Artificial Sweetened Beverages and Pediatric Obesity: The Controversy Continues
Abstract
:1. Introduction
2. Artificial Sweetened Beverages and Subsequent Food Intake
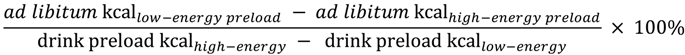
Summary
3. Observational Studies of Artificial Sweetened Beverages
3.1. Prospective Cohort Studies
3.2. Cross-Sectional Studies
3.3. Summary
4. Interventional Studies of Artificial Sweetened Beverages
Summary
5. Mechanism of Artificial Sweeteners
6. Conclusion
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; Lamb, M.M.; Flegal, K.M. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010, 303, 242–249. [Google Scholar] [CrossRef]
- Ogden, C.L. Prevalence of Obesity and trends in body mass index among US Children and adolescents, 1999–2010. JAMA 2012, 307, 483–490. [Google Scholar] [CrossRef]
- Hannon, T.S.; Rao, G.; Arslanian, S.A. Childhood obesity and type 2 diabetes mellitus. Pediatrics 2005, 116, 473–480. [Google Scholar] [CrossRef]
- Martin, L.E.; Holsen, L.M.; Chambers, R.J.; Bruce, A.S.; Brooks, W.M.; Zarcone, J.R.; Butler, M.G.; Savage, C.R. Neural mechanisms associated with food Motivation in obese and healthy weight adults. Obesity 2009, 18, 254–260. [Google Scholar]
- Lamb, M.M.; Ogden, C.L.; Carroll, M.D.; Lacher, D.A.; Flegal, K.M. Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999–2004. Am. J. Clin. Nutr. 2011, 94, 877–883. [Google Scholar] [CrossRef]
- Kapinos, K.A.; Yakusheva, O.; Eisenberg, D. Obesogenic environmental influences on young adults: Evidence from college dormitory assignments. Econ. Hum. Biol. 2014, 12, 98–109. [Google Scholar] [CrossRef]
- Virdis, A.; Ghiadoni, L.; Masi, S.; Versari, D.; Daghini, E.; Giannarelli, C.; Salvetti, A.; Taddei, S. Obesity in the childhood: A link to adult hypertension. Curr. Pharm. Des. 2009, 15, 1063–1071. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Zimmet, P.Z.; Welborn, T.A.; de Courten, M.P.; Cameron, A.J.; Sicree, R.A.; Dwyer, T.; Colagiuri, S.; Jolley, D.; Knuiman, M.; et al. The rising prevalence of diabetes and impaired glucose tolerance: The Australian diabetes, obesity and lifestyle study. Diabetes Care 2002, 25, 829–834. [Google Scholar] [CrossRef]
- Sinatra, F.R. Nonalcoholic fatty liver disease in pediatric patients. J. Parenter. Enter. Nutr. 2012, 36, 43S–48S. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Caprio, S. Calories from soft drinks—do they matter? N. Engl. J. Med. 2012, 367, 1462–1463. [Google Scholar] [CrossRef]
- Guthrie, J.F.; Morton, J.F. Food sources of added sweeteners in the diets of Americans. J. Am. Diet. Assoc. 2000, 100, 43–51. [Google Scholar] [CrossRef]
- Han, E.; Powell, L.M. Consumption patterns of sugar-sweetened beverages in the United States. JAND 2013, 113, 43–53. [Google Scholar]
- de la Peña, C. Artificial sweetener as a historical window to culturally situated health. Ann. N. Y. Acad. Sci. 2010, 1190, 159–165. [Google Scholar]
- Brown, R.J.; de Banate, M.A.; Rother, K.I. Artificial sweeteners: A systematic review of metabolic effects in youth. Int. J. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2012, 16, 1894–1900. [Google Scholar]
- Stellman, S.D.; Garfinkel, L. Artificial sweetener use and one-year weight change among women. Prev. Med. 1986, 15, 195–202. [Google Scholar] [CrossRef]
- Colditz, G.A.; Willett, W.C.; Stampfer, M.J.; London, S.J.; Segal, M.R.; Speizer, F.E. Patterns of weight change and their relation to diet in a cohort of healthy women. Am. J. Clin. Nutr. 1990, 51, 1100–1105. [Google Scholar]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Chomitz, V.R.; Antonelli, T.A.; Gortmaker, S.L.; Osganian, S.K.; Ludwig, D.S. A randomized trial of sugar-sweetened beverages and adolescent body weight. N. Engl. J. Med. 2012, 367, 1407–1416. [Google Scholar] [CrossRef]
- de Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A Trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [CrossRef]
- Johnson, S.L.; Birch, L.L. Parents“ and children”s adiposity and eating style. Pediatrics 1994, 94, 653–661. [Google Scholar]
- Johnson, S.L.; Taylor-Holloway, L.A. Non-Hispanic white and Hispanic elementary school children's self-regulation of energy intake. Am. J. Clin. Nutr. 2006, 83, 1276–1282. [Google Scholar]
- Faith, M.S.; Keller, K.L.; Johnson, S.L.; Pietrobelli, A.; Matz, P.E.; Must, S.; Jorge, M.A.; Cooperberg, J.; Heymsfield, S.B.; Allison, D.B. Familial aggregation of energy intake in children. Am. J. Clin. Nutr. 2004, 79, 844–850. [Google Scholar]
- Bellissimo, N.; Thomas, S.G.; Goode, R.C.; Anderson, G.H. Effect of short-duration physical activity and ventilation threshold on subjective appetite and short-term energy intake in boys. Appetite 2007, 49, 644–651. [Google Scholar] [CrossRef]
- Bellissimo, N.; Pencharz, P.B.; Thomas, S.G.; Anderson, G.H. Effect of television viewing at mealtime on food intake after a glucose preload in boys. Pediatr. Res. 2007, 61, 745–749. [Google Scholar] [CrossRef]
- Birch, L.L.; McPhee, L.; Sullivan, S. Children’s food intake following drinks sweetened with sucrose or aspartame: Time course effects. Physiol. Behav. 1989, 45, 387–395. [Google Scholar] [CrossRef]
- Anderson, G.H.; Saravis, S.; Schacher, R.; Zlotkin, S.; Leiter, L.A. Aspartame: effect on lunch-time food intake, appetite and hedonic response in children. Appetite 1989, 13, 93–103. [Google Scholar] [CrossRef]
- Vanselow, M.S.; Pereira, M.A.; Neumark-Sztainer, D.; Raatz, S.K. Adolescent beverage habits and changes in weight over time: findings from Project EAT. Am. J. Clin. Nutr. 2009, 90, 1489–1495. [Google Scholar] [CrossRef]
- Blum, J.W.; Jacobsen, D.J.; Donnelly, J.E. Beverage consumption patterns in elementary school aged children across a two-year period. J. Am. Coll. Nutr. 2005, 24, 93–98. [Google Scholar] [CrossRef]
- Berkey, C.S.; Rockett, H.R.H.; Field, A.E.; Gillman, M.W.; Colditz, G.A. Sugar-added beverages and adolescent weight change. Obes. Res. 2004, 12, 778–788. [Google Scholar] [CrossRef]
- Striegel-Moore, R.H.; Thompson, D.; Affenito, S.G.; Franko, D.L.; Obarzanek, E.; Barton, B.A.; Schreiber, G.B.; Daniels, S.R.; Schmidt, M.; Crawford, P.B. Correlates of beverage intake in adolescent girls: The National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. 2006, 148, 183–187. [Google Scholar] [CrossRef]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition 2007, 23, 557–563. [Google Scholar] [CrossRef]
- Kral, T.V.E.; Stunkard, A.J.; Berkowitz, R.I.; Stallings, V.A.; Moore, R.H.; Faith, M.S. Beverage consumption patterns of children born at different risk of obesity. Obes. 2008, 16, 1802–1808. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between consumption of sugar-sweetened drinks and childhood obesity: A prospective, observational analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- O’Connor, T.M.; Yang, S.-J.; Nicklas, T.A. Beverage intake among preschool children and its effect on weight status. Pediatrics 2006, 118, e1010–e1018. [Google Scholar] [CrossRef]
- Forshee, R.A.; Storey, M.L. Total beverage consumption and beverage choices among children and adolescents. Int. J. Food Sci. Nutr. 2003, 54, 297–307. [Google Scholar] [CrossRef]
- Giammattei, J.; Blix, G.; Marshak, H.H.; Wollitzer, A.O.; Pettitt, D.J. Television watching and soft drink consumption: Associations with obesity in 11- to 13-year-old schoolchildren. Arch. Pediatr. Adolesc. Med. 2003, 157, 882–886. [Google Scholar] [CrossRef]
- Pereira, M.A. Diet beverages and the risk of obesity, diabetes, and cardiovascular disease: A review of the evidence. Nutr. Rev. 2013, 71, 433–440. [Google Scholar] [CrossRef]
- Williams, C.L.; Strobino, B.A.; Brotanek, J. Weight control among obese adolescents: A pilot study. Int. J. Food Sci. Nutr. 2007, 58, 217–230. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Osganian, S.K.; Chomitz, V.R.; Ellenbogen, S.J.; Ludwig, D.S. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: A randomized, controlled pilot study. Pediatrics 2006, 117, 673–680. [Google Scholar] [CrossRef]
- De Ruyter, J.C.; Katan, M.B.; Kuijper, L.D.J.; Liem, D.G.; Olthof, M.R. The effect of sugar-free versus sugar-sweetened beverages on satiety, liking and wanting: an 18 month randomized double-blind trial in children. PLoS One 2013, 8, e78039. [Google Scholar]
- Mace, O.J.; Affleck, J.; Patel, N.; Kellett, G.L. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J. Physiol. 2007, 582, 379–392. [Google Scholar] [CrossRef]
- Brown, R.J.; Walter, M.; Rother, K.I. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009, 32, 2184–2186. [Google Scholar] [CrossRef]
- Mattes, R. Effects of aspartame and sucrose on hunger and energy intake in humans. Physiol. Behav. 1990, 47, 1037–1044. [Google Scholar] [CrossRef]
- Lavin, J.H.; French, S.J.; Read, N.W. The effect of sucrose- and aspartame-sweetened drinks on energy intake, hunger and food choice of female, moderately restrained eaters. Int. J. Obes. (Lond) 1997, 21, 37–42. [Google Scholar]
- Van Vugt, D.A. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum. Reprod. Update 2010, 16, 276–292. [Google Scholar] [CrossRef]
- Chambers, E.S.; Bridge, M.W.; Jones, D.A. Carbohydrate sensing in the human mouth: Effects on exercise performance and brain activity. J. Physiol. (Lond.) 2009, 587, 1779–1794. [Google Scholar] [CrossRef]
- Green, E.; Murphy, C. Altered processing of sweet taste in the brain of diet soda drinkers. Physiol. Behav. 2012, 107, 560–567. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Weijzen, P.; de Graaf, C.; Viergever, M.A. Consumption of caloric and non-caloric versions of a soft drink differentially affects brain activation during tasting. NeuroImage 2011, 54, 1367–1374. [Google Scholar] [CrossRef]
- Frank, G.K.W.; Oberndorfer, T.A.; Simmons, A.N.; Paulus, M.P.; Fudge, J.L.; Yang, T.T.; Kaye, W.H. Sucrose activates human taste pathways differently from artificial sweetener. NeuroImage 2008, 39, 1559–1569. [Google Scholar] [CrossRef]
- Yang, Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Freswick, P.N. Artificial Sweetened Beverages and Pediatric Obesity: The Controversy Continues. Children 2014, 1, 31-39. https://doi.org/10.3390/children1010031
Freswick PN. Artificial Sweetened Beverages and Pediatric Obesity: The Controversy Continues. Children. 2014; 1(1):31-39. https://doi.org/10.3390/children1010031
Chicago/Turabian StyleFreswick, Peter N. 2014. "Artificial Sweetened Beverages and Pediatric Obesity: The Controversy Continues" Children 1, no. 1: 31-39. https://doi.org/10.3390/children1010031



