Neural Oscillatory Correlates for Conditioning and Extinction of Fear
Abstract
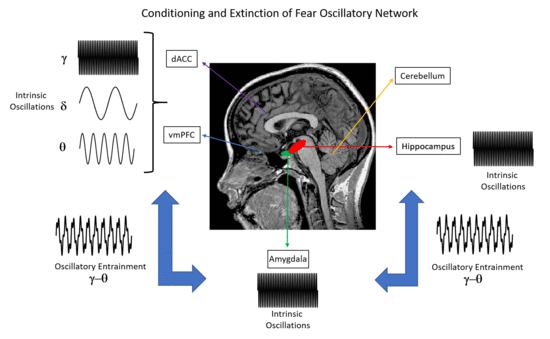
:1. Introduction
2. Fear Conditioning Correlates
2.1. Animal Models
2.2. Humans
3. Fear Extinction Correlates
3.1. Animal Models
3.2. Humans
4. Summary of Oscillatory Neural Correlates
5. Translational Perspectives
Acknowledgments
Conflicts of Interest
References
- Bitterman, M.E. Classical conditioning since Pavlov. Rev. Gen. Psychol. 2006, 10, 365–376. [Google Scholar] [CrossRef]
- Flor, H.; Birbaumer, N. Fear conditioning. In International Encyclopedia of the Social & Behavioral Sciences; Elsevier: Amsterdan, The Netherlands; New York, NY, USA, 2001. [Google Scholar]
- Furini, C.; Myskiw, J.; Izquierdo, I. The learning of fear extinction. Neurosci. Biobehav. Rev. 2014, 47, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Thompson, R.F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997, 20, 177–181. [Google Scholar] [CrossRef]
- Seidenbecher, T.; Laxmi, T.R.; Stork, O.; Pape, H.C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 2003, 301, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Delgado, M.R.; Nearing, K.I.; LeDoux, J.E. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 2004, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Anderson, K.L.; Leal, S.L.; Shestyuk, A.; Gulsen, G.; Mnatsakanyan, L.; Vadera, S.; Hsu, F.P.K.; Yassa, M.A.; Knight, R.T.; et al. Amygdala-hippocampal dynamics during salient information processing. Nat. Commun. 2017, 8, 14413. [Google Scholar] [CrossRef] [PubMed]
- Taub, A.H.; Perets, R.; Kahana, E.; Paz, R. Oscillations synchronize amygdala-to-prefrontal primate circuits during aversive learning. Neuron 2018, 97, 291–298. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Dahlgren, M.K.; Davis, F.C.; Dubois, S.; Shin, L.M. From pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014, 113, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.S.; Lee, A.; McGrath, A.G.; Graybiel, A.M.; Goosens, K.A. Amygdala-ventral striatum circuit activation decreases long-term fear. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Baldi, E.; Lorenzini, C.A.; Bucherelli, C. Cerebellar role in fear-conditioning consolidation. Proc. Natl. Acad. Sci. USA 2002, 99, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Utz, A.; Thürling, M.; Ernst, T.M.; Hermann, A.; Stark, R.; Wolf, O.T.; Timmann, D.; Merz, C.J. Cerebellar vermis contributes to the extinction of conditioned fear. Neurosci. Lett. 2015, 604, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Buehlmann, A.; Masquelier, T.; Hugues, E. The role of rhythmic neural synchronization in rest and task conditions. Front. Hum. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.T.; Seidenbecher, T.; Sangha, S.; Stork, O.; Pape, H.C. Theta resynchronization during reconsolidation of remote contextual fear memory. NeuroReport 2007, 18, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.T.; Seidenbecher, T.; Kluge, C.; Bergado, J.; Stork, O.; Pape, H.C. Dissociated theta phase synchronization in amygdalohippocampal circuits during various stages of fear memory. Eur. J. Neurosci. 2007, 25, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Barkus, C.; Line, S.J.; Huber, A.; Capitao, L.; Lima, J.; Jennings, K.; Lowry, J.; Sharp, T.; Bannerman, D.M.; McHugh, S.B. Variation in serotonin transporter expression modulates fear-evoked hemodynamic responses and theta-frequency neuronal oscillations in the amygdala. Biol. Psychiatry 2014, 75, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Lesting, J.; Daldrup, T.; Narayanan, V.; Himpe, C.; Seidenbecher, T.; Pape, H.C. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear Extinction. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Likhtik, E.; Stujenske, J.M.; Topiwala, M.A.; Harris, A.Z.; Gordon, J.A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 2014, 17, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Senn, V.; Wolff, S.B.E.; Herry, C.; Grenier, F.; Ehrlich, I.; Gründemann, J.; Fadok, J.P.; Müller, C.; Letzkus, J.J.; Lüthi, A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 2014, 81, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Karalis, N.; Dejean, C.; Chaudun, F.; Khoder, S.; Rozeske, R.R.; Wurtz, H.; Bagur, S.; Benchenane, K.; Sirota, A.; Courtin, J.; et al. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat. Neurosci. 2016, 19, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Fenton, G.E.; Halliday, D.M.; Mason, R.; Bredy, T.W.; Stevenson, C.W. Sex differences in learned fear expression and extinction involve altered gamma oscillations in medial prefrontal cortex. Neurobiol. Learn. Mem. 2016, 135, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Livneh, U.; Paz, R. Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron 2012, 75, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Klavir, O.; Genud-Gabai, R.; Paz, R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron 2013, 80, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Courtin, J.; Karalis, N.; Gonzalez-Campo, C.; Wurtz, H.; Herry, C. Persistence of amygdala gamma oscillations during extinction learning predicts spontaneous fear recovery. Neurobiol. Learn. Mem. 2014, 113, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Headley, D.B.; Weinberger, N.M. Fear conditioning enhances gamma oscillations and their entrainment of neurons representing the conditioned stimulus. J. Neurosci. 2013, 33, 5705–5717. [Google Scholar] [CrossRef] [PubMed]
- Stujenske, J.M.; Likhtik, E.; Topiwala, M.A.; Gordon, J.A. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 2014, 83, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Miltner, W.H.; Braun, C.; Arnold, M.; Witte, H.; Taub, E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature 1999, 397, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.M.; Panitz, C.; Hermann, C.; Pizzagalli, D.A. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J. Neurosci. 2014, 34, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, B.L.; Spence, J.S.; Shakal, S.K.; Motes, M.A.; Calley, C.S.; Calley, V.I.; Hart, J., Jr.; Kraut, M.A. Electrophysiological spatiotemporal dynamics during implicit visual threat processing. Brain Cogn. 2014, 91, 54–61. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Gruss, L.F.; Keil, A. Aversive learning shapes neuronal orientation tuning in human visual cortex. Nat. Commun. 2015, 6, 7823. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.H.; Colloca, L.; Korzeniewska, A.; Cheng, J.J.; Campbell, C.M.; Hillis, A.E.; Lenz, F.A. Oscillatory EEG activity induced by conditioning stimuli during fear conditioning reflects Salience and Valence of these stimuli more than Expectancy. Neuroscience 2017, 346, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Lithari, C.; Moratti, S.; Weisz, N. Limbic areas are functionally decoupled and visual cortex takes a more central role during fear conditioning in humans. Sci. Rep. 2016, 6, 29220. [Google Scholar] [CrossRef] [PubMed]
- Balderston, N.L.; Hale, E.; Hsiung, A.; Torrisi, S.; Holroyd, T.; Carver, F.W.; Coppola, R.; Ernst, M.; Grillon, C. Threat of shock increases excitability and connectivity of the intraparietal sulcus. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.C.; Narayanan, R.T.; Lesting, J.; Stork, O.; Seidenbecher, T.; Kluge, C.; Sangha, S. Distinctive patterns of theta synchronization in amygdalo-hippocampal-prefrontal cortical circuits during fear memory consolidation and extinction. Soc. Neurosci. Abstr. 2009, 8, 680. [Google Scholar]
- Sangha, S.; Narayanan, R.T.; Bergado-Acosta, J.R.; Stork, O.; Seidenbecher, T.; Pape, H.C. Deficiency of the 65-kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear. J. Neurosci. 2009, 29, 15713–15720. [Google Scholar] [CrossRef] [PubMed]
- Lesting, J.; Narayanan, R.T.; Kluge, C.; Sangha, S.; Seidenbecher, T.; Pape, H.C. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Fenton, G.E.; Halliday, D.M.; Mason, R.; Stevenson, C.W. Medial prefrontal cortex circuit function during retrieval and extinction of associative learning under anesthesia. Neuroscience 2014, 265, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.; Zaki, Y.; Maguire, J.; Reijmers, L.G. Cellular and oscillatory substrates of fear extinction learning. Nat. Neurosci. 2017, 20, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Whittle, N.; Flynn, S.M.; Graybeal, C.; Pinard, C.R.; Gunduz-Cinar, O.; Kravitz, A.V.; Singewald, N.; Holmes, A. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol. Learn. Mem. 2014, 113, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Sperl, M.F.J.; Panitz, C.; Rosso, I.M.; Dillon, D.G.; Kumar, P.; Hermann, A.; Whitton, A.E.; Hermann, C.; Pizzagalli, D.A.; Mueller, E.M. Fear extinction recall modulates human frontomedial theta and amygdala activity. Cereb. Cortex. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abend, R.; Jalon, I.; Gurevitch, G.; Sar-El, R.; Shechner, T.; Pine, D.S.; Hendler, T.; Bar-Haim, Y. Modulation of fear extinction processes using transcranial electrical stimulation. Transl. Psychiatry 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Van’t Wout, M.; Mariano, T.Y.; Garnaat, S.L.; Reddy, M.K.; Rasmussen, S.A.; Greenberg, B.D. Can Transcranial Direct Current Stimulation Augment Extinction of Conditioned Fear? Brain Stimul. 2016, 9, 529–536. [Google Scholar] [CrossRef] [PubMed]
- van’t Wout, M.; Longo, S.M.; Reddy, M.K.; Philip, N.S.; Bowker, M.T.; Greenberg, B.D. Transcranial direct current stimulation may modulate extinction memory in posttraumatic stress disorder. Brain Behav. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Noble, L.J.; Gonzalez, I.J.; Meruva, V.B.; Callahan, K.A.; Belfort, B.D.; Ramanathan, K.R.; Meyers, E.; Kilgard, M.P.; Rennaker, R.L.; McIntyre, C.K. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; Verkuil, B.; Van Diest, I.; Van der Does, W.; Thayer, J.F.; Brosschot, J.F. The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol. Learn. Mem. 2016, 132, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Genheimer, H.; Andreatta, M.; Asan, E.; Pauli, P. Reinstatement of contextual conditioned anxiety in virtual reality and the effects of transcutaneous vagus nerve stimulation in humans. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.D.; Gabriels, L.A.; Malone, D.A.; Rezai, A.R.; Friehs, G.M.; Okun, M.S.; Nuttin, B.J. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol. Psychiatry 2010, 15, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Wojtecki, L.; Hirschmann, J.; Elben, S.; Boschheidgen, M.; Trenado, C.; Vesper, J.; Schnitzler, A. Oscillatory coupling of the subthalamic nucleus in obsessive compulsive disorder. Brain. 2017, 140. [Google Scholar] [CrossRef]
- Rodriguez-Romaguera, J.; Do Monte, F.H.M.; Quirk, G.J. Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc. Natl. Acad. Sci. USA 2012, 109, 8764–8769. [Google Scholar] [PubMed]
- Do-Monte, F.H.; Rodriguez-Romaguera, J.; Rosas-Vidal, L.E.; Quirk, G.J. Deep brain stimulation of the ventral striatum increases BDNF in the fear extinction circuit. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, A.; Amano, K.; Cortese, A.; Shibata, K.; Yoshida, W.; Seymour, B.; Kawato, M.; Lau, H. Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nat. Hum. Behav. 2016, 1. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trenado, C.; Pedroarena-Leal, N.; Cif, L.; Nitsche, M.; Ruge, D. Neural Oscillatory Correlates for Conditioning and Extinction of Fear. Biomedicines 2018, 6, 49. https://doi.org/10.3390/biomedicines6020049
Trenado C, Pedroarena-Leal N, Cif L, Nitsche M, Ruge D. Neural Oscillatory Correlates for Conditioning and Extinction of Fear. Biomedicines. 2018; 6(2):49. https://doi.org/10.3390/biomedicines6020049
Chicago/Turabian StyleTrenado, Carlos, Nicole Pedroarena-Leal, Laura Cif, Michael Nitsche, and Diane Ruge. 2018. "Neural Oscillatory Correlates for Conditioning and Extinction of Fear" Biomedicines 6, no. 2: 49. https://doi.org/10.3390/biomedicines6020049