siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. siRNA Sequence
2.3. Cell Culture and Seeding
2.4. Imaging of Particles with Scanning Elentron Microscope (SEM)
2.5. Generation of Target siRNAs/CA Complexes and Transfection of MCF-7, MDA-MB-231 and 4T1 Cell Lines
2.6. Cell Viability Assessment with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.7. Western Blotting
2.8. Effects of siRNA-Loaded Nanoparticles on Tumor Regression in 4T1-Induced Breast Cancer Model
2.9. Statistical Analysis
3. Results and Discussion
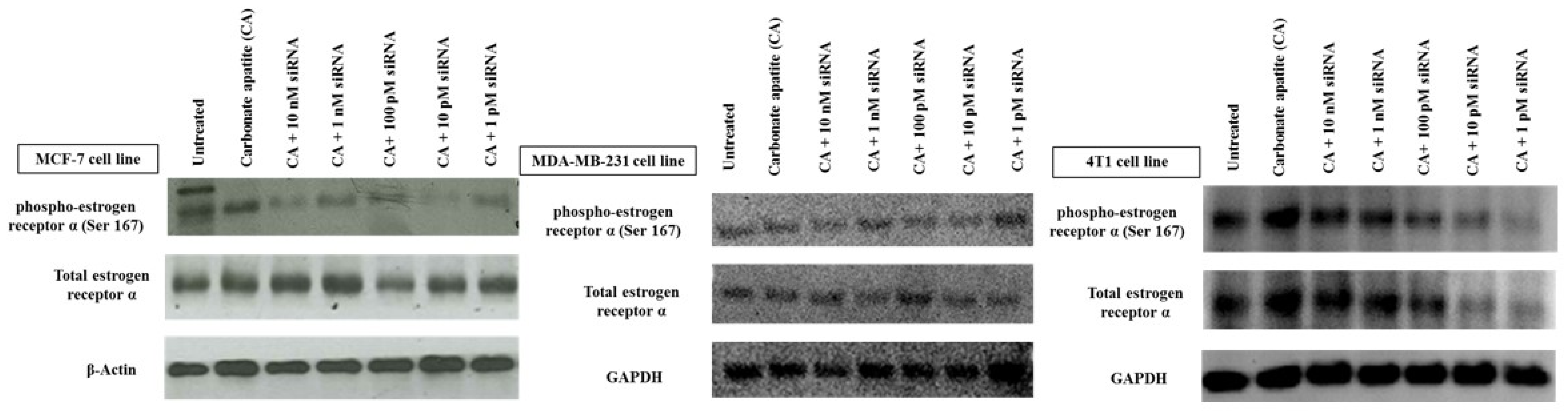
3.1. Roles of ER in Proliferation/Survival of Breast Cancer Cells
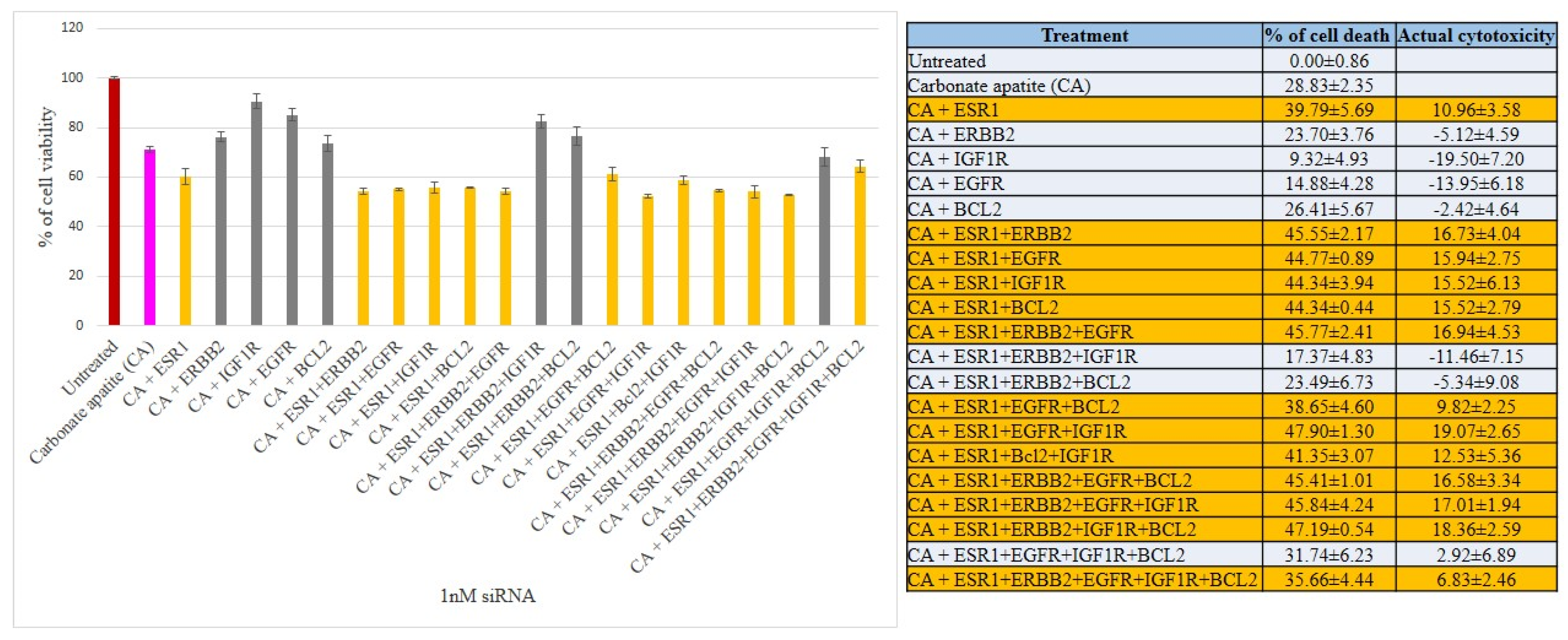
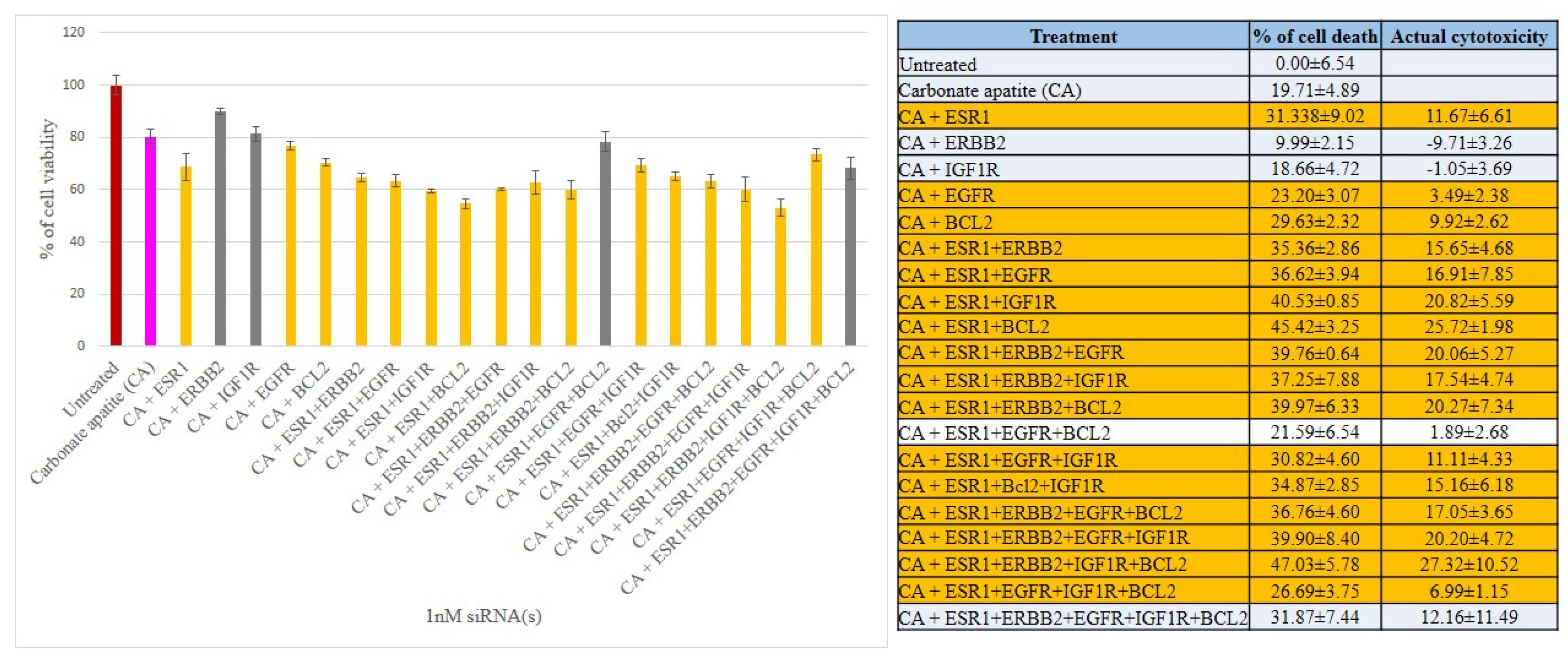
3.2. Cross-Talks of ER, HER2, EGFR, IGF1R and BCL-2 Signaling Cascades Affecting Proliferation/Survival of Breast Cancer Cells
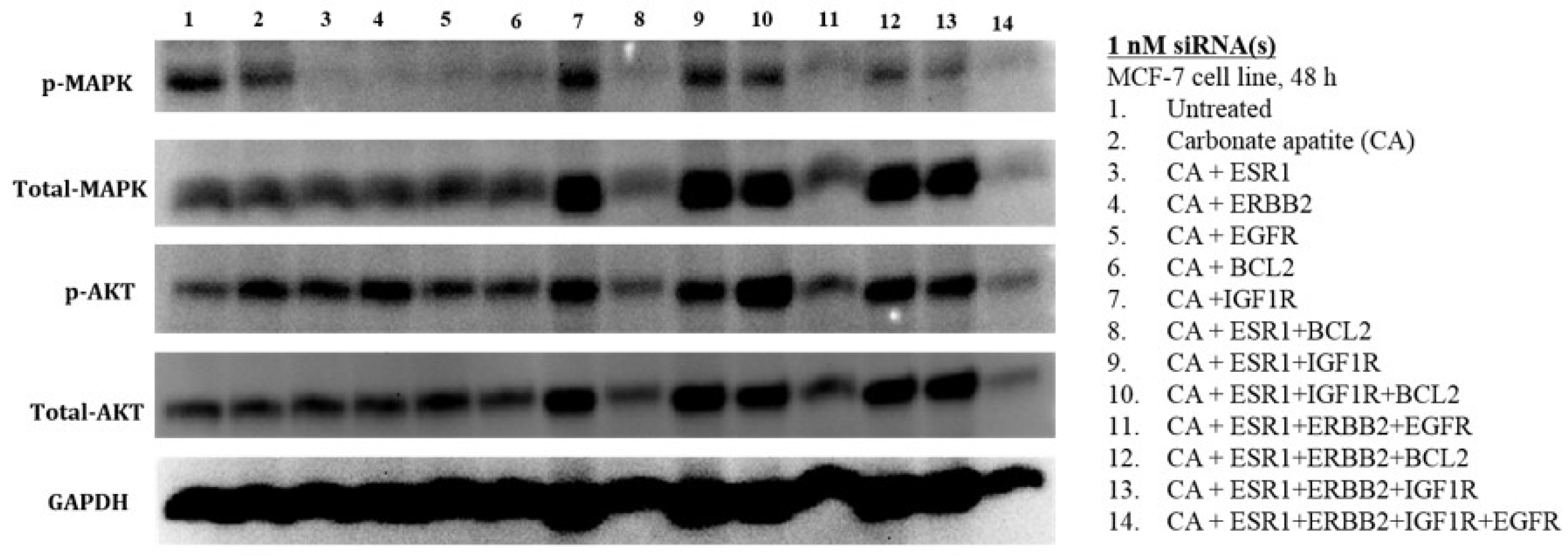
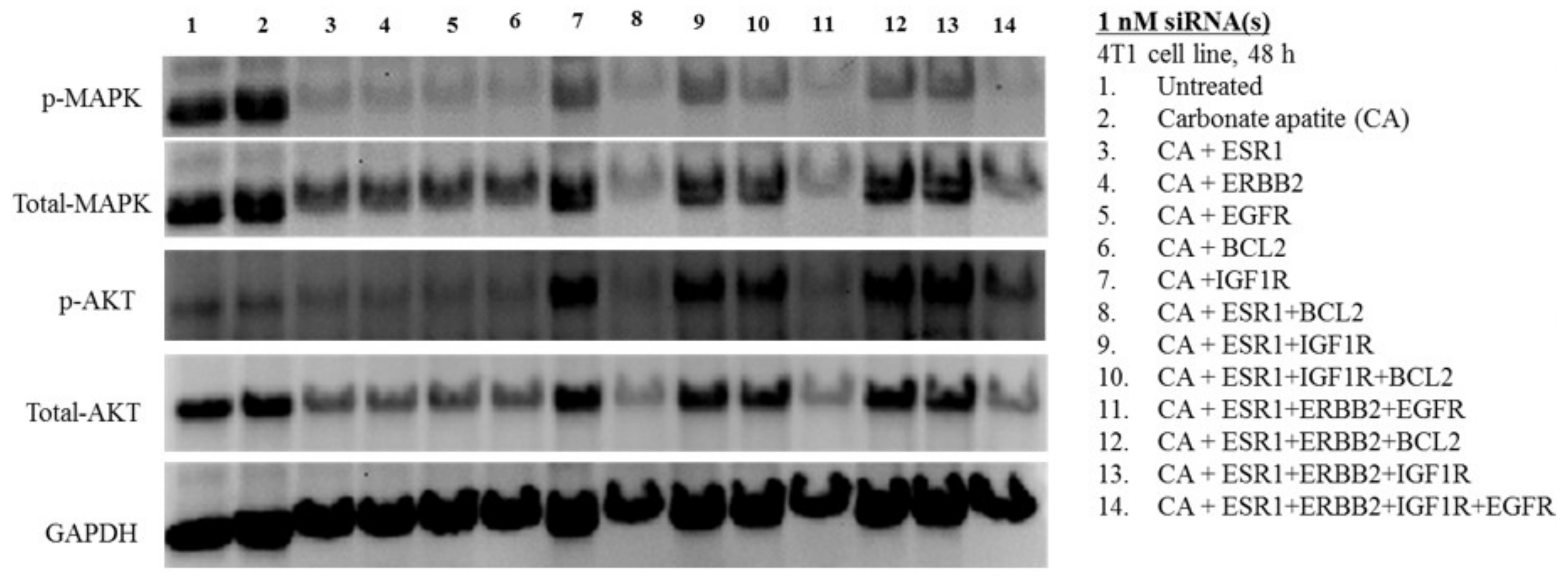
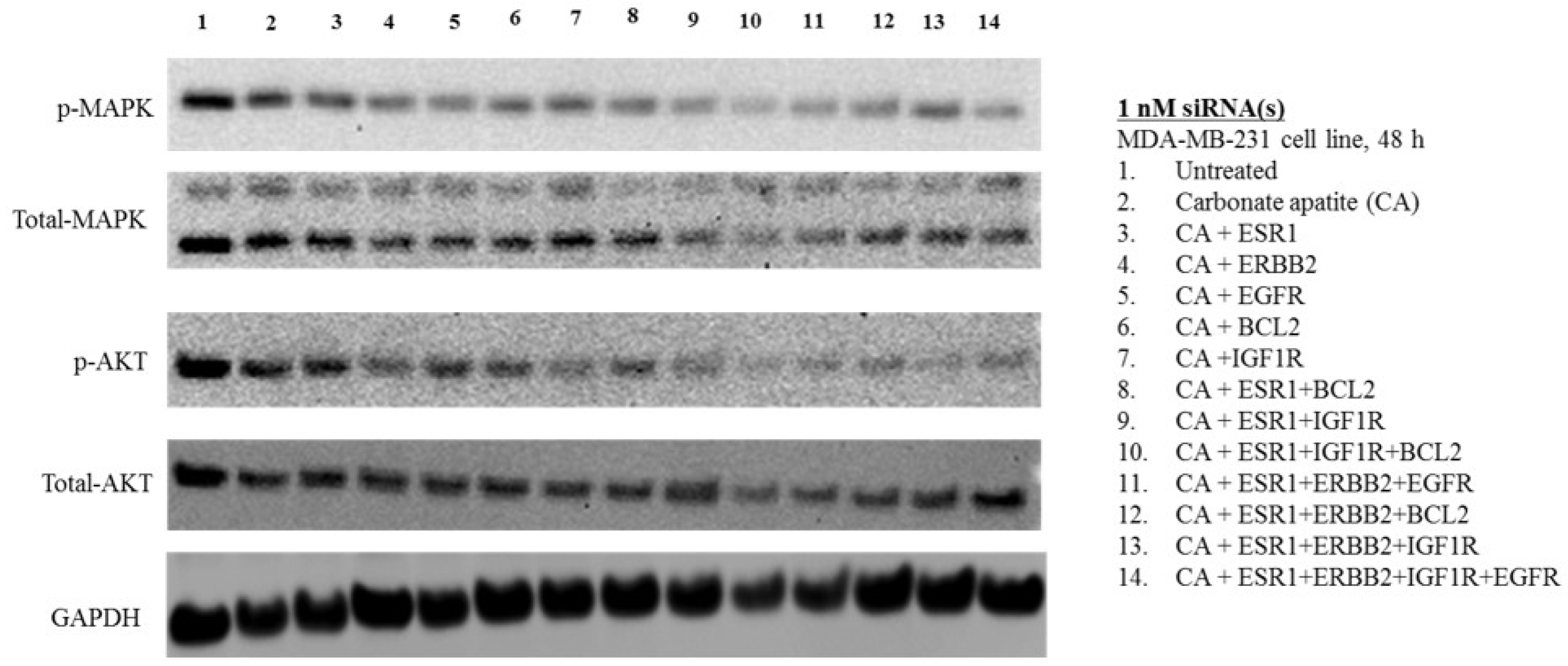
3.3. MAPK and AKT Expression and Activation Following Treatment with CA-siRNA(s) Complexes
3.4. Intracellular Delivery of Negative Control siRNA Using CA Nanoparticles In Vitro and In Vivo
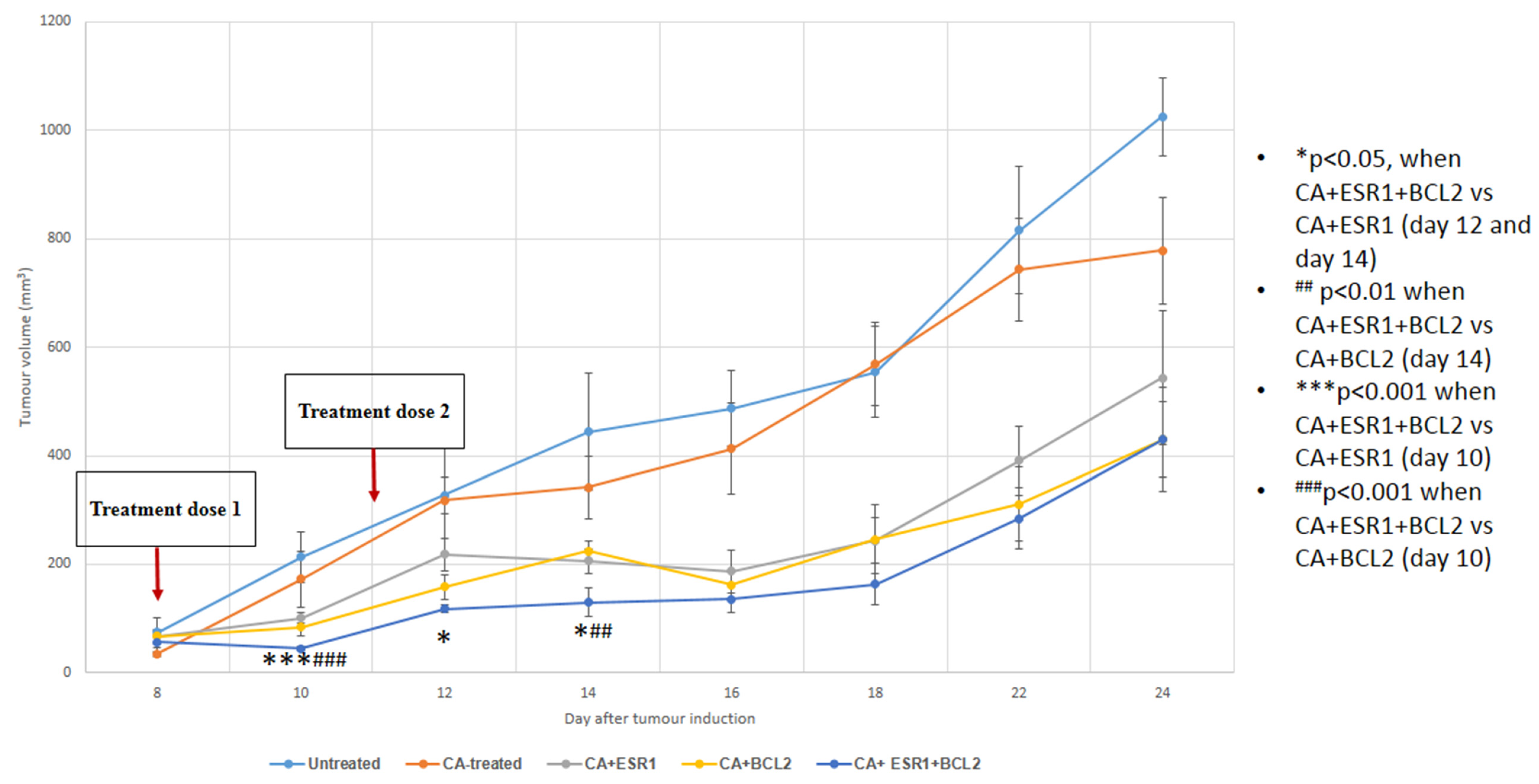
3.5. Intravenous Delivery of CA Nanoformulations of siRNAs Targeting ESR1 and BCL2
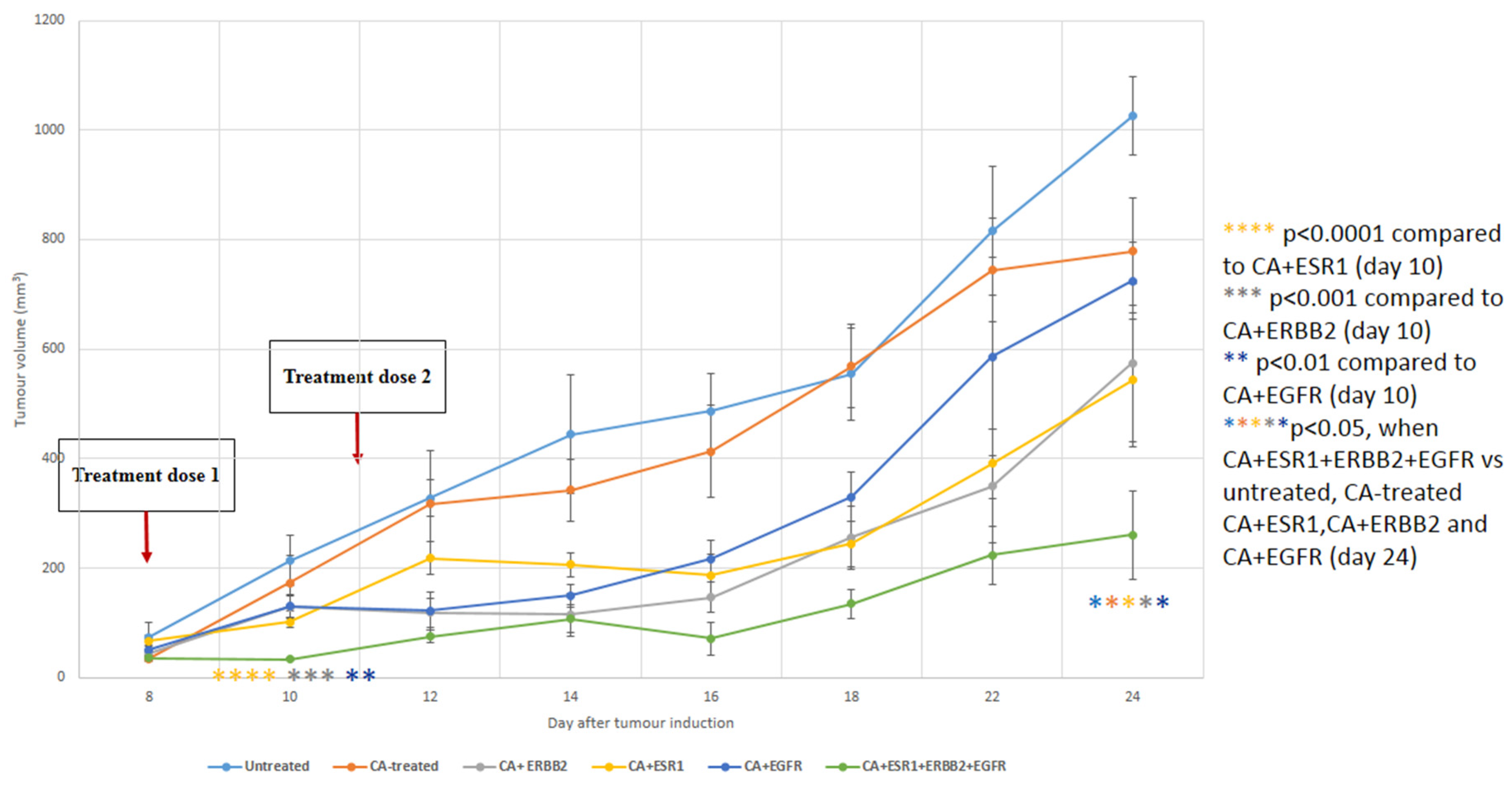
3.6. Intravenous Delivery of CA Nanoformulations of siRNAs Targeting ESR1, ERBB2 and EGFR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, G.N.; Delbue, D.; Silva, K.L.; Robaina, M.C.; Khongkow, P.; Gomes, A.R.; Zona, S.; Crocamo, S.; Mencalha, A.L.; Magalhães, L.M.; et al. FOXM1 targets XIAP and survivin to modulate breast cancer survival and chemoresistance. Cell Signal. 2015, 27, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Fatemian, T.; Chowdhury, E.H. Targeting oncogenes and tumor suppressors genes to mitigate chemoresistance. Curr. Cancer Drug Targets 2014, 14, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Masood, S. Estrogen and progesteron receptors in cytology: A comprehensive review. Diagn. Cytopathol. 1992, 8, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Cellular functions of plasma membrane estrogen receptors. Steroids 2002, 67, 471–475. [Google Scholar] [CrossRef]
- Osborne, C.K.; Shou, J.; Massarweh, S.; Schiff, R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin. Cancer Res. 2005, 11, 865–870. [Google Scholar]
- Sommer, S.; Fuqua, S.A.W. Estrogen receptor and breast cancer. Semin. Cancer Biol. 2001, 11, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Tiash, S.; Chowdhury, E.H. Growth factor receptors: Promising drug targets in cancer. J. Cancer Metastasis Treat 2015, 1, 190–200. [Google Scholar]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rettig, W.J.; Old, L.J. Immunogenetics of human cell surface differentiation. Annu. Rev. Immunol. 1989, 7, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for treatment of metastatic breast cancer. Clin. Ther. 1999, 21, 309–318. [Google Scholar] [CrossRef]
- Chowdhury, E.H. Strategies for tumor-directed delivery of siRNA. Expert Opin. Drug Deliv. 2011, 8, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H.; Akaike, T. High performance DNA nano-carriers of carbonate apatite: Multiple factors in regulation of particle synthesis and transfection efficiency. Int. J. Nanomed. 2007, 2, 101–106. [Google Scholar] [CrossRef]
- Chowdhury, E.H. pH-sensitive nano-crystals of carbonate apatite for smart and cell-specific transgene delivery. Expert Opin. Drug Deliv. 2007, 4, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Stanislaus, A.; Chua, M.J.; Tada, S.; Tagawa, Y.; Chowdhury, E.H.; Akaike, T. Carbonate apatite-facilitated intracellularly delivered siRNA for efficient knockdown of functional genes. J. Control. Release 2010, 147, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tiash, S.; Kamaruzman, N.I.B.; Chowdhury, E.H. Carbonate apatite nanoparticles carry siRNA(s) targeting growth factor receptor genes egfr1 and erbb2 to regress mouse breast tumor. Drug Deliv. 2017, 24, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.J.; Tiash, S.; Fatemian, T.; Noordin, M.I.; Keng, C.S.; Chowdhury, E.H. Carbonate apatite-facilitated intracellular delivery of c-ROS1 small interfering RNA sensitises MCF-7 breast cancer cells to cisplatin and paclitaxel. OA Cancer 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Tiash, S.; Chua, M.J.; Chowdhury, E.H. Knockdown of ROS1 gene sensitizes breast tumor growth to doxorubicin in a syngeneic mouse model. Int. J. Oncol. 2016, 48, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Afratis, N.; Smirlaki, G.; Nikitovic, D.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. Cross-talk between estradiol receptor and EGFR/IGF-IR signaling pathways in estrogen-responsive breast cancers: Focus on the role and impact of proteoglycans. Matrix Biol. 2014, 35, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, P.; Zhu, B.; Sun, L.; Huang, Q.; Wang, J. Induction of estrogen receptor α-36 expression by bone morphogenetic protein 2 in breast cancer cell lines. Mol. Med. Rep. 2012, 6, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.H.J.; Maie, A.B.; Bushra, A.A.; Francis, I. Reassessment of estrogen receptor expression in human breast cancer cell lines. Anticancer Res. 2011, 31, 521–527. [Google Scholar] [PubMed]
- Jeong, H.; Kim, J.; Lee, Y.; Seo, J.H.; Hong, S.R.; Kim, A. Neuregulin-1 induces cancer stem cell characteristics in breast cancer cell lines. Oncol. Rep. 2014, 32, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Moerkens, M.; Zhang, Y.; Wester, L.; van de Water, B.; Meerman, J.H. Epidermal growth factor receptor signalling in human breast cancer cells operates parallel to estrogen receptor α signalling and results in tamoxifen insensitive proliferation. BMC Cancer 2014, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gaben, A.M.; Sabbah, M.; Redeuilh, G.; Bedin, M.; Mester, J. Ligand-free estrogen receptor activity complements IGF1R to induce the proliferation of the MCF-7 breast cancer cells. BMC Cancer 2012, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Holly, J.M.P.; Perks, C.M. Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front. Endocrinol. 2014, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Okollie, B.; Artemov, D. Controlled internalization of Her-2/neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 2007, 6, 1960–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dool, C.J.; Mashhedi, H.; Zakikhani, M.; David, S.; Zhao, Y.; Birman, E.; Carboni, J.M.; Gottardis, M.; Blouin, M.J.; Pollak, M. IGF1/insulin receptor kinase inhibition by BMS-536924 is better tolerated than alloxan-induced hypoinsulinemia and more effective than metformin in the treatment of experimental insulinresponsive breast cancer. Endocr. Relat. Cancer 2011, 18, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Czarnek, M.; Bzowska, M.; Mężyk-Kopeć, R.; Stalińska, K.; Wyroba, B.; Sroka, J.; Jucha, J.; Deneka, D.; Stokłosa, P.; et al. ADAM17 Silencing in Mouse Colon Carcinoma Cells: The Effect on Tumoricidal Cytokines and Angiogenesis. PLoS ONE 2012, 7, e50791. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, L.; He, T.; Xiao, X.; Liu, X.; Wang, L.; Yang, L.; Yang, M.; Zhang, T.; CHen, R.; et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int. J. Biol. Sci. 2012, 8, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Horii, R.; Ito, Y.; Saji, S.; Younes, M.; Iwase, T.; Akiyama, F. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer 2015, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Joos, S.; Zornig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 2004, 1644, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Klasa, R.J.; Gillum, A.M.; Klem, R.E.; Frankel, S.R. Oblimersen Bcl-2 antisense: Facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002, 12, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.L.; Lo, H.W. Regulation of Apoptosis by HER2 in Breast Cancer. J. Carcinog. Mutagen. 2013, 2013 (Suppl. 7). [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaruzman, N.I.; Tiash, S.; Ashaie, M.; Chowdhury, E.H. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines 2018, 6, 73. https://doi.org/10.3390/biomedicines6030073
Kamaruzman NI, Tiash S, Ashaie M, Chowdhury EH. siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways. Biomedicines. 2018; 6(3):73. https://doi.org/10.3390/biomedicines6030073
Chicago/Turabian StyleKamaruzman, Nur Izyani, Snigdha Tiash, Maeirah Ashaie, and Ezharul Hoque Chowdhury. 2018. "siRNAs Targeting Growth Factor Receptor and Anti-Apoptotic Genes Synergistically Kill Breast Cancer Cells through Inhibition of MAPK and PI-3 Kinase Pathways" Biomedicines 6, no. 3: 73. https://doi.org/10.3390/biomedicines6030073




