The Effect of Detectable HIV Viral Load among HIV-Infected Children during Antiretroviral Treatment: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Testing
2.3. Statistical Analysis
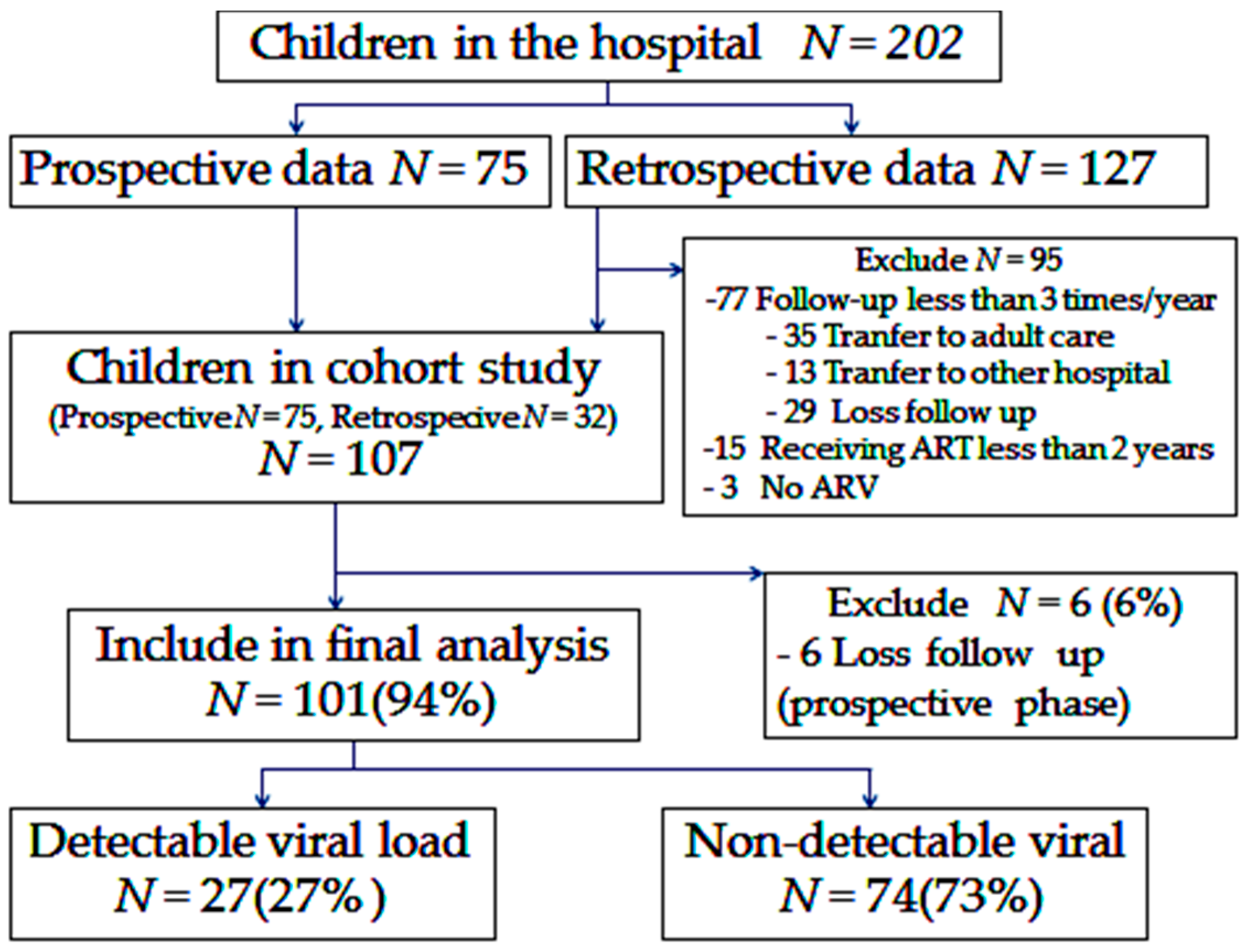
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ciaranello, A.L.; Chang, Y.; Margulis, A.V.; Bernstein, A.; Bassett, I.V.; Losina, E.; Walensky, R.P. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: A systematic review and meta-analysis. Clin. Infect. Dis. 2009, 49, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Collins, I.J.; Jourdain, G.; Hansudewechakul, R.; Kanjanavanit, S.; Hongsiriwon, S.; Ngampiyasakul, C.; Sriminiphant, S.; Technakunakorn, P.; Ngo-Giang-Huong, N.; Duong, T.; et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: A 5-year observational cohort study. Clin. Infect. Dis. 2010, 51, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, A.; Yotebieng, M.; Lusiama, J.; Matumona, Y.; Kitetele, F.; Napravnik, S.; Cole, S.R.; van Rie, A.; Behets, F. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: A cohort study. PLoS Med. 2011, 8, e100104. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.B.; Rimland, D. Effect of Expanded HIV Testing Programs on the status of newly diagnosed HIV-infected patients in Two Veterans Health Administration facilities: 1999–2009. J. Acquir. Immune Defic. Syndr. 2011, 57, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach World Health Organization. 2015. Available online: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf (accessed on 8 April 2015).
- Fonsah, J.Y.; Njamnshi, A.K.; Kouanfack, C.; Qiu, F.; Njamnshi, D.M.; Tagny, C.T.; Nchindap, E.; Kenmogne, L.; Mbanya, D.; Heaton, R.; et al. Adherence to Antiretroviral Therapy (ART) in Yaoundé-Cameroon: Association with Opportunistic Infections, Depression, ART Regimen and Side Effects. PLoS ONE 2017, 12, e0170893. [Google Scholar] [CrossRef] [PubMed]
- AIDSinfo. Management of Children Receiving Antiretroviral Therapy. Available online: https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv-guidelines/438/recognizing-and-managing-antiretroviral-treatment-failure (accessed on 17 October 2017).
- Thitilertdecha, P.; Khowawisetsut, L.; Ammaranond, P.; Poungpairoj, P.; Tantithavorn, V.; Onlamoon, N. Impact of Vaccination on Distribution of T Cell Subsets in Antiretroviral-Treated HIV-Infected Children. Dis. Mark. 2017, 2017, 5729639. [Google Scholar] [CrossRef] [PubMed]
- Granwehr, B. Review: Influenza vaccine reduces influenza-like illness in healthy adults. Ann. Intern. Med. 2014, 161, JC6. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; McAllister, D.A.; Reed, C.; Adeloye, D.O.; Rudan, I.; Muhe, L.M.; Madhi, S.A.; Campbell, H.; Nair, H. Global, regional, and national estimates of pneumonia burden in HIV-infected children in 2010: A meta-analysis and modelling study. Lancet Infect. Dis. 2014, 14, 1250–1258. [Google Scholar] [CrossRef]
- Venturini, E.; Turkova, A.; Chiappini, E.; Galli, L.; de Martino, M.; Thorne, C. Tuberculosis and HIV co-infection in children. BMC Infect. Dis. 2014, 14 (Suppl. 1), S5. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.; Mudenda, V.; Mwaba, P.; Zumla, A. Deaths due to respiratory tract infections in Africa: A review of autopsy studies. Curr. Opin. Pulm. Med. 2013, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Chintu, C.; Mudenda, V.; Lucas, S.; Nunn, A.; Lishimpi, K.; Maswahu, D.; Kasolo, F.; Mwaba, P.; Bhat, G.; Terunuma, H.; et al. Lung diseases at necropsy in African children dying from respiratory illnesses: A descriptive necropsy study. Lancet 2002, 360, 985–990. [Google Scholar] [CrossRef]
- Gupta, R.K.; Lucas, S.B.; Fielding, K.L.; Lawn, S.D. Prevalence of tuberculosis in postmortem studies of HIV-infected adults and children in resource-limited settings: A systematic review and meta-analysis. AIDS 2015, 29, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.M.; Zar, H.J. Community-acquired pneumonia in HIV-infected children: A global perspective. Curr. Opin. Pulm. Med. 2010, 16, 208–216. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report. Contract No. WHO/ HTM/TB/2014.08. 2014. Available online: http://www.who.int/tb/publications/global_report/en/ (accessed on 1 August 2014).
- Rebouças, M.C.; Silva, M.O.D.; Haguihara, T.; Brites, C.; Netto, E.M. Tuberculosis incidence among people living with HIV/AIDS with virologic failure of antiretroviral therapy in Salvador, Bahia, Brazil. Braz. J. Infect. Dis. 2017, 21, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Amani-Bosse, C.; Dahourou, D.L.; Malateste, K.; Amorissani-Folquet, M.; Coulibaly, M.; Dattez, S.; Emieme, A.; Barry, M.; Rouzioux, C.; N’gbeche, S.; et al. Virologic response and resistances over 12 months among HIV-infected children less than two years receiving first-line lopinavir/ritonavir-based antiretroviral therapy in Cote d’Ivoire and Burkina Faso: The MONOD ANRS 12206 cohort. J. Int. AIDS Soc. 2017, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mosoko, J.J.; Akam, W.; Weidle, P.; Brooks, J.; Aweh, A. Survival and adherence to ART in an era of decreasing drug cost in Limbe, Cameroon. In Proceedings of the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA, USA, 25–28 February 2007. Abstract 536. [Google Scholar]
- ECDC. Influenza Case Definitions. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012D0506&qid=1428573336660&from=EN#page=16 (accessed on 27 September 2012).
- Thomas, S.; Theodore, S. Community-acquired pneumonia. In Nelson Textbook of Pediatrics, 19th ed.; Kliegman, R.M., Stanton, B.M.D., Geme, J.S.T., Schor, N., Behrman, R.E., Eds.; Saunders: Philadelphia, PA, USA, 2011; pp. 1474–1479. [Google Scholar]
- CDC. Opportunistic Infections. Available online: https://www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html (accessed on 30 May 2017).
- World Health Organization. Child Growth Standards (Girls). Available online: http://www.who.int/childgrowth/standards/chts_bfa_girls_z/en/ (accessed on 13 February 2006).
- World Health Organization. Child Growth Standards (Boys). Available online: http://www.who.int/childgrowth/standards/b_f_a_tables_z_boys/en/ (accessed on 31 March 2006).
- World Health Organization. Growth Reference 5–19 Years. Available online: http://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 4 April 2007).
- Lima, V.; Harrigan, R.; Murray, M.; Moore, D.M.; Wood, E.; Hogg, R.S.; Montaner, J.S. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS 2008, 22, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Gachara, G.; Mavhandu, L.G.; Rogawski, E.T.; Manhaeve, C.; Bessong, P.O. Evaluating adherence to antiretroviral therapy using pharmacy refill records in a rural treatment site in South Africa. AIDS Res. Treat. 2017, 2017, 5456219. [Google Scholar] [CrossRef] [PubMed]
- Mitiku, H.; Abdosh, T.; Teklemariam, Z. Factors affecting adherence to antiretroviral treatment in Harari national regional state, eastern Ethiopia. ISRN AIDS 2013, 2013, 960954. [Google Scholar] [CrossRef] [PubMed]
- Gondrie, I.P.E.; Bastiaans, D.E.T.; Fraaij, P.L.A.; Driessen, G.J.A.; van der Knaap, L.C.; Visser, E.G.; van Jaarsveld, P.; de Groot, R.; Hartwig, N.G.; Burger, D.M.; et al. Sustained Viral Suppression in HIV-Infected Children on Once-Daily Lopinavir/ritonavir in Clinical Practice. Pediatr. Infect. Dis. J. 2017, 36, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Moolasart, V.; Manosuthi, W.; Ausavapipit, J.; Chottanapund, S.; Likanonsakul, S.; Uttayamakul, S.; Srisopha, S.; Lerdsamran, H.; Puthavathana, P. Long-term seroprotective response of trivalent seasonal influenza vaccine in HIV-infected children, regardless of immunogenicity before immunisation. Int. J. WendSalvador AIDS 2016, 27, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.R.; Goode, M.; Lynam, P.; Gavin, P.J.; Butler, K.M. HIV virological suppression influences response to the AS03-adjuvanted monovalent pandemic influenza A H1N1 vaccine in HIV-infected children. Influenza Respir. Viruses 2014, 8, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Halloran, M.E.; Longini, I.M., Jr. Public health. Community studies for vaccinating schoolchildren against influenza. Science 2006, 311, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Nebot Giralt, A.; Nöstlinger, C.; Lee, J.; Salami, O.; Lallemant, M.; Ouma, O.; Nyamongo, I.; Marchal, B. Understanding the acceptability and adherence to paediatric antiretroviral treatment in the new formulation of pellets (LPV/r): The protocol of a realist evaluation. BMJ Open 2017, 7, e014528. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.E. Strategies for optimizing adherence to highly active antiretroviral therapy: Lessons from research and clinical practice. Clin. Infect. Dis. 2001, 33, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Bulage, L.; Ssewanyana, I.; Nankabirwa, V.; Nsubuga, F.; Kihembo, C.; Pande, G.; Ario, A.R.; Matovu, J.K.; Wanyenze, R.K.; Kiyaga, C. Factors associated with virologic non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014–July 2015. BMC Infect. Dis. 2017, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.A.; Harris, D.R.; Tassiopoulos, K.; Leister, E.; Negrini, S.F.; Ferreira, F.F.; Cruz, M.L.; Pinto, J.; Allison, S.; Hazra, R.; et al. Relationship between viral load and behavioral measures of adherence to antiretroviral therapy in children living with human immunodeficiency virus in Latin America. Braz. J. Infect. Dis. 2015, 19, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.D.; Fink, V.; Yip, B.; Hogg, R.S.; Harrigan, P.R.; Montaner, J.S. Association between HIV-1 RNA level and CD4 cell count among untreated HIV-infected individuals. Am. J. Public Health 2009, 99 (Suppl. 1), S193–S196. [Google Scholar] [CrossRef] [PubMed]
- Joram, S.L.; Paul, G.; Moses, K.; Stanley, B.; Isaac, M.; Allan, G.; Tom, M.; Lilian, K.; Mildred, M. Misdiagnosis of HIV treatment failure based on clinical and immunological criteria in Eastern and Central Kenya. BMC Infect. Dis. 2017, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Keiser, O.; Tweya, H.; Boulle, A.; Braitstein, P.; Schecter, M.; Brinkhof, M.W.; Dabis, F.; Tuboi, S.; Sprinz, E.; Pujades-Rodriguez, M.; et al. Switching to second-line antiretroviral therapy in resource-limited settings: Comparison of programmes with and without viral load monitoring. AIDS 2009, 23, 1867–1874. [Google Scholar] [PubMed]
- Vallabhaneni, S.; Chandy, S.; Heylen, E.; Ekstrand, M.L. Evaluation of WHO immunologic criteria for treatment failure: Implications for detection of virologic failure, evolution of drug resistance and choice of second-line therapy in India. J. Int. AIDS Soc. 2013, 16, 18449. [Google Scholar] [CrossRef] [PubMed]
| Factor | Group A Detectable Viral Load (N = 27) | Group B Non-Detectable Viral Load (N = 74) | p-Value |
|---|---|---|---|
| Age (years) | 0.695 | ||
| Mean ± SD | 12.0 ± 4.3 | 12.3 ± 4.3 | |
| Sex, n | 1.00 | ||
| Male | 14 | 37 | |
| Female | 13 | 37 | |
| BMI for age z-scores (standard deviation scores) | 0.100 | ||
| Obesity (>+2 SD) | 2 | 5 | |
| Overweight (>+1 SD) | 0 | 6 | |
| Recommended (−2 SD to +1 SD) | 20 | 46 | |
| Thinness (<−2 SD) | 4 | 15 | |
| Severe thinness (<−3 SD) | 1 | 2 | |
| Previous TB disease history, n (%) | 5 (18.5) | 8 (10.8) | 0.325 |
| Parent TB history, n (%) | 23 (85.2) | 59 (79.7) | 0.584 |
| Factor | Group A Detectable Viral Load (N = 27) | Group B Non-Detectable Viral Load (N = 74) | p-Value |
|---|---|---|---|
| Adherence ≥ 95%, n (%) | 17 (63.0) | 65 (87.8) | 0.008 |
| Last WHO stage, n (%) | |||
| 1, 2 | 20 (74.1) | 47 (63.5) | 0.353 |
| 3, 4 | 7 (25.9) | 27 (36.5) | |
| HAART, n (%) | |||
| PI | 15 (55.6) | 37 (50.0) | 0.659 |
| Non-PI | 12 (44.4) | 37 (50.0) | |
| Nadir CD4 cell count during the study, mean ± SD, cell/µL | 646.0 ± 326.2 | 867.9 ± 341.1 | 0.025 |
| Nadir hematocrit, mean ± SD, g/dL | 37.1 ± 4.3 | 38.3 ± 3.1 | 0.222 |
| Peak cholesterol, mean ± SD, mg/dL | 186.0 ± 35.0 | 193.4 ± 37.6 | 0.478 |
| Peak triglyceride, mean ± SD, mg/dl | 135.8 ± 75.1 | 126.8 ± 59.0 | 0.608 |
| Hospitalization, n (%) | 7 (25.9) | 15 (20.3) | 0.590 |
| Pneumonia, n (%) | 5 (18.5) | 18 (24.3) | 0.604 |
| ILI, n (%) | 11 (40.7) | 13 (17.6) | 0.020 |
| OIs, n (%) | 12 (44.4) | 16 (21.6) | 0.028 |
| Study status, n (%) | |||
| Yes | 22 (81.5) | 63 (85.1) | 0.759 |
| No | 5 (18.5) | 11 (14.9) | |
| Distance of residence, n (%) | |||
| Short | 25 (92.6) | 64 (86.5) | 0.501 |
| Long | 2 (7.4) | 10 (13.5) | |
| Caregiver, n (%) | |||
| Parent | 14 (51.9) | 36 (48.6) | 0.712 |
| Relative | 11 (40.7) | 30 (40.5) | |
| Care Center | 2 (7.4) | 8 (10.8) | |
| Father as main caregiver, n (%) | 3 (11.1) | 10 (13.5) | 1.00 |
| Significant Factors | Odds Ratio | p-Value | 95% CI |
|---|---|---|---|
| ILI | 9.629 | 0.025 | 1.337–69.359 |
| Adherence | 0.195 | 0.025 | 0.047–0.811 |
| Lower nadir CD4 during the study | 1.102 | 0.038 | 1.100–1.305 |
| OIs | 0.773 | 0.795 | 0.111–5.364 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moolasart, V.; Chottanapund, S.; Ausavapipit, J.; Likanonsakul, S.; Uttayamakul, S.; Changsom, D.; Lerdsamran, H.; Puthavathana, P. The Effect of Detectable HIV Viral Load among HIV-Infected Children during Antiretroviral Treatment: A Cross-Sectional Study. Children 2018, 5, 6. https://doi.org/10.3390/children5010006
Moolasart V, Chottanapund S, Ausavapipit J, Likanonsakul S, Uttayamakul S, Changsom D, Lerdsamran H, Puthavathana P. The Effect of Detectable HIV Viral Load among HIV-Infected Children during Antiretroviral Treatment: A Cross-Sectional Study. Children. 2018; 5(1):6. https://doi.org/10.3390/children5010006
Chicago/Turabian StyleMoolasart, Visal, Suthat Chottanapund, Jarurnsook Ausavapipit, Sirirat Likanonsakul, Sumonmal Uttayamakul, Don Changsom, Hatairat Lerdsamran, and Pilaipan Puthavathana. 2018. "The Effect of Detectable HIV Viral Load among HIV-Infected Children during Antiretroviral Treatment: A Cross-Sectional Study" Children 5, no. 1: 6. https://doi.org/10.3390/children5010006





