Parental Protectiveness Mediates the Association between Parent-Perceived Child Self-Efficacy and Health Outcomes in Pediatric Functional Abdominal Pain Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1 Child Self-Efficacy Scale (parent-report) (CSES; [13])
2.2.2. Adult Responses to Children’s Symptoms Scale (ARCS; [17,18,19])
2.2.3. Children’s Somatization Inventory (parent-report) (CSI; [20])
2.2.4. Functional Disability Inventory (parent-report) (FDI; [23])
2.3. Data Analysis
3. Results
3.1. Descriptive Statistics
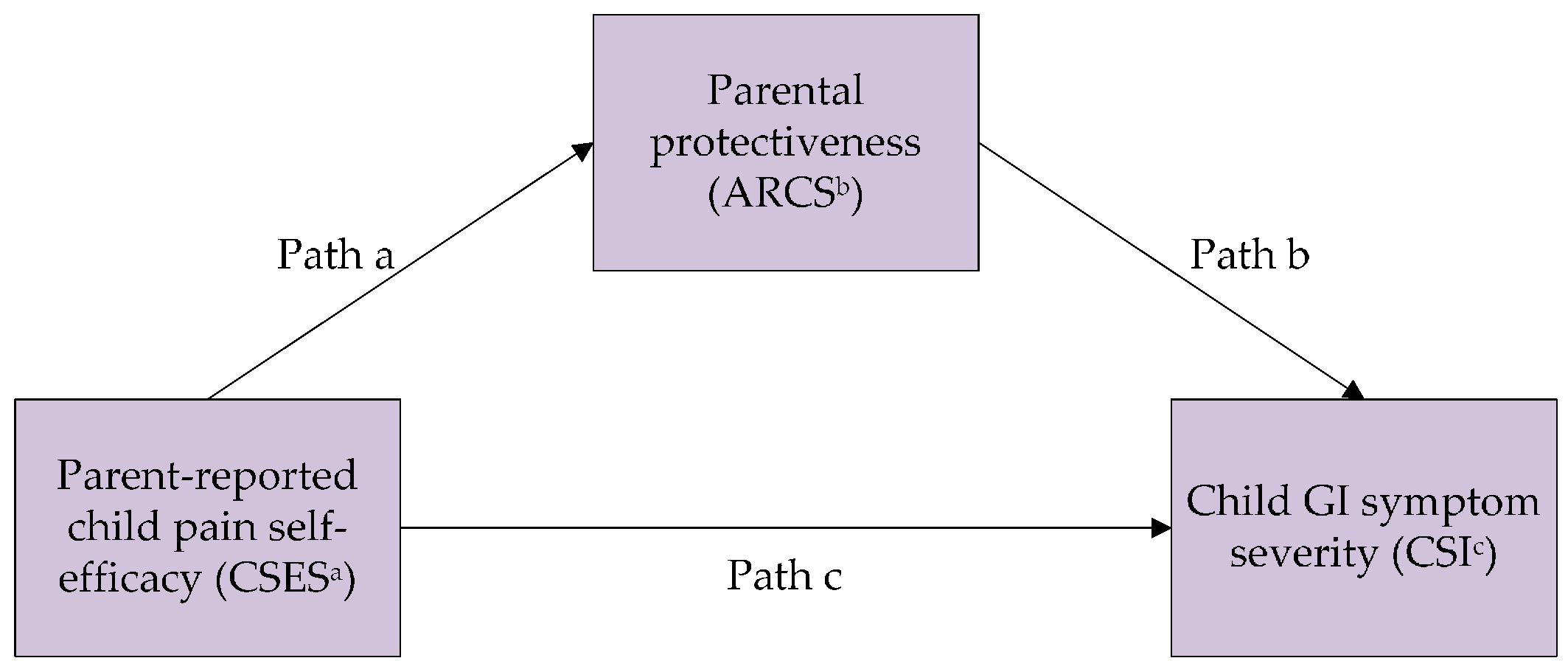
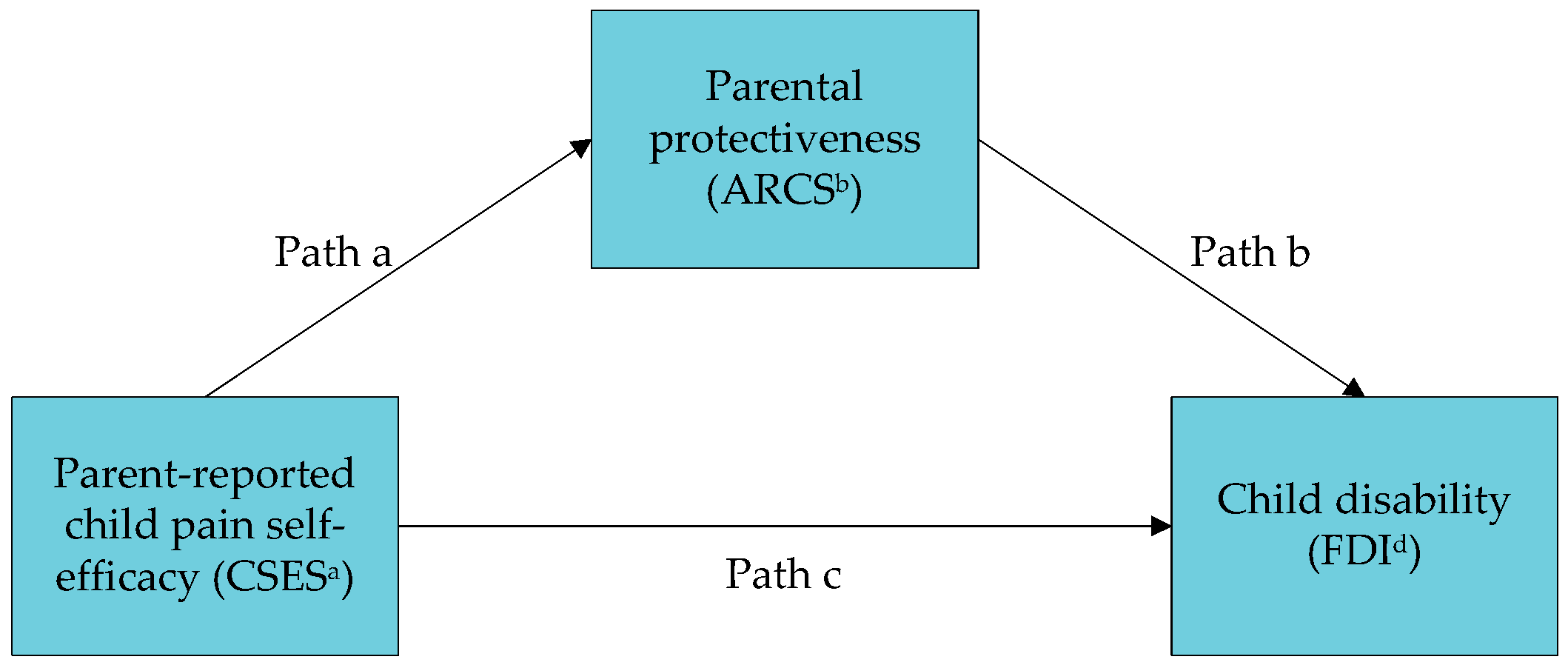
3.2. Mediation Analyses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Korterink, J.J.; Diederen, K.; Benninga, M.A.; Tabbers, M.M. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE 2015, 10, e0126982. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional disorders: Children and adolescents. Gastroenterology 2016, 150, 1456.e1452–1468.e1452. [Google Scholar] [CrossRef] [PubMed]
- Starfield, B.; Gross, E.; Wood, M.; Pantell, R.; Allen, C.; Gordon, I.B.; Moffatt, P.; Drachman, R.; Katz, H. Psychosocial and psychosomatic diagnoses in primary care of children. Pediatrics 1980, 66, 159–167. [Google Scholar] [PubMed]
- Rouster, A.S.; Karpinski, A.C.; Silver, D.; Monagas, J.; Hyman, P.E. Functional gastrointestinal disorders dominate pediatric gastroenterology outpatient practice. J. Pediatr. Gastroenterol. Nutr. 2015, 62, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.A.; Mannalithara, A.; Singh, G.; Pasricha, P.J.; Ladabaum, U. Clinical and economic burden of emergency department visits due to gastrointestinal diseases in the united states. Am. J. Gastroenterol. 2013, 108, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.L.; Romano, J.M.; Levy, R.L.; Walker, L.S.; Whitehead, W.E. Catastrophizing and parental response to child symptom complaints. Child Health Care 2009, 38, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Foundations of Thought and Action: A Social Cognitive Theory; Prentice-Hall: New Jersey, NJ, USA, 1986. [Google Scholar]
- Bandura, A. Human agency in social cognitive theory. Am. Psychol. 1989, 44, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, I.M.; Strecher, V.J.; Becker, M.H. Social learning theory and the health belief model. Health Educ. Q. 1988, 15, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H.; Rosenstock, I.M.; Drachman, R.H. Compliance with a medical regimen for asthma: A test of the health belief model. Public Health Rep. 1978, 93, 268–277. [Google Scholar] [PubMed]
- Asghari, A.; Nicholas, M.K. Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain 2001, 94, 85–100. [Google Scholar] [CrossRef]
- Bursch, B.; Tsao, J.C.; Meldrum, M.; Zeltzer, L.K. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions). Pain 2006, 125, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kalapurakkel, S.; Carpino, E.A.; Lebel, A.; Simons, L.E. “Pain can’t stop me”: Examining pain self-efficacy and acceptance as resilience processes among youth with chronic headache. J. Pediatr. Psychol. 2015, 40, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; van Tilburg, M.; Langer, S.L.; Romano, J.; Mancl, L.; Whitehead, W.E.; Feld, S.; Walker, L.S. Parent-only intervention reduces symptoms and disability in abdominal pain patients. J. Pediatr. Gastroenterol. Nutr. 2015, 61, S224. [Google Scholar] [CrossRef]
- Drossman, D.A. Rome III: The Functional Gastrointestinal Disorders; Degnon Associates: McLean, VA, USA, 2006. [Google Scholar]
- Walker, L.S.; Levy, R.L.; Whitehead, W.E. Validation of a measure of protective parent responses to children’s pain. Clin. J. Pain 2006, 22, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Van Slyke, D.A.; Walker, L.S. Mothers’ responses to children’s pain. Clin. J. Pain 2006, 22, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Palermo, T.M.; Essner, B.; Zhou, C.; Levy, R.L.; Langer, S.L.; Sherman, A.L.; Walker, L.S. A developmental analysis of the factorial validity of the parent-report version of the adult responses to children’s symptoms in children versus adolescents with chronic pain or pain-related chronic illness. J. Pain 2015, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Beck, J.E.; Garber, J.; Lambert, W. Children’s somatization inventory: Psychometric properties of the revised form (csi-24). J. Pediatr. Psychol. 2009, 34, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Dengler-Crish, C.M.; Horst, S.N.; Walker, L.S. Somatic complaints in childhood functional abdominal pain are associated with functional gastrointestinal disorders in adolescence and adulthood. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Acra, S.; Bruehl, S.; Walker, L.S. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Greene, J.W. The functional disability inventory: Measuring a neglected dimension of child health status. J. Pediatr. Psychol. 1991, 16, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; The Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Levy, R.L.; Whitehead, W.E.; Walker, L.S.; Von Korff, M.; Feld, A.; Garner, M.; Christie, D. Increased somatic complaints and health-care utilization in children: Effects of parent ibs status and parent response to gastrointestinal symptoms. Am. J. Gastroenterol. 2004, 99, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Claar, R.L.; Simons, L.E.; Logan, D.E. Parental response to children’s pain: The moderating impact of children’s emotional distress on symptoms and disability. Pain 2008, 138, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.; Langer, S.L.; Romano, J.M.; Labus, J.; Walker, L.S.; Murphy, T.B.; Van Tilburg, M.; Feld, L.D.; Christie, D.L.; Whitehead, W.E. Cognitive mediators of treatment outcomes in pediatric functional abdominal pain. Clin. J. Pain 2014, 30, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
| Protect (ARCS) | GI Symptoms (CSI-GI) | Disability (FDI) | M (SD) | Scale | |
|---|---|---|---|---|---|
| CSES a | −0.55 ** | −0.25 ** | −0.43 ** | 3.30 (0.91) | 1–5 |
| ARCS b | 0.25 ** | 0.39 ** | 1.17 (0.65) | 0–4 | |
| CSI-GI c | 0.46 ** | 1.42 (0.68) | 0–4 | ||
| FDI d | 0.70 (0.73) | 0–4 |
| Estimate (SE) | p | 95% CI | |
|---|---|---|---|
| Path c (effect of predictor on outcome) | −0.19 (0.04) | <0.001 | |
| Path a (effect of predictor on mediator) | −0.39 (0.03) | <0.001 | |
| Path b (effect of mediator on outcome) | 0.18 (0.07) | 0.011 | |
| Indirect effect: Path a × b | −0.07 (0.03) | −0.13, −0.01 | |
| Direct effect: Path c’ | −0.12 (0.05) | 0.016 | |
| Ratio of indirect effect to total effect | 37% | ||
| Estimate (SE) | p | 95% CI | |
|---|---|---|---|
| Path c (effect of predictor on outcome) | −0.34 (0.04) | <0.001 | |
| Path a (effect of predictor on mediator) | −0.39 (0.03) | <0.001 | |
| Path b (effect of mediator on outcome) | 0.24 (0.07) | <0.001 | |
| Indirect effect: Path a × b | −0.10 (0.03) | −0.16, −0.04 | |
| Direct effect: Path c’ | −0.25 (0.05) | <0.001 | |
| Ratio of indirect effect to total effect | 28% | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuPen, M.M.; Van Tilburg, M.A.L.; Langer, S.L.; Murphy, T.B.; Romano, J.M.; Levy, R.L. Parental Protectiveness Mediates the Association between Parent-Perceived Child Self-Efficacy and Health Outcomes in Pediatric Functional Abdominal Pain Disorder. Children 2016, 3, 15. https://doi.org/10.3390/children3030015
DuPen MM, Van Tilburg MAL, Langer SL, Murphy TB, Romano JM, Levy RL. Parental Protectiveness Mediates the Association between Parent-Perceived Child Self-Efficacy and Health Outcomes in Pediatric Functional Abdominal Pain Disorder. Children. 2016; 3(3):15. https://doi.org/10.3390/children3030015
Chicago/Turabian StyleDuPen, Melissa M., Miranda A. L. Van Tilburg, Shelby L. Langer, Tasha B. Murphy, Joan M. Romano, and Rona L. Levy. 2016. "Parental Protectiveness Mediates the Association between Parent-Perceived Child Self-Efficacy and Health Outcomes in Pediatric Functional Abdominal Pain Disorder" Children 3, no. 3: 15. https://doi.org/10.3390/children3030015






