Improved Methods for the Rapid Formation and Prevention of Advanced Glycation End Products (AGEs) In Vitro by Coupling to the Hypoxanthine/Xanthine Oxidase Assay System
Abstract
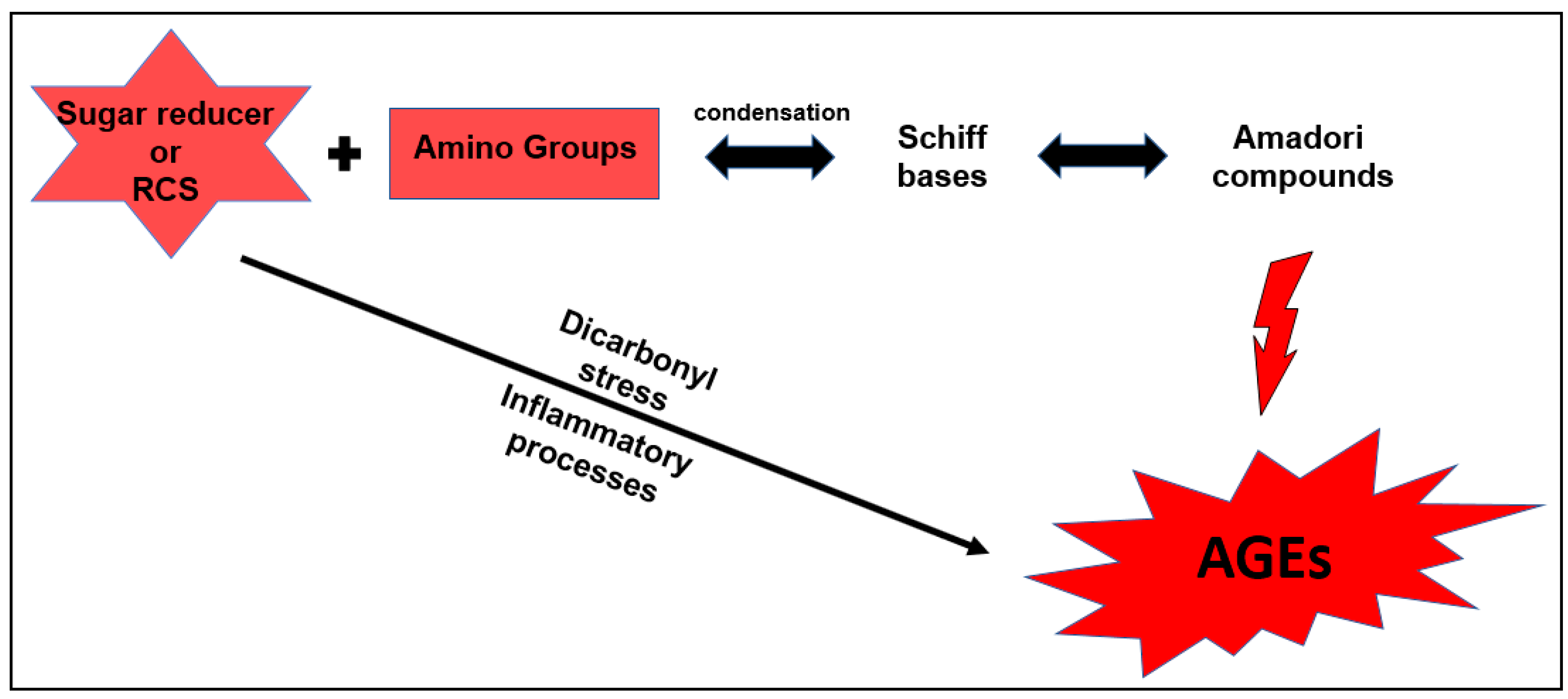
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. General Description of the Methods Developed for the Formation and Inhibition of Advanced Glycation Products (AGEs)
2.3. Formation of AGEs Induced by the Hypoxanthine/XO–BSA–Glucose/Fructose System
2.4. Formation of AGEs Induced by the System Hypoxanthine/XO–BSA–MGO/GO
2.5. Inhibition of AGEs Formation
3. Results
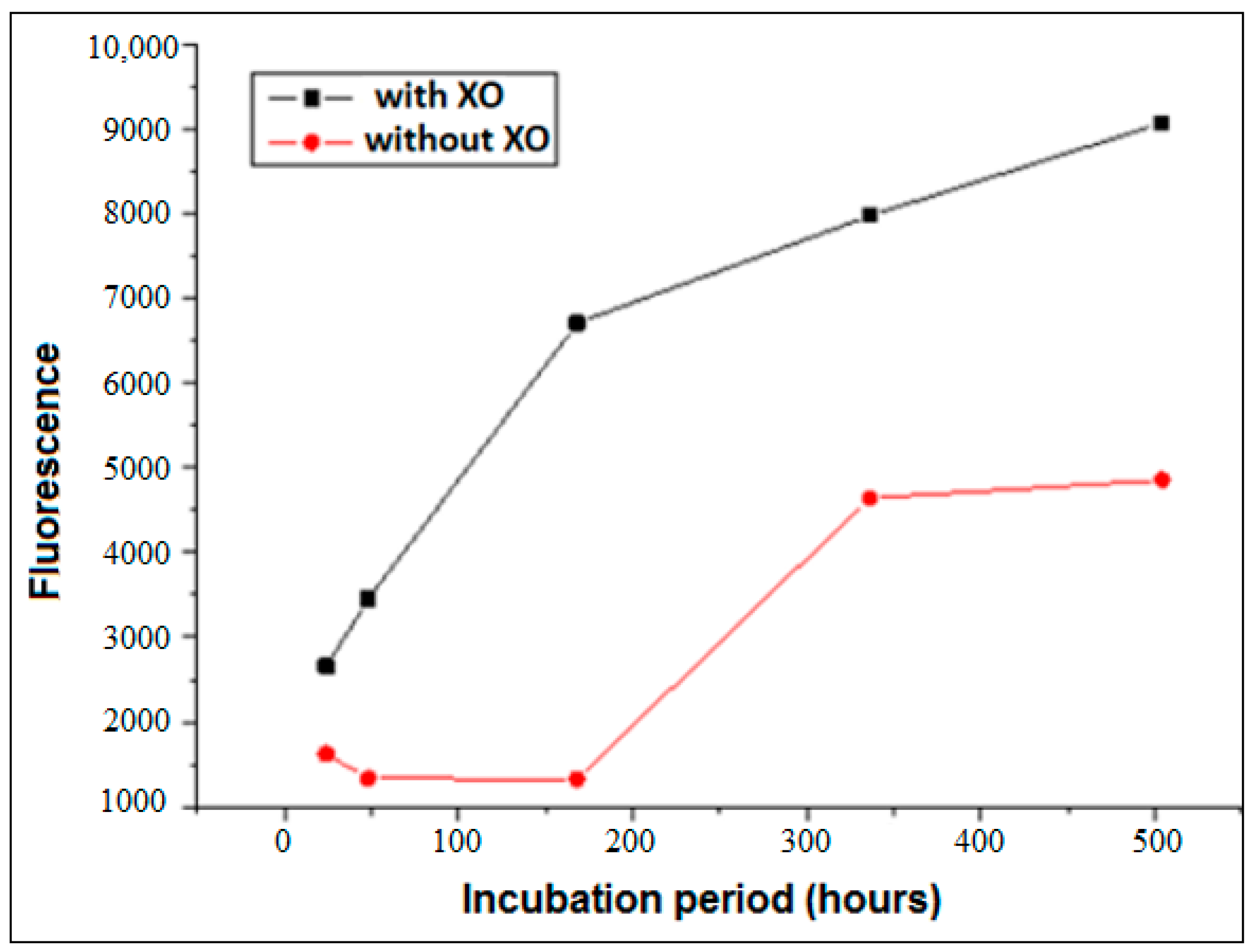
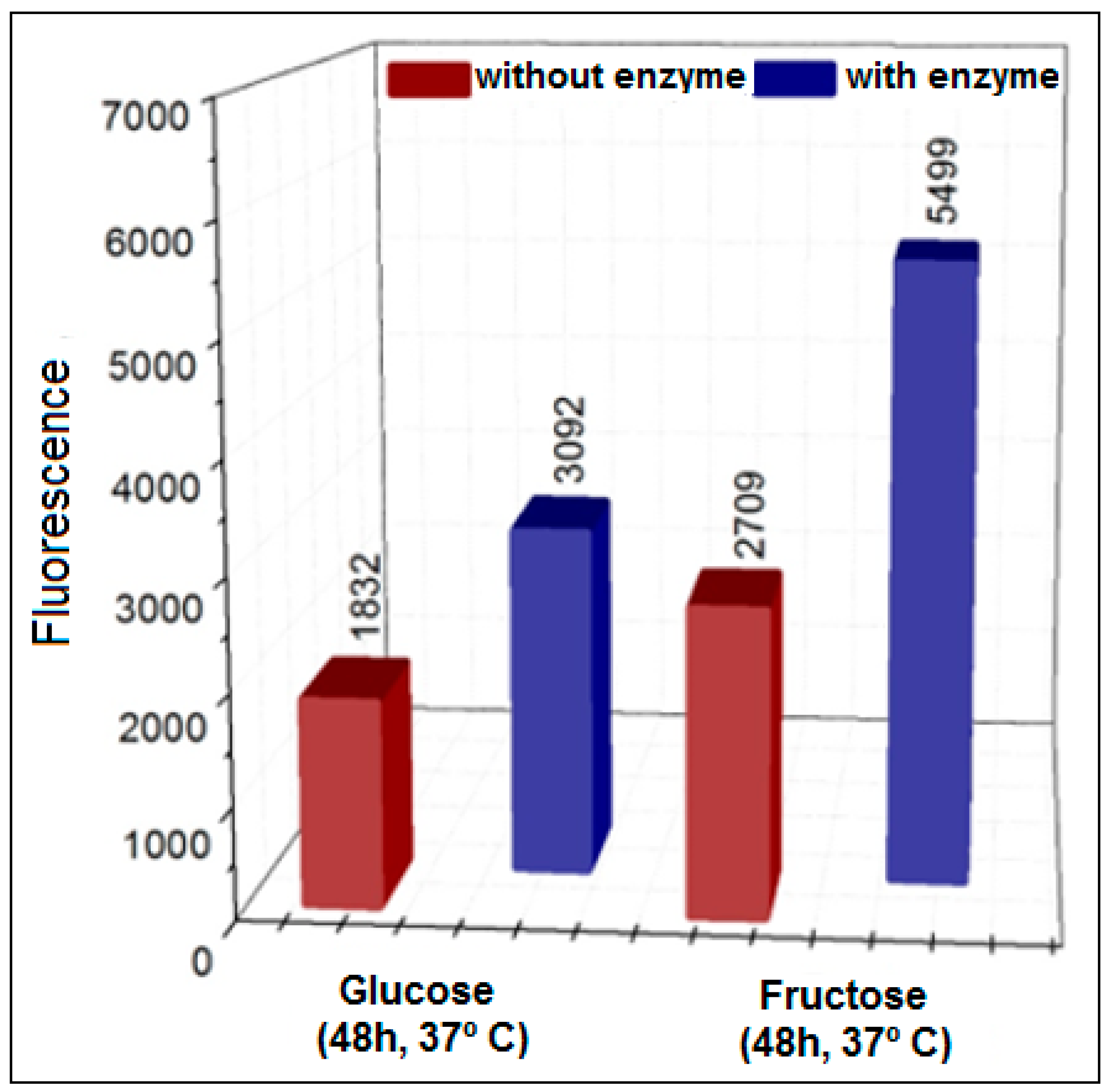
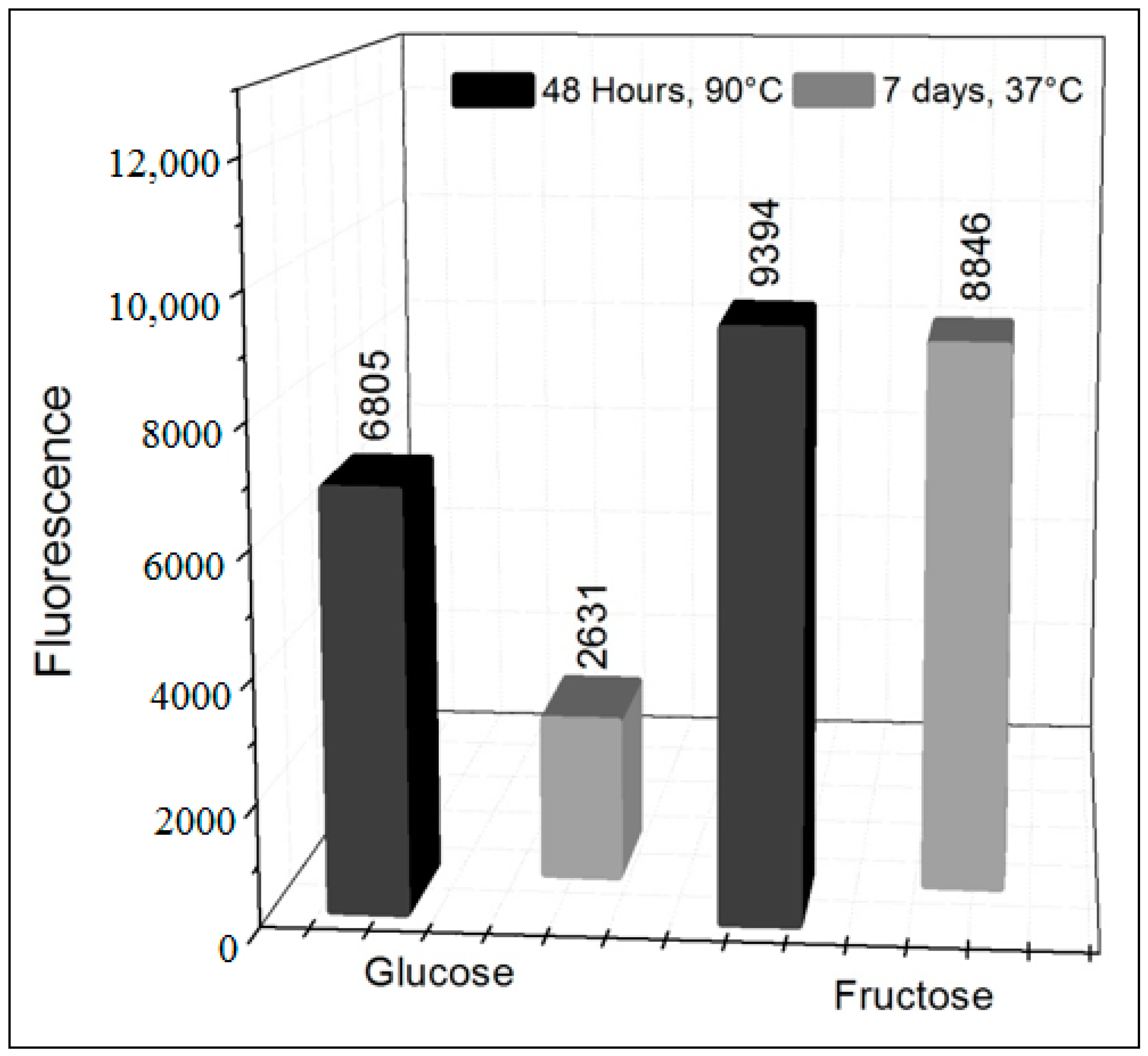
3.1. Formation of AGEs Induced by the System Hypoxanthine/XO–BSA–Glucose/Fructose
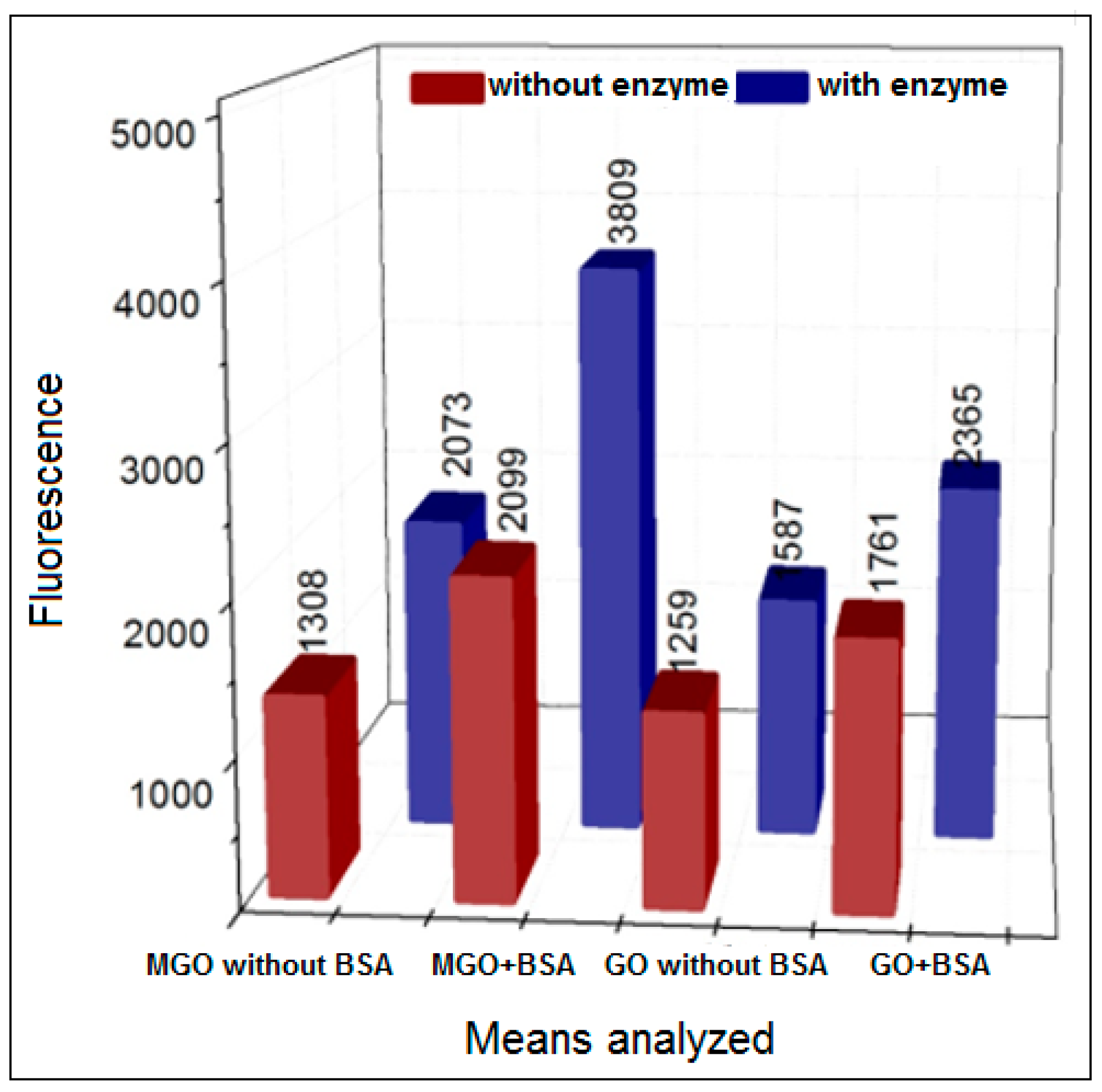
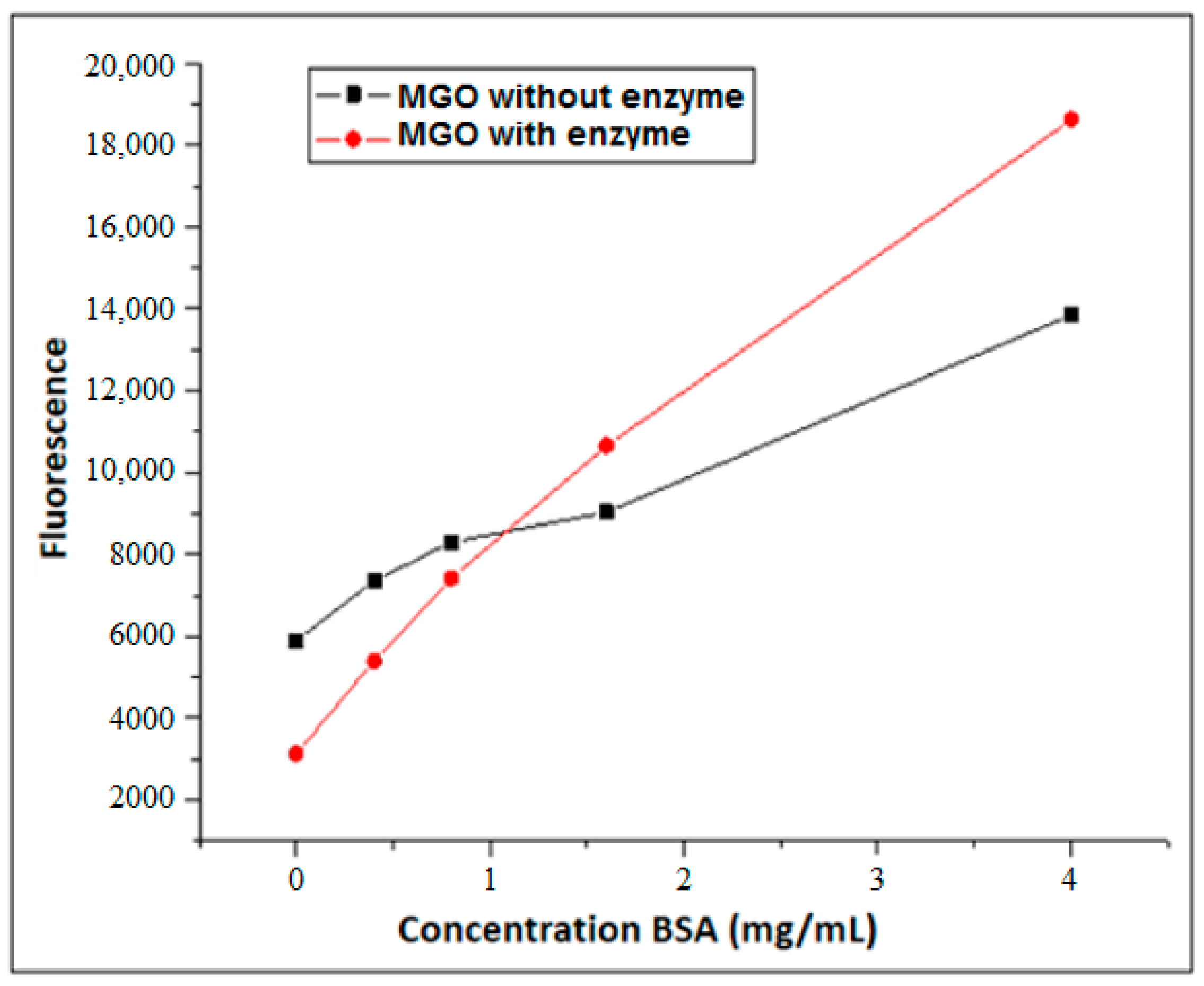
3.2. Formation of AGEs Induced by the Hypoxanthine/XO–BSA–MGO/GO System
3.3. Inhibition of AGEs Using Pure Compounds and Methanol Extracts Plus SPE Fractions of HS
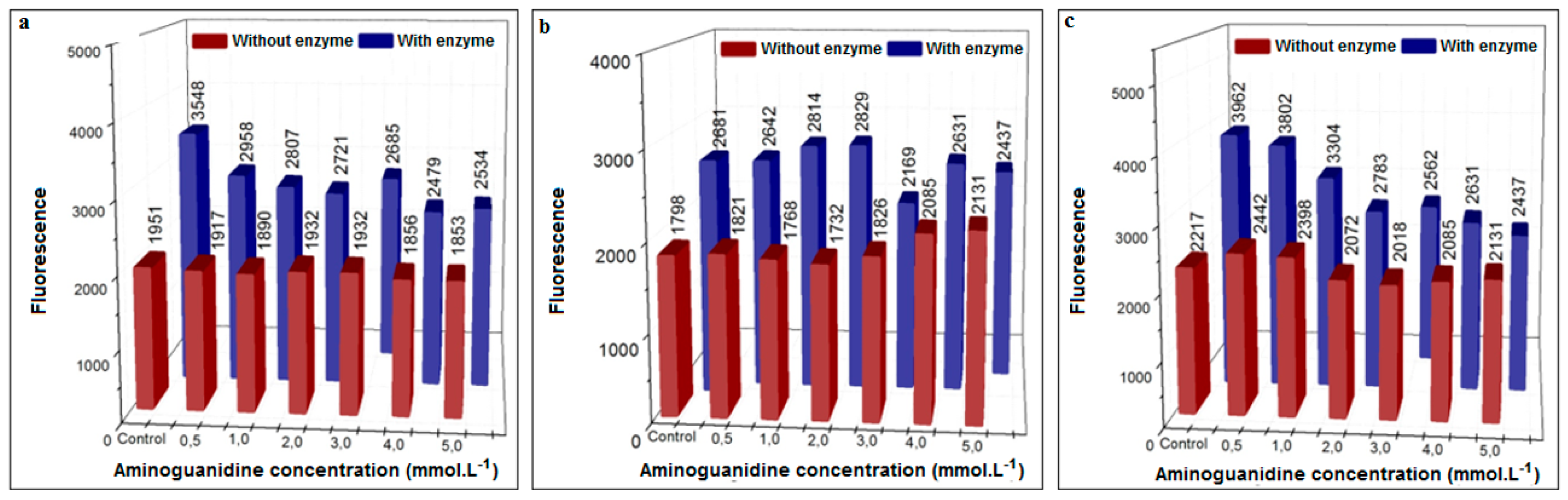
3.3.1. Inhibition Using Aminoguanidine (Positive Control)
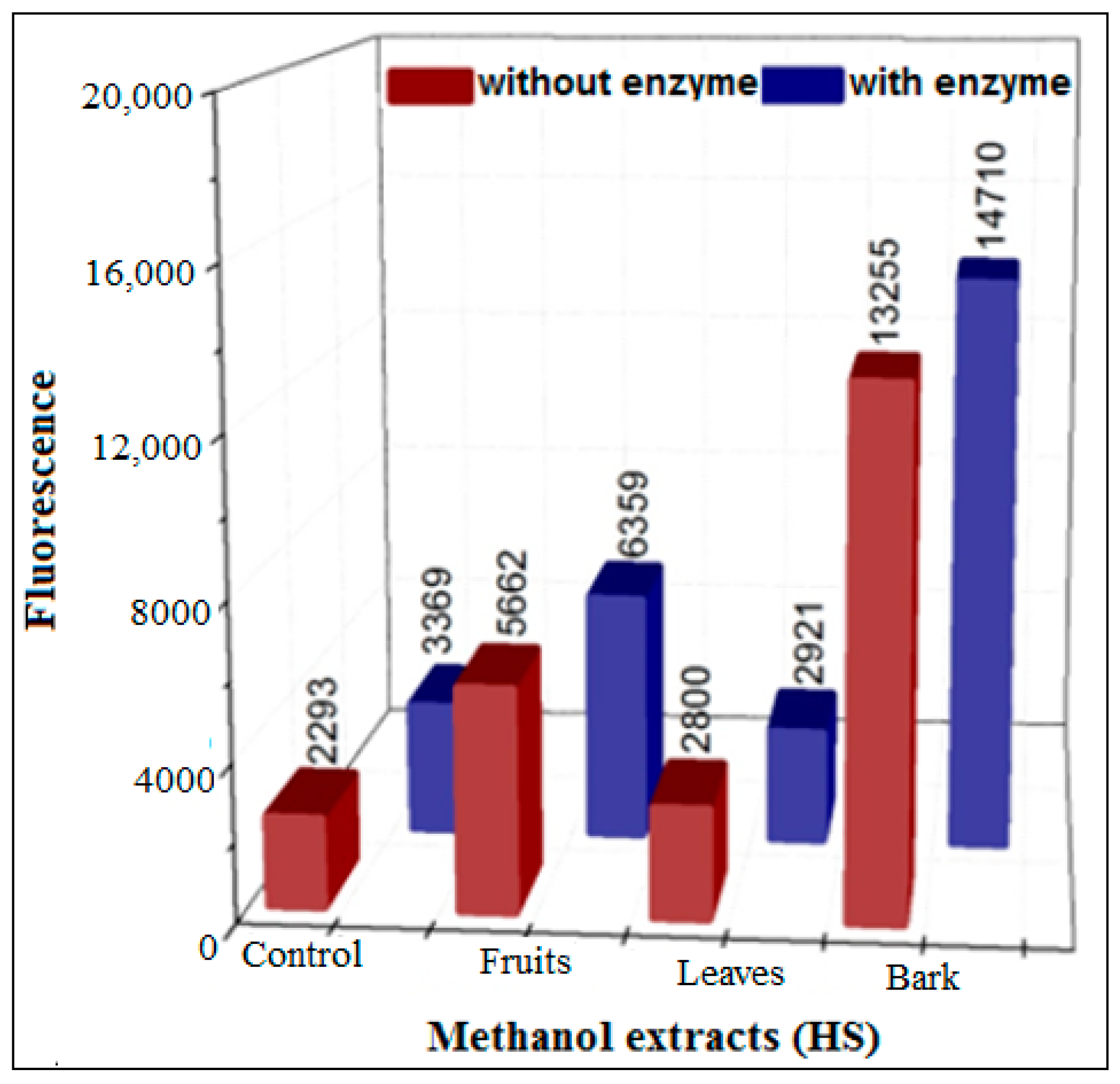
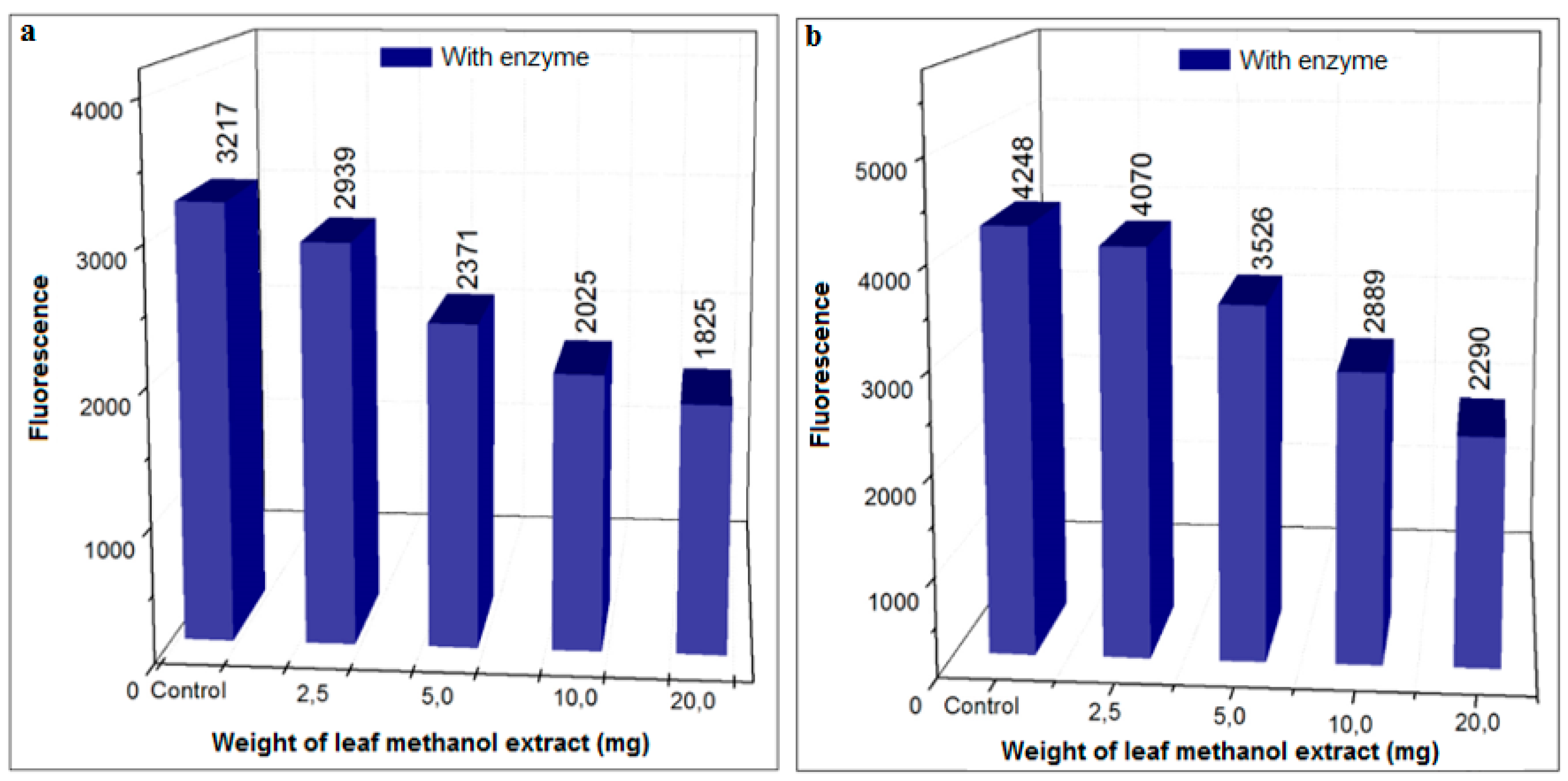
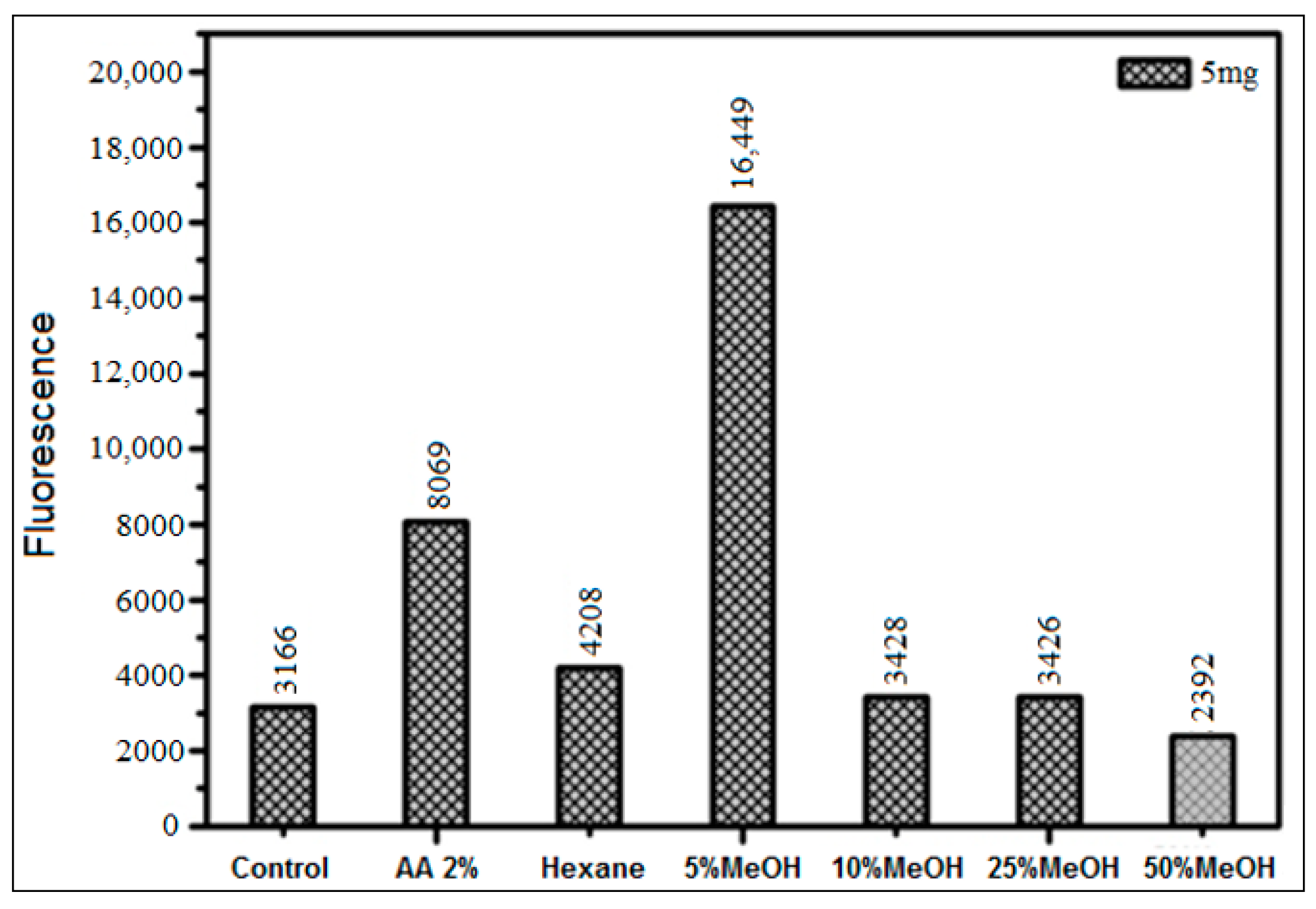
3.3.2. Inhibition Using HS Extracts and SPE Fractions
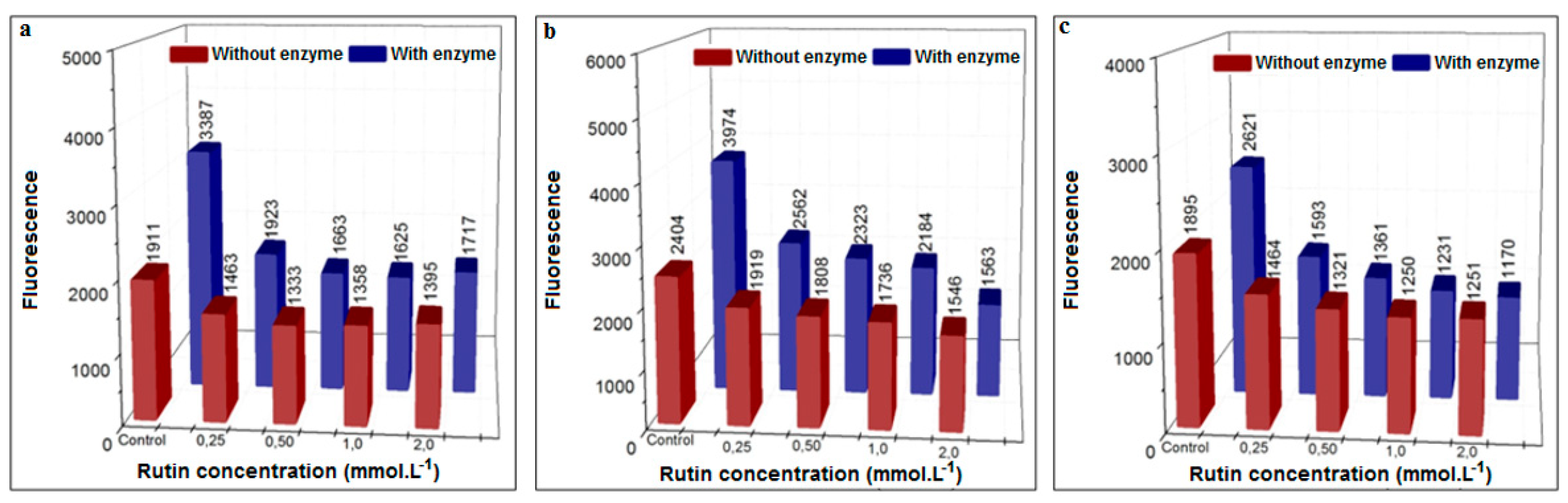
3.3.3. Inhibition Using the Flavonoid Rutin
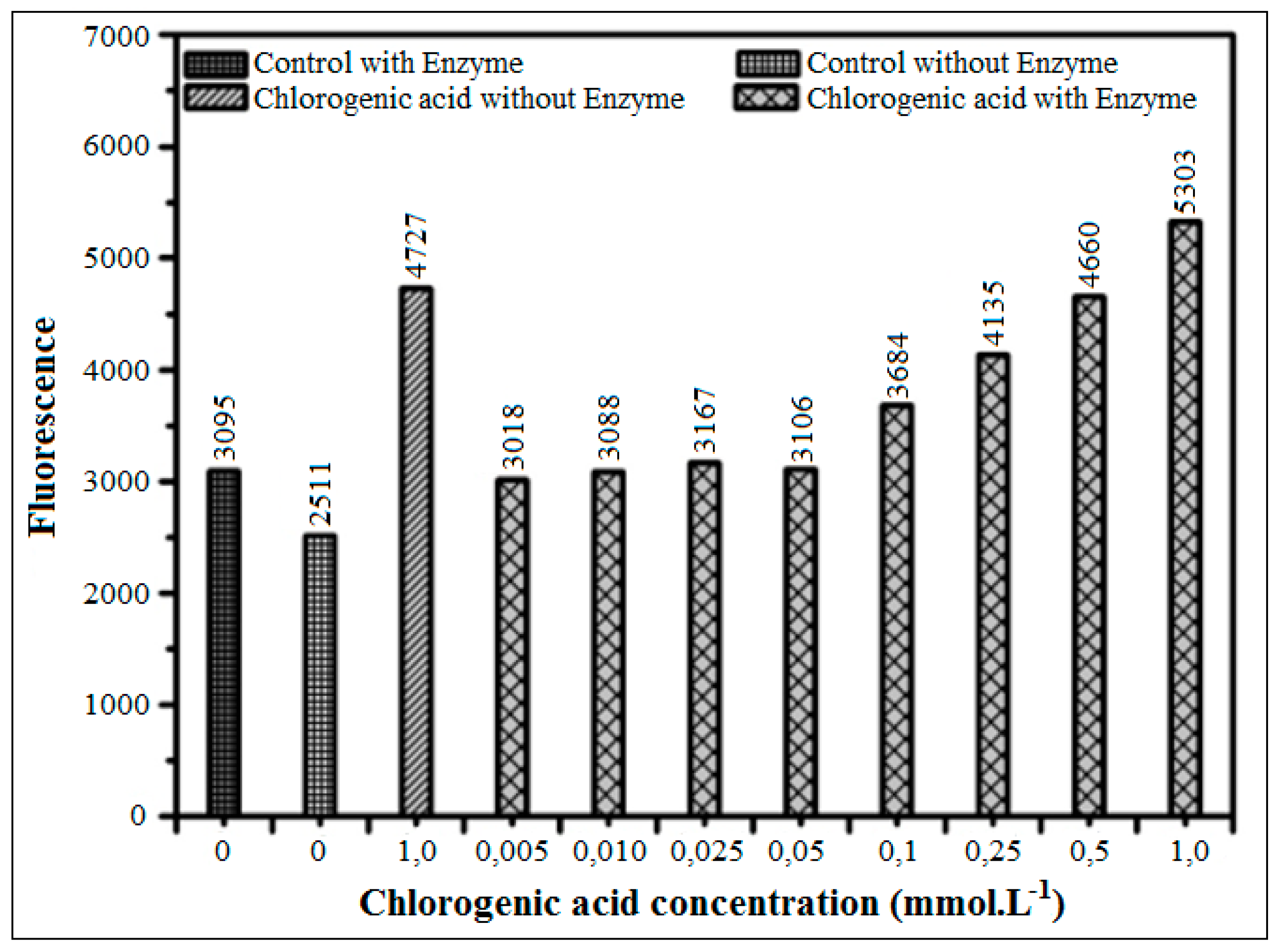
3.3.4. Inhibition Using Chlorogenic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R.J.; Blomhoff, R. Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferruci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Wang, S.; Sun, B.G. Food Safety Chemistry: Toxicant Occurrence, Analisys and Mitigation, 1st ed.; CRC Press: New York, NY, USA, 2014; 334p, ISBN 10 1466597941. [Google Scholar]
- Barbosa, J.H.P.; Souza, I.T.; Santana, A.E.G.; Goulart, M.O.F. A determinação dos produtos avançados de glicação (AGES) e de lipoxidação (ALES) em alimentos e em sistemas biológicos: Avanços, desafios e perspectivas. Quim. Nova 2016, 39, 608–620. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Interplay between the maillard reaction and lipid peroxidation in biochemical systems. Ann. N. Y. Acad. Sci. 2005, 1043, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N. The Maillard Reaction Reconsidered: Cooking and Eating for Health, 1st ed.; CRC Press: New York, NY, USA, 2016; 438p, ISBN 9781482248210. [Google Scholar]
- Marques, S.P.D. Nova Proposta para Rápida Formação/Combate dos Produtos de Glicação Avançada (AGEs) e Propriedades Farmacológicas de Hancornia speciosa Gomes. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 5 May 2017. [Google Scholar]
- Bruice, P.Y. Química Orgânica, 4rd ed.; Pearson: São Paulo, Brazil, 2006; pp. 747–751. ISBN 3226132261. [Google Scholar]
- Fan, X.; Reneker, L.W.; Obrenovich, M.E.; Strauch, C.; Cheng, R.; Jarvis, S.M.; Ortwerth, B.J.; Monnier, V.M. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 16912–16917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledl, F.; Schleicher, E. New aspects of the Maillard reactions in foods and in the human body. Angew. Chem. Int. Ed. Engl. 1990, 29, 565–706. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; Courten, B.; Bugel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food. Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Howard, T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996, 7, 659–663. [Google Scholar] [CrossRef]
- Bousova, I.; Pruchova, Z.; Trnkova, L.; Drsata, J. Comparison of glycation of glutathione S-transferase by methylglyoxal, glucose or fructose. Mol. Cell. Biochem. 2011, 357, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hengel, M.; Pan, C.; Seiber, J.N.; Shibamoto, T. Determination of toxic alpha-dicarbonyl compounds, glyoxal, methylglyoxal, and diacetyl, released to the headspace of lipid commodities upon heat treatment. J. Agric. Food Chem. 2013, 61, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Bai, N.; He, K.; Ho, C.T.; Yang, C.S.; Sang, S. Apple polyphenols, phloretin and phloridzin: New trapping agents of reactive dicarbonyl species. Chem. Res. Toxicol. 2008, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Lo, C.Y.; Ho, C.T. Methylglyoxal: Its presence in beverages and potential scavengers. Ann. N. Y. Acad. Sci. 2008, 1126, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.Y.; Hsiao, W.T.; Chen, X.Y. Efficiency of trapping methylglyoxal by phenols and phenolic acid. J. Food Sci. 2011, 76, H90–H96. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food. Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y.; Liu, C.L.; Chyau, C.C.; Hu, M.L. Trapping of methylglyoxal by curcumin in cell-free system and in human umbilical vein endotelial cells. J. Agric. Food Chem. 2012, 60, 8190–8196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ho, C.T. Flavour chemistry of methylglyoxal and glyoxal. Chem. Soc. Rev. 2012, 41, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Sekiguchi, K.; Aso-o, R.; Ayuha, K.; Ni-iyama, H.; Kato, T.; Kikugawa, K. DNA strand breaks induced through active oxygen radicals by fragrant component 4-hydroxy-2-hydroxymethyl-5-methyl-3(2h)-furanone in Maillard reaction of hexose/amino acid. Food Chem. Toxicol. 1995, 33, 803–814. [Google Scholar] [CrossRef]
- Yim, H.; Kang, S.; Hah, Y.; Chock, P.B.; Yim, M.B. Free radicals generated during the glycation reaction of amino acids by methylglyoxal. J. Biol. Chem. 1995, 270, 28228–28233. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Okano, Y.; Sakurai, H. Generation of active oxygen species from advanced glycation end-products (AGEs) during ultraviolet light A (UVA) irradiation and a possible mechanism for cell damaging. Biochim. Biophys. Acta 1999, 1428, 45–56. [Google Scholar] [CrossRef]
- Spiteller, G. Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products. Ann. N. Y. Acad. Sci. 2008, 1126, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lunceford, N.; GugliuccI, A. Ilex paraguariensis extracts inhibit AGE formation more efficiently than green tea. Fitoterapia 2005, 76, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Vishwanath, V.; Frank, K.E.; Elmets, C.A.; Dauchot, P.; Kohn, R.R. Relation between complications of type I diabetes mellitus and collagen linked fluorecence. N. Engl. J. Med. 1986, 314, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Lapolla, A.; Fedele, D.; Reitano, R.; Arico, N.C.; Seraglia, R.; Traldi, P.; Marotta, E.; Tonani, R. Enzymatic digestion and mass spectrometry in the studies of advanced glycation end products/peptides. J. Am. Soc. Mass Spectrom. 2004, 15, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.T.S.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006, 44, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Smith, J.S. Determination of advanced glycation end products in cooked meat products. Food Chem. 2015, 168, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.; Morales, J. Mechanism of reactive carbonyl species trapping by hydroxytyrosol under simulated physiological conditions. Food Chem. 2015, 175, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, B.O. Biological effects of aminoguanidine: An update. Inflamm. Res. 1999, 48, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Use of aminoguanidine (pimagedine) to prevent the formation of advanced glycation end products. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.M.; Panaskar, S.N.; Arvindekar, A.U. Inhibition of advanced glycation end product formation by cymene—A common food constituent. J. Funct. Foods 2013, 6, 107–115. [Google Scholar] [CrossRef]
- Lo, C.Y.; Li, S.; Tan, D.; Pan, M.H.; Sang, S.; Ho, C.T. Trapping reactions of reactive carbonyl species with tea polyphenols in simulated physiological conditions. Mol. Nutr. Food Res. 2006, 50, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhu, J.X.; Wu, A.G.; Xu, Y.H.; Duan, T.T.; Zheng, Z.G.; Wang, R.S.; Li, D.; Zhu, Q. Pre-column incubation followed by fast liquid chromatography analysis for rapid screening of natural methylglyoxal scavengers directly from herbal medicines: Case study of Polygonum cuspidatum. J. Chromatogr. A 2013, 1286, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, K.W.; Ma, J.; Chen, B.; Ho, C.T.; Lo, C.; Chen, F.; Wang, M. Cinnamon bark proanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J. Agric. Food Chem. 2008, 56, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Mcpherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, E.; Dufossé, L.; Guérard, F. Kinetic Study on the Maillard Reaction. Consideration of Sugar Reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Miric, D.J.; Kisic, B.J.; Filipovic-Danic, S.; Grbic, R.; Dragojevic, I.; Miric, M.B.; Puhalo-Sladoje, D. Xanthine oxidase activity in type 2 diabetes mellitus patients with and without diabetic peripheral neuropathy. J. Diabetes Res. 2016, 2016, 4370490. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, S.; Trevisan, T.; Maia, C.; Breuer, A.; Owen, R.W. Improved Methods for the Rapid Formation and Prevention of Advanced Glycation End Products (AGEs) In Vitro by Coupling to the Hypoxanthine/Xanthine Oxidase Assay System. Biomedicines 2018, 6, 88. https://doi.org/10.3390/biomedicines6030088
Marques S, Trevisan T, Maia C, Breuer A, Owen RW. Improved Methods for the Rapid Formation and Prevention of Advanced Glycation End Products (AGEs) In Vitro by Coupling to the Hypoxanthine/Xanthine Oxidase Assay System. Biomedicines. 2018; 6(3):88. https://doi.org/10.3390/biomedicines6030088
Chicago/Turabian StyleMarques, Samuel, Teresa Trevisan, Carlos Maia, Andrea Breuer, and Robert W. Owen. 2018. "Improved Methods for the Rapid Formation and Prevention of Advanced Glycation End Products (AGEs) In Vitro by Coupling to the Hypoxanthine/Xanthine Oxidase Assay System" Biomedicines 6, no. 3: 88. https://doi.org/10.3390/biomedicines6030088





