Sleep Disorders in Childhood Neurological Diseases
Abstract
:1. Introduction
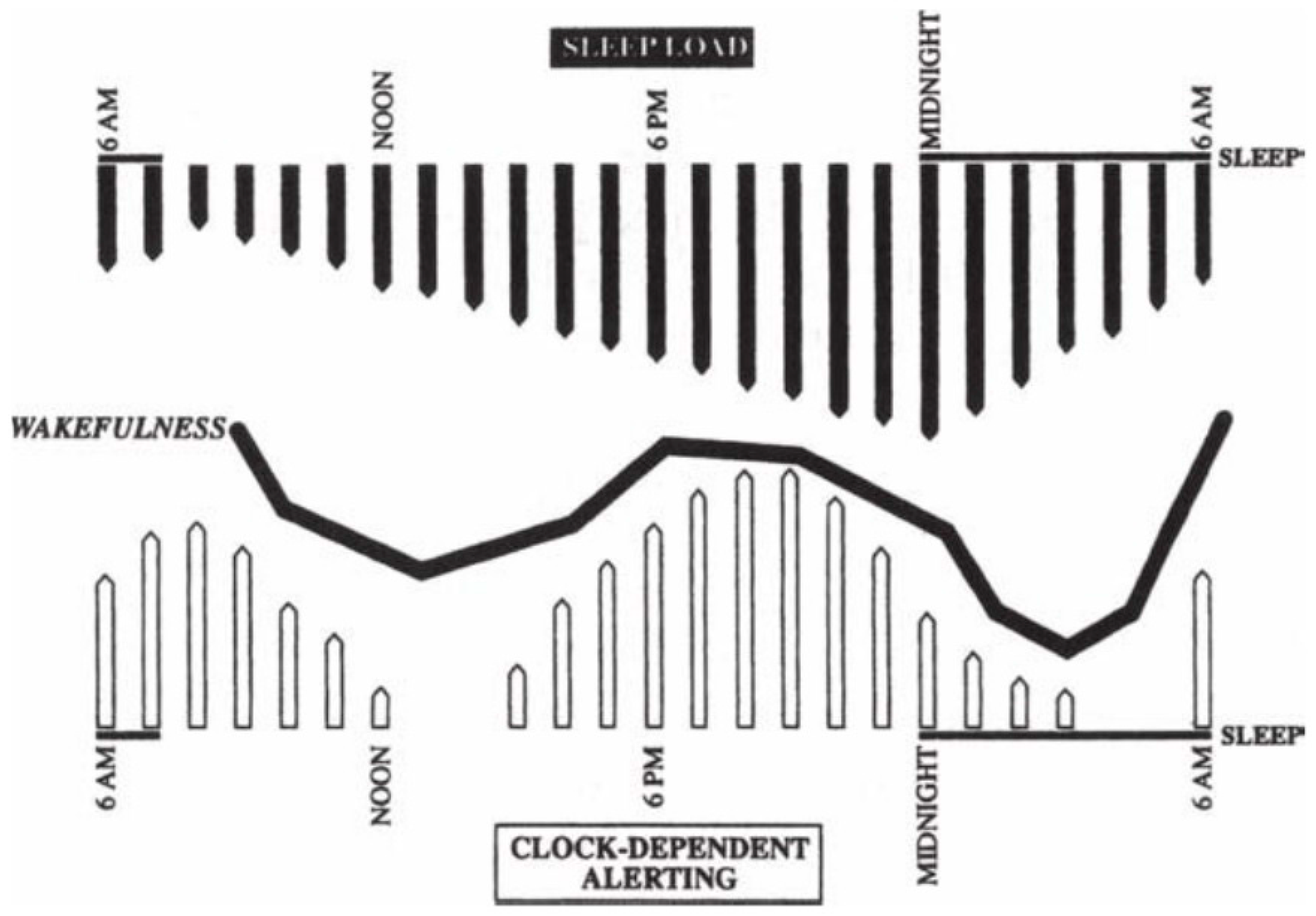
2. Sleep-Wake Cycles Regulation
3. Central Nervous System Malformations
4. Achondroplasia
5. Autism Spectrum and Neurodevelopmental Disorders
6. Neuromuscular Diseases
7. Sleep and Epilepsy
8. Cerebral Palsy
9. Headaches
10. Conclusion
Acknowledgment
Conflicts of Interest
References
- Owens, J. Classification and epidemiology of childhood sleepdisorders. Prim. Care 2008, 35, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Richdale, A.L.; Schreck, K.A. Sleep problems in autism spectrumdisorders: Prevalence, nature, & possible biopsychosocialaetiologies. Sleep Med. Rev. 2009, 13, 403–411. [Google Scholar] [PubMed]
- Hvolby, A.; Jørgensen, J.; Bilenberg, N. Actigraphic and parentalreports of sleep difficulties in children with attention-deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 2008, 162, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Dosi, C.; Figura, M.; Ferri, R.; Bruni, O. Sleep and headache. Semin. Pediatr. Neurol. 2015, 22, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Damberger, M.M.; Foldvary-Schaefer, N. Primary sleep disorders in people with epilepsy: Clinical questions and answers. Child. Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 145–176. [Google Scholar] [CrossRef] [PubMed]
- Dement, W.C.; Vaughan, C. The Promise of Sleep: A Pioneer in Sleep Medicine Explores the Vital Connection between Health, Happiness, and a Good Night’s Sleep; Delacorte Press: New York, NY, USA, 1999. [Google Scholar]
- España, R.A.; Scammell, T.E. Sleep neurobiology for the clinician. Sleep 2004, 27, 811–820. [Google Scholar] [PubMed]
- Fallone, G.; Owens, J.A.; Deane, J. Sleepiness in children and adolescents: Clinical implications. Sleep Med. Rev. 2002, 6, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Jenni, O.; Carskadon, M. Sleep behavior and sleep regulation from infancy through adolescence. Sleep Med. Clin. 2007, 2, 321–329. [Google Scholar] [CrossRef]
- Sadeh, A.; Gruber, R.; Raviv, A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Dev. 2003, 74, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Arens, R.; Omlin, K.J.; Jacobs, R.A.; Keens, T.G. Peripheral chemoreceptor function in children with myelomeningocele and Arnold-Chiari malformation type 2. Chest 1995, 108, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Maria, B.L.; Boltshauser, E.; Palmer, S.C.; Tran, T. Clinical features and revised diagnostic criteria in Joubert syndrome. J. Child Neurol. 1999, 14, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Shiang, R.; Thompson, L.M.; Zhu, Y.Z.; Church, D.M.; Fielder, T.J.; Bocian, M.; Winokur, S.T.; Wasmuth, J.J. Mutations in the transmembrane domain of FGFR-3 cause the most common genetic form of dwarfism, achondroplasia. Cell 1994, 78, 335–342. [Google Scholar] [CrossRef]
- Zucconi, M.; Weber, G.; Castronovo, V.; Ferini-Strambi, L.; Russo, F.; Chiumello, G.; Smirne, S. Sleep and upper airway obstruction in children with achondroplasia. J. Pediatr. 1996, 129, 743–749. [Google Scholar] [CrossRef]
- Tasker, R.; Dundas, I.; Laverty, A.; Fletcher, M.; Lane, R.; Stocks, J. Distinct patterns of respiratory difficulty in young children with achondroplasia: A clinical, sleep and lung function study. Arch. Dis. Child. 1998, 79, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Posserud, M.B.; Gillberg, C.; Lundervold, A.J.; Hysing, M. Sleep problems in children with autism spectrum problems: A longitudinal population-based study. Autism 2012, 16, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Souders, M.C.; Mason, T.B.A.; Valladares, O.; Bucan, M.; Levy, S.E.; Mandell, D.S.; Weaver, T.E.; Pinto-Martin, J. Sleep behaviors and sleep quality in children withautismspectrum disorders. Sleep 2009, 32, 1566–1578. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, L.; Stores, G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Dev. Med. Child Neurol. 2004, 46, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.W.; Rodriguez, A.J.; Jennison, K.; Buckley, J.; Thurm, A.; Sato, S.; Swedo, S. Rapid eye movement sleep percentage in children with autism compared with children with developmental delay and typical development. Arch. Pediatr. Adolesc. Med. 2010, 164, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Stickgold, R. Sleep-dependent memory consolidation. Nature 2005, 437, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Maquet, P. The role of sleep in learning and memory. Science 2001, 294, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.P.; Giannotti, F.; Cortesi, F. Sleep patterns in autistic spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 917. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.E.; Malow, B.A. Autism and other neurodevelopmental disorders. In Sleep in Childhood Neurological Disorders; Kothare, S.V., Kotagal, S., Eds.; Demos Medical: New York, NY, USA, 2011; pp. 143–152. [Google Scholar]
- Daoust, A.M.; Lusignan, F.A.; Braun, C.M.J.; Mottron, L.; Godbout, R. Dream content analysis in persons with an autism spectrum disorder. J. Autism Dev. Disord. 2008, 38, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Daoust, A.M.; Limoges, E.; Bolduc, C.; Mottron, L.; Godbout, R. EEG spectral analysis of wakefulness and REM sleep in high functioning autistic spectrum disorders. Clin. Neurophysiol. 2004, 115, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, L.; Ljunggren, E.; Bremer, A.; Pedersen, C.; Landén, M.; Thuresson, K.; Giacobini, M.; Melke, J. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med. Genom. 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, R.; Cuccaro, M. Epilepsy and autism: Neurodevelopmental perspective. Curr. Neurol. Neurosci. Rep. 2011, 11, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Dosman, C.F.; Brian, J.A.; Drmic, I.E.; Senthilselvan, A.; Harford, M.M.; Smith, R.W.; Sharieff, W.; Zlotkin, S.H.; Moldofsky, H.; Roberts, S.W. Children with autism: Effect of iron supplementation on sleep and ferritin. Pediatr. Neurol. 2007, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P.; Gamble, C.; Jones, A.; Wiggs, L.; Williamson, P.; Sutcliffe, A.; Montgomery, P.; Whitehouse, W.P.; Choonara, I.; Allport, T.; Edmond, A.; Appleton, R. Melatonin for sleep problems in children with neurodevelopmental disorders: Randomised double masked placebo controlled trial. BMJ 2012, 345, e6664. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, P.; Stores, G.; Wiggs, L. The relative efficacy of two brief treatments for sleep problems in young learning disabled (mentally retarded) children: A randomised controlled trial. Arch. Dis. Child. 2004, 89, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Sebastiani, T.; Panunzi, S.; Valente, D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: A randomized placebo-controlled trial. J. Sleep Res. 2012, 21, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Gordon, E.; Kang, N.; Wagner, G.C. Use of clonidine in children with autism spectrum disorders. Brain Dev. 2008, 30, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P. When to use drugs to help sleep. Arch. Dis. Child. 2008, 93, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, C. Gillberg: Rettvariants—Retoidphenotypes. Clin. Dev. Med. 1993, 127, 40–60. [Google Scholar]
- Hagberg, B.; Goutieres, F.; Rett, A.; Wilson, J. Rett syndrome: Criteria for inclusion and exclusion. Brain Dev. 1985, 7, 372–373. [Google Scholar] [CrossRef]
- Scala, E.; Ariani, F.; Mari, F.; Caselli, R.; Pescucci, C.; Longo, I.; Meloni, I.; Giachino, D.; Bruttini, M.; Hayek, G.; et al. CDKL5/STK9 is mutated in Rett syndrome variant with infantile spasms. J. Med. Genet. 2005, 42, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Kalscheuer, V.M.; Tao, J.; Donnelly, A.; Hollway, G.; Schwinger, E.; Kübart, S.; Menzel, C.; Hoeltzenbein, M.; Tommerup, N.; Eyre, H.; et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am. J. Hum. Genet. 2003, 72, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; Archer, H.L.; Colley, J.P.; Ravn, K.; Nielsen, J.B.; Kerr, A.; Williams, E.; Christodoulou, J.; Gecz, J.; Jardine, P.E.; et al. Early onset seizures and Rett-like features associated with mutations in CDKL5. Eur. J. Hum. Genet. 2005, 13, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Borg, I.; Freude, K.; Kübart, S.; Hoffmann, K.; Menzel, C.; Laccone, F.; Firth, H.; Ferguson-Smith, M.A.; Tommerup, N.; Ropers, H.H.; et al. Disruption of Netrin G1 by a balanced chromosome translocation in a girl with Rett syndrome. Eur. J. Hum. Genet. 2005, 13, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Ariani, F.; Hayek, G.; Rondinella, D.; Artuso, R.; Mencarelli, M.A.; Spanhol-Rosseto, A.; Pollazzon, M.; Buoni, S.; Spiga, O.; Ricciardi, S.; et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 2008, 83, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Nagarajan, L.; De Klerk, N.; Jacoby, P.; Ellaway, C.; Leonard, H. Sleep problems in Rett syndrome. Brain Dev. 2007, 29, 609–616. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, G.; Demaio, V.; Scarpelli, F.; Calvario, T.; Minervini, M.G. Central sleep apnea in Rett syndrome. Neurol. Sci. 2009, 30, 389–391. [Google Scholar] [CrossRef] [PubMed]
- d’Orsi, G.; Demaio, V.; Minervini, M.G. Myoclonic status misdiagnosed as movement disorders in Rettsyndrome: A video-polygraphic study. Epilepsy Behav. 2009, 15, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.K.; Glaze, D.G.; Schultz, R.J.; Zoghbi, H.Y.; Williamson, D.; Frost, J.D., Jr.; Jankovic, J.J.; Del Junco, D.; Skender, M.; Waring, S.; et al. Rett syndrome: Controlled study of an oral opiate antagonist, naltrexone. Ann. Neurol. 1994, 35, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Givan, D.C. Sleep and breathing in children with neuromuscular disease. In Sleep and Breathing in Children: A Developmental Approach; Loughlin, G.M., Carrol, J.L., Marcus, C.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 691–735. [Google Scholar]
- Zucconi, M. Sleep disorders in children with neurologic diseases. In Sleep and Breathing in Children: A Developmental Approach; Loughlin, G.M., Carrol, J.L., Marcus, C.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 363–383. [Google Scholar]
- Khan, Y.; Heckrnatt, J.Z. Obstructive apnoeas in Duchenne muscular dystrophy. Thorax 1994, 49, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Calverley, P.M.; Edwards, R.H. Hypoxemia during sleep in Duchenne muscular dystrophy. Am. Rev. Respir. Dis. 1988, 137, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Edwards, R.H.; Calverley, P.M. Mechanisms of sleep-disordered breathing in chronic neuromuscular disease: Implications for management. Q. J. Med. 1991, 81, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Kirk, V.G.; Flemons, W.W.; Adams, C.; Rimmer, K.P.; Montgomery, M.D. Sleep-disordered breathing in Duchenne muscular dystrophy: A preliminary study of the role of portable monitoring. Pediatr. Pulmonol. 2000, 29, 135–140. [Google Scholar] [CrossRef]
- Kotagal, P.; Yardi, N. The relationship between sleep and epilepsy. Semin. Pediatr. Neurol. 2008, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Nickels, K.; Wirrell, W. Electrical status epilepticus in sleep. Semin.Pediatr. Neurol. 2008, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Liukkonen, E.; Kantola-Sorsa, E.; Paetau, R.; Gaily, E.; Peltola, M.; Granström, M.L. Long term outcome of 32 children with encephalopathy with status epilepticus during sleep, or ESES syndrome. Epilepsia 2010, in press. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, R.J.; Wilken, K.; Bennet, D.R. Efficacy of sleep deprivation as an activating procedure in epilepsy patients. J. Clin. Neurophysiol. 1984, 1, 83. [Google Scholar] [CrossRef] [PubMed]
- Britton, T.C.; O’Donoghue, M.; Duncan, J.S. Exacerbation of epilepsy by sleep apnea. J. Neurol. Neurosurg. Psychiatry 1997, 63, 808. [Google Scholar] [CrossRef] [PubMed]
- Kosko, J.K.; Derkay, C.S. Uvulopalatopharyngoplasty: Treatment of obstructive sleep apnea in neurologically impaired pediatric patients. Int. J. Pediatr. Otorhinolaryngol. 1995, 32, 241–246. [Google Scholar] [CrossRef]
- Cohen, S.R.; Lefaivre, J.F.; Burstein, F.; Simms, C.; Kattos, A.V.; Scott, P.H.; Montgomery, G.L.; Graham, L. Surgical treatment of obstructive sleep apnea in neurologically compromised patients. Plast. Reconstr. Surg. 1997, 99, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Kirk, V.; Kahn, A.; Brouillette, R.T. Diagnostic approach to obstructivesleep apnea in children. Sleep Med. Rev. 1998, 2, 255–269. [Google Scholar] [CrossRef]
- Shintani, T.; Asakura, K.; Ishi, K.; Yoshida, M.; Kataura, A.; Ogasawara, H. Obstructive sleep apnea in children with cerebral palsy. Nippon Jibiinkoka Gakkai Kaiho 1998, 101, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, M.; Kiyono, S.; Takenchi, T. Nocturnal sleep in mentally retarded infants with cerebral palsy. Electroencephalogr. Clin. Nenrophysiol. 1985, 61, 465–471. [Google Scholar] [CrossRef]
- Leger, D.; Prevot, E.; Philip, P.; Yence, C.; Labaye, N.; Paillard, M.; Guilleminault, C. Sleep disorders in children with blindness. Ann. Neurol. 1999, 46, 648. [Google Scholar] [CrossRef]
- Levi, R.; Hultling, C.; Nash, M.S.; Seiger, A. The Stockholm spinal cord injury study: Medical problems in a regional SCI population. Paraplegia 1995, 33, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Tancredi, A.; Yundt, B.; Larin, H. Sleep issues in children with physical disabilities and their families. Phys. Occup. Ther. Pediatr. 2006, 26, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Lundy, C.T.; Doherty, G.M.; Fairhurst, C.B. Botulinum toxin type A injection can be an effective treatment for pain in children with hip spasms and cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ramstad, K.; Jahnsen, R.; Lofterod, B.; Skjeldal, O.H. Continuous intrathecal baclofen therapy in children with cerebral palsy: When does improvement emerge? Acta Paediatr. 2010, 99, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.; Olness, K.N. Clinical and demographic characteristics of migraine in urban children. Headache 1997, 37, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Split, W.; Neuman, W. Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache 1999, 39, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Heyer, G.L.; Rose, S.C.; Merison, K.; Perkins, S.Q.; Lee, J.E. Specific headache factors predict sleep disturbances among youth with migraine. Pediatr. Neurol. 2014, 514, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Abu-Arafeh, I.; Howells, R. Primary headaches in children under the age of 7 years. Curr. Pain Headache Rep. 2014, 18, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Abu-Arefeh, I.; Russell, G. Prevalence of headache andmigraine in schoolchildren. BMJ 1994, 309, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, V.; Galli, F.; Fabrizi, P.; Giannantoni, A.S.; Napoli, L.; Bruni, O.; Trillo, S. Headache and psychiatric comorbidity: Clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998, 18, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Barabas, G.; Ferrari, M.; Matthews, W.S. Childhood migraine and somnambulism. Neurology 1986, 33, 948–1048. [Google Scholar] [CrossRef]
- Giround, M.; D’Athis, P.; Guard, O.; Dumas, R. Migraine et somnambulisme: Une enquete portant sur 122 migraineux. Rev. Neurol. 1986, 142, 42–46. [Google Scholar]
- Pradalier, A.; Guittard, M.; Dry, J. Somnambulism, migraine and propranolol. Headache 1987, 27, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Dexter, J.D. The relationship between disorders of arousal from sleep and migraine. Headache 1986, 26, 322. [Google Scholar]
- Bruni, O.; Fabrizi, P.; Ottaviano, S.; Cortesi, F.; Giannotti, F.; Guidetti, V. Prevalence of sleep disorders in childhood and adolescence headache: A case-control study. Cephalalgia 1997, 17, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kohrman, M.H.; Carney, P.R. Sleep-related disorders in neurologic disease during childhood. Pediatr. Neurol. 2000, 23, 107–113. [Google Scholar] [CrossRef]
- Bruni, O.; Russo, P.M.; Violani, C.; Guidetti, V. Sleep and migraine: An actigraphic study. Cephalalgia 2004, 24, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Littner, M.; Kushida, C.A.; Anderson, M.W.; Bailey, D.; Berry, R.B.; Davila, D.G.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; Loube, D.; et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: An update for 2002. Am. Acad. Sleep Med. 2003, 26, 337–341. [Google Scholar] [CrossRef]
- De Giorgis, G.; Miletto, R.; Iannuccelli, M.; Camuffo, M.; Scerni, S. Headache in association with sleep disorders in children: A psychodiagnostic evaluation and controlled clinical study—l-5-HTP versus placebo. Drugs Exp. Clin. Res. 1987, 13, 425–433. [Google Scholar] [PubMed]
- Bruni, O.; Galli, F.; Guidetti, V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999, 19, 58–60. [Google Scholar] [CrossRef]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolaymat, A.; Liu, Z. Sleep Disorders in Childhood Neurological Diseases. Children 2017, 4, 84. https://doi.org/10.3390/children4100084
Tolaymat A, Liu Z. Sleep Disorders in Childhood Neurological Diseases. Children. 2017; 4(10):84. https://doi.org/10.3390/children4100084
Chicago/Turabian StyleTolaymat, Abdullah, and Zhao Liu. 2017. "Sleep Disorders in Childhood Neurological Diseases" Children 4, no. 10: 84. https://doi.org/10.3390/children4100084




