Semaphorin 3C and Its Receptors in Cancer and Cancer Stem-Like Cells
Abstract
:1. Introduction
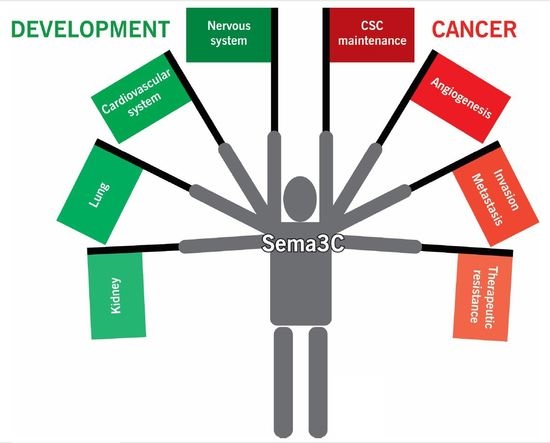
2. Sema3C Function in Development
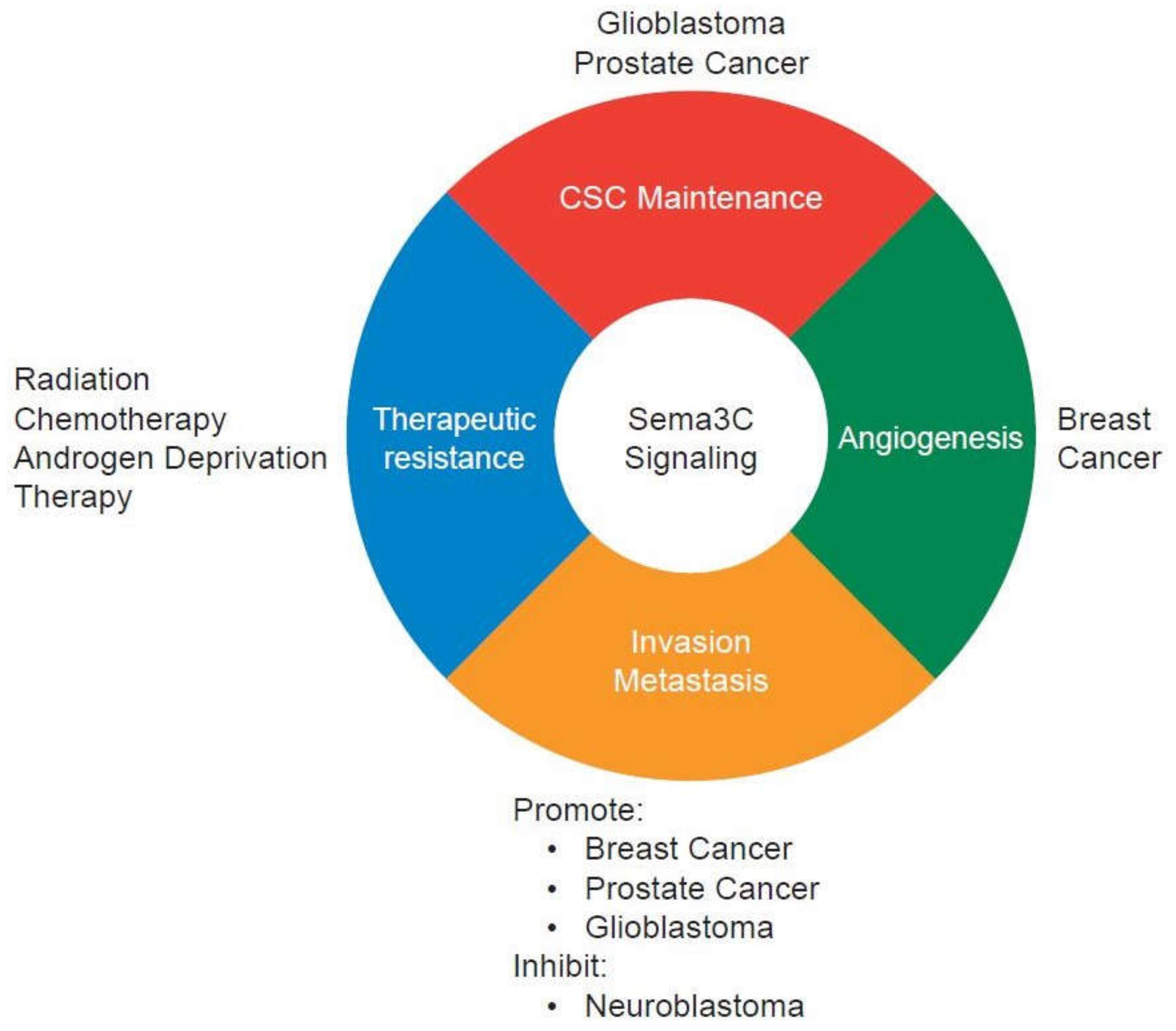
3. Sema3C in Cancer and Cancer Stem-Like Cells
4. Sema3C Receptors in Carcinogenesis
5. Therapeutic Strategies Targeting Sema3C and Its Receptors
6. Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Venkatesh, H.; Monje, M. Neuronal activity in ontogeny and oncology. Trends Cancer 2017, 3, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Lodola, A.; Giorgio, C.; Incerti, M.; Zanotti, I.; Tognolini, M. Targeting Eph/ephrin system in cancer therapy. Eur. J. Med. Chem. 2017, 142, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gara, R.K.; Kumari, S.; Ganju, A.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Slit/Robo pathway: A promising therapeutic target for cancer. Drug Discov. Today 2015, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Chopin, V.; Lagadec, C.; Toillon, R.A.; Le Bourhis, X. Neurotrophin signaling in cancer stem cells. Cell. Mol. Life Sci. 2016, 73, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, P.; Potiron, V.; Drabkin, H.; Roche, J. Guidance molecules in lung cancer. Cell Adh. Migr. 2010, 4, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, U.; Ucuncu Kefeli, A.; Cabuk, D.; Isik, U.; Sonkaya, A.; Acikgoz, O.; Ozden, E.; Uygun, K. Netrin-1 in cancer: Potential biomarker and therapeutic target? Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, P.; Gemmill, R.M.; Drabkin, H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther. 2014, 7, 1663–1687. [Google Scholar] [PubMed]
- Worzfeld, T.; Offermanns, S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014, 13, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Cagnoni, G.; Tamagnone, L. Semaphorin receptors meet receptor tyrosine kinases on the way of tumor progression. Oncogene 2014, 33, 4795–4802. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Tamagnone, L. Semaphorins in cancer: Biological mechanisms and therapeutic approaches. Semin. Cell Dev. Biol. 2013, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Tamagnone, L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell 2012, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Mumblat, Y.; Smolkin, T.; Toledano, S.; Nir-Zvi, I.; Ziv, K.; Kessler, O. The role of the semaphorins in cancer. Cell Adh. Migr. 2016, 10, 652–674. [Google Scholar] [CrossRef] [PubMed]
- Capparuccia, L.; Tamagnone, L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J. Cell Sci. 2009, 122, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.A.; Bashaw, G.J. Axon guidance pathways and the control of gene expression. Dev. Dyn. 2017. [Google Scholar] [CrossRef] [PubMed]
- Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 1999, 97, 551–552. [Google Scholar]
- Gu, C.; Yoshida, Y.; Livet, J.; Reimert, D.V.; Mann, F.; Merte, J.; Henderson, C.E.; Jessell, T.M.; Kolodkin, A.L.; Ginty, D.D. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 2005, 307, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Bagnard, D.; Thomasset, N.; Lohrum, M.; Puschel, A.W.; Bolz, J. Spatial distributions of guidance molecules regulate chemorepulsion and chemoattraction of growth cones. J. Neurosci. 2000, 20, 1030–1035. [Google Scholar] [PubMed]
- Bagnard, D.; Lohrum, M.; Uziel, D.; Puschel, A.W.; Bolz, J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 1998, 125, 5043–5053. [Google Scholar] [PubMed]
- Carballo-Molina, O.A.; Sanchez-Navarro, A.; Lopez-Ornelas, A.; Lara-Rodarte, R.; Salazar, P.; Campos-Romo, A.; Ramos-Mejia, V.; Velasco, I. Semaphorin 3C released from a biocompatible hydrogel guides and promotes axonal growth of rodent and human dopaminergic neurons. Tissue Eng. Part A 2016, 22, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Montiel, H.L.; Tamariz, E.; Sandoval-Minero, M.T.; Varela-Echavarria, A. Semaphorins 3A, 3C, and 3F in mesencephalic dopaminergic axon pathfinding. J. Comp. Neurol. 2008, 506, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Wiegreffe, C.; Simon, R.; Peschkes, K.; Kling, C.; Strehle, M.; Cheng, J.; Srivatsa, S.; Liu, P.; Jenkins, N.A.; Copeland, N.G.; et al. Bcl11a (Ctip1) controls migration of cortical projection neurons through regulation of Sema3c. Neuron 2015, 87, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Ruediger, T.; Zimmer, G.; Barchmann, S.; Castellani, V.; Bagnard, D.; Bolz, J. Integration of opposing semaphorin guidance cues in cortical axons. Cereb. Cortex 2013, 23, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Steup, A.; Lohrum, M.; Hamscho, N.; Savaskan, N.E.; Ninnemann, O.; Nitsch, R.; Fujisawa, H.; Puschel, A.W.; Skutella, T. Sema3C and netrin-1 differentially affect axon growth in the hippocampal formation. Mol. Cell. Neurosci. 2000, 15, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Sanyas, I.; Bozon, M.; Moret, F.; Castellani, V. Motoneuronal Sema3C is essential for setting stereotyped motor tract positioning in limb-derived chemotropic semaphorins. Development 2012, 139, 3633–3643. [Google Scholar] [CrossRef] [PubMed]
- Oschipok, L.W.; Teh, J.; McPhail, L.T.; Tetzlaff, W. Expression of semaphorin3C in axotomized rodent facial and rubrospinal neurons. Neurosci. Lett. 2008, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Arnold, S.; Heanue, T.; Kilambi, K.P.; Doan, B.; Kapoor, A.; Ling, A.Y.; Sosa, M.X.; Guy, M.; Burzynski, G.; et al. Functional loss of semaphorin 3C and/or semaphorin 3D and their epistatic interaction with ret are critical to Hirschsprung disease liability. Am. J. Hum. Genet. 2015, 96, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H.; Grum, F.; Strauch, U.; Capellino, S.; Bataille, F.; Bleich, A.; Falk, W.; Scholmerich, J.; Obermeier, F. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 2008, 57, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Feiner, L.; Webber, A.L.; Brown, C.B.; Lu, M.M.; Jia, L.; Feinstein, P.; Mombaerts, P.; Epstein, J.A.; Raper, J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001, 128, 3061–3070. [Google Scholar] [PubMed]
- Plein, A.; Calmont, A.; Fantin, A.; Denti, L.; Anderson, N.A.; Scambler, P.J.; Ruhrberg, C. Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J. Clin. Investig. 2015, 125, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Valdembri, D.; Regano, D.; Maione, F.; Giraudo, E.; Serini, G. Class 3 semaphorins in cardiovascular development. Cell Adh. Migr. 2016, 10, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Yoshida, J.; Sugimoto, T.; Yamamoto, M.; Makino, N.; Takamatsu, H.; Takegahara, N.; Suto, F.; Hori, M.; Fujisawa, H.; et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 2008, 321, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Yabuki, M.; Harada, K.; Hori, M.; Kikutani, H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 2004, 6, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Nishizawa, T.; Furutani, M.; Arai, S.; Yamamura, E.; Joo, K.; Takahashi, T.; Matsuoka, R.; Yamagishi, H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 13933–13938. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Shibata, S.; Miyagawa-Tomita, S.; Ong, S.G.; Takahashi, H.; Kume, T.; Okano, H.; Matsuoka, R.; Yamagishi, H. Regulation of Sema3c and the interaction between cardiac neural crest and second heart field during outflow tract development. Sci. Rep. 2017, 7, 6771. [Google Scholar] [CrossRef] [PubMed]
- Theveniau-Ruissy, M.; Perez-Pomares, J.M.; Parisot, P.; Baldini, A.; Miquerol, L.; Kelly, R.G. Coronary stem development in wild-type and Tbx1 null mouse hearts. Dev. Dyn. 2016, 245, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Ta-Shma, A.; Pierri, C.L.; Stepensky, P.; Shaag, A.; Zenvirt, S.; Elpeleg, O.; Rein, A.J. Isolated truncus arteriosus associated with a mutation in the plexin-D1 gene. Am. J. Med. Genet. A 2013, 161A, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Kagoshima, M.; Ito, T. Diverse gene expression and function of semaphorins in developing lung: Positive and negative regulatory roles of semaphorins in lung branching morphogenesis. Genes Cells 2001, 6, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Vadivel, A.; Alphonse, R.S.; Collins, J.J.; van Haaften, T.; O’Reilly, M.; Eaton, F.; Thebaud, B. The axonal guidance cue semaphorin 3C contributes to alveolar growth and repair. PLoS ONE 2013, 8, e67225. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Tufro, A. Semaphorins in kidney development and disease: Modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr. Nephrol. 2011, 26, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Banu, N.; Teichman, J.; Dunlap-Brown, M.; Villegas, G.; Tufro, A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006, 20, 2150–2152. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhu, K.; Liu, J.; Chen, J.; Tang, J.; Liang, Y.; Jin, R.; Liang, X.; Cai, X. The evaluative value of Sema3C and MFN2 co-expression detected by immunohistochemistry for prognosis in hepatocellular carcinoma patients after hepatectomy. Onco Targets Ther. 2016, 9, 3213–3221. [Google Scholar] [PubMed]
- Miyato, H.; Tsuno, N.H.; Kitayama, J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012, 103, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Z.; Guo, S.; Li, J.; Liu, S.; You, Y.; Ni, B.; Wang, H.; Bie, P. Increased semaphorin 3c expression promotes tumor growth and metastasis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathway. Cancer Lett. 2017, 397, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Evanno, E.; Godet, J.; Piccirilli, N.; Guilhot, J.; Milin, S.; Gombert, J.M.; Fouchaq, B.; Roche, J. Tri-methylation of H3K79 is decreased in TGF-beta1-induced epithelial-to-mesenchymal transition in lung cancer. Clin. Epigenet. 2017, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Cole-Healy, Z.; Vergani, P.; Hunter, K.; Brown, N.J.; Reed, M.W.; Staton, C.A. The relationship between semaphorin 3C and microvessel density in the progression of breast and oral neoplasia. Exp. Mol. Pathol. 2015, 99, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.J.; Dalal, K.; Hsing, M.; Cheng, C.W.; Khosravi, S.; Yenki, P.; Tse, C.; Peacock, J.W.; Sharma, A.; Chiang, Y.T.; et al. Androgen receptor transcriptionally regulates semaphorin 3C in a GATA2-dependent manner. Oncotarget 2017, 8, 9617–9633. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.J.; Hui, D.H.F.; Lee, W.W.; Dong, M.; Tombe, T.; Jiao, I.Z.F.; Khosravi, S.; Takeuchi, A.; Peacock, J.W.; Ivanova, L.; et al. Semaphorin 3 C drives epithelial-to-mesenchymal transition, invasiveness, and stem-like characteristics in prostate cells. Sci. Rep. 2017, 7, 11501. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.W.; Takeuchi, A.; Hayashi, N.; Liu, L.; Tam, K.J.; Al Nakouzi, N.; Khazamipour, N.; Tombe, T.; Dejima, T.; Lee, K.C.; et al. SEMA3C drives cancer growth by transactivating multiple receptor tyrosine kinases via Plexin B1. EMBO Mol. Med. 2018, 10, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.; Wick, W.; Weller, M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia 2003, 42, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Man, J.; Shoemake, J.; Zhou, W.; Fang, X.; Wu, Q.; Rizzo, A.; Prayson, R.; Bao, S.; Rich, J.N.; Yu, J.S. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014, 9, 1812–1826. [Google Scholar] [CrossRef] [PubMed]
- Vaitkiene, P.; Skiriute, D.; Steponaitis, G.; Skauminas, K.; Tamasauskas, A.; Kazlauskas, A. High level of Sema3C is associated with glioma malignancy. Diagn. Pathol. 2015, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Cheng, L.; Guryanova, O.A.; Wu, Q.; Bao, S. Cancer stem cells in glioblastoma—Molecular signaling and therapeutic targeting. Protein Cell 2010, 1, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Schonberg, D.L.; Lubelski, D.; Miller, T.E.; Rich, J.N. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol. Asp. Med. 2014, 39, 82–101. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, M.K.; Li, L.Y.; Lu, M.H.; Shao Ch, K.; Su, Z.L.; He, D.; Pang, J.; Gao, X. The predictive value of semaphorins 3 expression in biopsies for biochemical recurrence of patients with low- and intermediate-risk prostate cancer. Neoplasma 2013, 60, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.G.; Meadows, G.G. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int. J. Oncol. 2007, 30, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Q.; Li, G.; Zeng, X.; Kuang, S.; Li, X.; Yue, Y. TET1-mediated different transcriptional regulation in prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 203–211. [Google Scholar] [PubMed]
- Malik, M.F.; Satherley, L.K.; Davies, E.L.; Ye, L.; Jiang, W.G. Expression of semaphorin 3C in breast cancer and its impact on adhesion and invasion of breast cancer cells. Anticancer Res. 2016, 36, 1281–1286. [Google Scholar] [PubMed]
- Zhu, X.; Zhang, X.; Ye, Z.; Chen, Y.; Lv, L.; Hu, H. Silencing of semaphorin 3C suppresses cell proliferation and migration in MCF-7 breast cancer cells. Oncol. Lett. 2017, 14, 5913–5917. [Google Scholar] [CrossRef] [PubMed]
- Esselens, C.; Malapeira, J.; Colome, N.; Casal, C.; Rodriguez-Manzaneque, J.C.; Canals, F.; Arribas, J. The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J. Biol. Chem. 2010, 285, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Mumblat, Y.; Kessler, O.; Ilan, N.; Neufeld, G. Full-length semaphorin-3C is an inhibitor of tumor lymphangiogenesis and metastasis. Cancer Res. 2015, 75, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Delloye-Bourgeois, C.; Bertin, L.; Thoinet, K.; Jarrosson, L.; Kindbeiter, K.; Buffet, T.; Tauszig-Delamasure, S.; Bozon, M.; Marabelle, A.; Combaret, V.; et al. Microenvironment-driven shift of cohesion/detachment balance within tumors induces a switch toward metastasis in neuroblastoma. Cancer Cell 2017, 32, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Lu, M.M.; Epstein, J.A. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell 2004, 7, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Rodriguez, E.R.; Reimert, D.V.; Shu, T.; Fritzsch, B.; Richards, L.J.; Kolodkin, A.L.; Ginty, D.D. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 2003, 5, 45–57. [Google Scholar] [CrossRef]
- Yang, W.J.; Hu, J.; Uemura, A.; Tetzlaff, F.; Augustin, H.G.; Fischer, A. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol. Med. 2015, 7, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Toledano, S.; Lu, H.; Palacio, A.; Ziv, K.; Kessler, O.; Schaal, S.; Neufeld, G.; Barak, Y. A Sema3C mutant resistant to cleavage by furin (FR-Sema3C) inhibits choroidal neovascularization. PLoS ONE 2016, 11, e0168122. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.; Driscoll, P.C. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today 2013, 18, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Vander Kooi, C.W. Neuropilin functions as an essential cell surface receptor. J. Biol. Chem. 2015, 290, 29120–29126. [Google Scholar] [CrossRef] [PubMed]
- Nishide, M.; Kumanogoh, A. The role of semaphorins in immune responses and autoimmune rheumatic diseases. Nat. Rev. Rheum. 2018, 14, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Endo, R.; Gotoh, M.; Hirohashi, S. Identification of semaphorin E as a non-MDR drug resistance gene of human cancers. Proc. Natl. Acad. Sci. USA 1997, 94, 14713–14718. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E. Neuropilin 1: Function and therapeutic potential in cancer. Cancer Immunol. Immunother. 2014, 63, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Fritz, J.; Pierdant-Mancera, M.; Bagnard, D. Current drug design to target the Semaphorin/Neuropilin/Plexin complexes. Cell Adh. Migr. 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.F.; Ye, L.; Jiang, W.G. The Plexin-B family and its role in cancer progression. Histol. Histopathol. 2014, 29, 151–165. [Google Scholar] [PubMed]
- Sun, S.; Lei, Y.; Li, Q.; Wu, Y.; Zhang, L.; Mu, P.P.; Ji, G.Q.; Tang, C.X.; Wang, Y.Q.; Gao, J.; et al. Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma. Oncotarget 2017, 8, 74019–74035. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, T.; Lu, X.; Zhou, H.; Huang, Y. RNA interference targeting NRP-1 inhibits human glioma cell proliferation and enhances cell apoptosis. Mol. Med. Rep. 2011, 4, 1261–1266. [Google Scholar] [PubMed]
- Castellani, V.; Chedotal, A.; Schachner, M.; Faivre-Sarrailh, C.; Rougon, G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 2000, 27, 237–249. [Google Scholar] [CrossRef]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J., Jr.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF-VEGFR2-neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Rabinowicz, N.; Rainero, E.; Lanzetti, L.; Serini, G.; Norman, J.; Neufeld, G.; Tamagnone, L. Neuropilin-1-dependent regulation of EGF-receptor signaling. Cancer Res. 2012, 72, 5801–5811. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, A.; Hirota-Komatsu, S.; Araki, M.; Komatsu, N. Platelet-derived growth factor receptors form complexes with neuropilin-1 during megakaryocytic differentiation of thrombopoietin-dependent UT-7/TPO cells. Biochem. Biophys. Res. Commun. 2015, 459, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Stoilova, S.; Mohammed, N.; Prud’homme, G.J. Neuropilin-1 exerts co-receptor function for TGF-beta-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-beta. Carcinogenesis 2011, 32, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Ben-Gigi, L.; Klein, H.; Behar, O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J. Neurosci. 2007, 27, 13000–13011. [Google Scholar] [CrossRef] [PubMed]
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745. [Google Scholar] [CrossRef]
- Kwiatkowski, S.C.; Guerrero, P.A.; Hirota, S.; Chen, Z.; Morales, J.E.; Aghi, M.; McCarty, J.H. Neuropilin-1 modulates TGFbeta signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS ONE 2017, 12, e0185065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, E.; Ryu, S.W.; Choi, C.; Choi, K. Combined inhibition of vascular endothelial growth factor receptor signaling with temozolomide enhances cytotoxicity against human glioblastoma cells via downregulation of Neuropilin-1. J. Neurooncol. 2016, 128, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, P.; Blank, A.E.; Franz, K.; Hattingen, E.; Dunst, M.; Zeiner, P.; Hoffmann, K.; Bahr, O.; Mader, L.; Goeppert, B.; et al. Differential expression of vascular endothelial growth factor A, its receptors VEGFR-1, -2, and -3 and co-receptors neuropilin-1 and -2 does not predict bevacizumab response in human astrocytomas. Neuro. Oncol. 2016, 18, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hou, C.; Hou, A.; Zhu, D. Concurrent expression of VEGF-C and neuropilin-2 is correlated with poor prognosis in glioblastoma. Tohoku J. Exp. Med. 2016, 238, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Roodink, I.; Verrijp, K.; Raats, J.; Leenders, W.P. Plexin D1 is ubiquitously expressed on tumor vessels and tumor cells in solid malignancies. BMC Cancer 2009, 9, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.; Wu, Q.; Sathornsumetee, S.; Hao, Y.; Li, Z.; Hjelmeland, A.B.; Shi, Q.; McLendon, R.E.; Bigner, D.D.; Rich, J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006, 66, 7843–7848. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Reynaud, D.; Kang, Y.A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Gottgens, B.; Passegue, E. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 2015, 17, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Lacal, P.M. Neuropilin-1 as therapeutic target for malignant melanoma. Front. Oncol. 2015, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpel, A.; Gamper, C.; Spenle, C.; Fernandez, A.; Jacob, L.; Baumlin, N.; Laquerriere, P.; Orend, G.; Cremel, G.; Bagnard, D. Inhibition of primary breast tumor growth and metastasis using a neuropilin-1 transmembrane domain interfering peptide. Oncotarget 2016, 7, 54723–54732. [Google Scholar] [CrossRef] [PubMed]
- Nasarre, C.; Roth, M.; Jacob, L.; Roth, L.; Koncina, E.; Thien, A.; Labourdette, G.; Poulet, P.; Hubert, P.; Cremel, G.; et al. Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene 2010, 29, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Iwanami, A.; Nakamura, M.; Kishino, A.; Kikuchi, K.; Shibata, S.; Okano, H.J.; Ikegami, T.; Moriya, A.; Konishi, O.; et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006, 12, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Omoto, M.; Yoshida, S.; Miyashita, H.; Kawakita, T.; Yoshida, K.; Kishino, A.; Kimura, T.; Shibata, S.; Tsubota, K.; Okano, H.; et al. The semaphorin 3A inhibitor SM-345431 accelerates peripheral nerve regeneration and sensitivity in a murine corneal transplantation model. PLoS ONE 2012, 7, e47716. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Kishino, A.; Konishi, O.; Kumagai, K.; Hosotani, N.; Saji, I.; Nakayama, C.; Kimura, T. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J. Biol. Chem. 2003, 278, 42985–42991. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, A.; Eliasen, A.M.; Chin, M.R.; Zlotkowski, K.; Siegel, D. Syntheses of xanthofulvin and vinaxanthone, natural products enabling spinal cord regeneration. Angew. Chem. Int. Ed. 2013, 52, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.R.; Zlotkowski, K.; Han, M.; Patel, S.; Eliasen, A.M.; Axelrod, A.; Siegel, D. Expedited access to vinaxanthone and chemically edited derivatives possessing neuronal regenerative effects through ynone coupling reactions. ACS Chem. Neurosci. 2015, 6, 542–550. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Yu, J.S. Semaphorin 3C and Its Receptors in Cancer and Cancer Stem-Like Cells. Biomedicines 2018, 6, 42. https://doi.org/10.3390/biomedicines6020042
Hao J, Yu JS. Semaphorin 3C and Its Receptors in Cancer and Cancer Stem-Like Cells. Biomedicines. 2018; 6(2):42. https://doi.org/10.3390/biomedicines6020042
Chicago/Turabian StyleHao, Jing, and Jennifer S. Yu. 2018. "Semaphorin 3C and Its Receptors in Cancer and Cancer Stem-Like Cells" Biomedicines 6, no. 2: 42. https://doi.org/10.3390/biomedicines6020042