yoghurt and fat by HPTLC-FLD, HPTLC-ESI/MS and HPTLC-DART/MS. Anal. Bioanal. Chem. 2006, 385, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Young, P.J. Direct znalysis of curcumin in turmeric by DART-MS. Phytochem. Anal. 2009, 20, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jee, E.H.; Ahn, K.S.; Choi, H.S.; Jang, Y.P. Identification of marker compounds in herbal drugs on TLC with DART-MS. Arch. Pharm. Res. 2010, 33, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, Ł.; Kryszeń, J.; Stochmal, A.; Oleszek, W.; Waksmundzka-Hajnos, M. Approach to develop a standardized TLC-DPPH• test for assessing free radical scavenging properties of selected phenolic compounds. J. Pharm. Biomed. Anal. 2012, 70, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E.; Klingelhöfer, I. Liquid chromatography-bioassay-mass spectrometry for profiling of physiologically active food. Anal. Chem. 2014, 86, 8289–8295. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choma, I.M.; Jesionek, W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Chromatography 2015, 2, 225-238. https://doi.org/10.3390/chromatography2020225
Choma IM, Jesionek W. TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants. Chromatography. 2015; 2(2):225-238. https://doi.org/10.3390/chromatography2020225
Chicago/Turabian StyleChoma, Irena M., and Wioleta Jesionek. 2015. "TLC-Direct Bioautography as a High Throughput Method for Detection of Antimicrobials in Plants" Chromatography 2, no. 2: 225-238. https://doi.org/10.3390/chromatography2020225




