Stereotactic Body Radiation Therapy in the Management of Upper GI Malignancies
Abstract
:1. Introduction
2. SBRT for Pancreatic Cancer
2.1. The Neoadjuvant Setting
2.2. The Definitive Setting
2.3. The Adjuvant Setting
3. SBRT for Hepatocellular Cancer
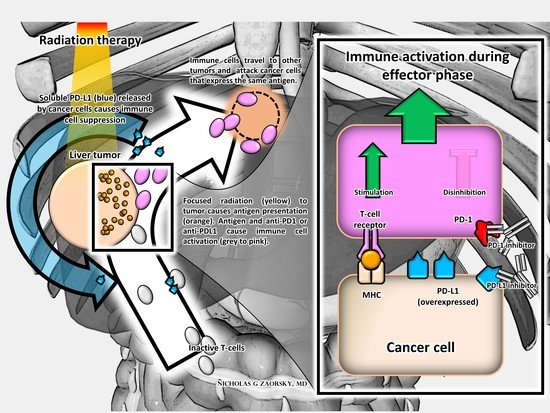
4. SBRT and Immunomodulatory Biomarkers in Upper GI Malignancies and Therapeutic Implications
5. Conclusions and Future Directions
Conflicts of Interest
References
- Rosati, L.M.; Kumar, R.; Herman, J.M. Integration of Stereotactic Body Radiation Therapy into the Multidisciplinary Management of Pancreatic Cancer. Semin. Radiat. Oncol. 2017, 27, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Bornschlegl, S.; Park, S.S.; Gastineau, D.A.; Roberts, L.R.; Dietz, A.B.; Hallemeier, C.L. Comprehensive assessment of circulating immune cell populations in response to stereotactic body radiation therapy in patients with liver cancer. Adv. Radiat. Oncol. 2017, 2, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, C.L.; Herman, J.M.; Laheru, D.A.; Klein, A.P.; Erdek, M.A.; Fishman, E.K.; Hruban, R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013, 63, 318–348. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Baine, M.J.; Souchek, J.J.; Menning, M.; Kaur, S.; Yan, Y.; Ouellette, M.M.; Jain, M.; Lin, C.; Batra, S.K. Biological determinants of radioresistance and their remediation in pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P. Focal Radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int. J. Radiat. Oncol. 2016, 94, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-S.; Liang, P.; Lu, H.-Z.; Liang, J.-N.; Liu, J.-M.; Zhou, Y.; Gao, Y.-C.; Tang, M.-Y. Stereotactic body radiotherapy using CyberKnife for locally advanced unresectable and metastatic pancreatic cancer. World J. Gastroenterol. 2015, 21, 8156. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yuan, Z.; Li, F.; Dong, Y.; Zhuang, H.; Wang, J.; Chen, H.; Wang, P. Analysis of clinical efficacy of CyberKnife® treatment for locally advanced pancreatic cancer. Onco Targets Ther. 2015, 8, 1427. [Google Scholar] [CrossRef] [PubMed]
- Moningi, S.; Dholakia, A.S.; Raman, S.P.; Blackford, A.; Cameron, J.L.; Le, D.T.; De Jesus-Acosta, A.M.C.; Hacker-Prietz, A.; Rosati, L.M.; Assadi, R.K.; et al. The role of stereotactic body radiation therapy for pancreatic cancer: A single-institution experience. Ann. Surg. Oncol. 2015, 22, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, J.-C.M.; Parikh, S.D.; Heron, D.E.; Howell, M.; Zeh, H.; Moser, A.J.; Bahary, N.; Quinn, A.; Burton, S.A. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am. J. Clin. Oncol. 2011, 34, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, M.S.; Heron, D.E.; Wegner, R.E.; Zeh, H.J.; Bahary, N.; Krasinskas, A.M.; Lembersky, B.; Brand, R.; Moser, A.J.; Quinn, A.E.; et al. Pathologic response with neoadjuvant chemotherapy and stereotactic body radiotherapy for borderline resectable and locally-advanced pancreatic cancer. Radiat. Oncol. 2013, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Polistina, F.; Costantin, G.; Casamassima, F.; Francescon, P.; Guglielmi, R.; Panizzoni, G.; Febbraro, A.; Ambrosino, G. Unresectable Locally Advanced Pancreatic Cancer: A Multimodal Treatment Using Neoadjuvant Chemoradiotherapy (Gemcitabine Plus Stereotactic Radiosurgery) and Subsequent Surgical Exploration. Ann. Surg. Oncol. 2010, 17, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Miksad, R.; Goldstein, M.; Sullivan, R.; Bullock, A.; Buchbinder, E.; Pleskow, D.; Sawhney, M.; Kent, T.; Vollmer, C.; et al. Induction Gemcitabine and Stereotactic Body Radiotherapy for Locally Advanced Nonmetastatic Pancreas Cancer. Int. J. Radiat. Oncol. 2011, 81, e615–e622. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-C.; Jen, Y.-M.; Li, M.-H.; Chao, H.-L.; Tsai, J.-T. Comparing outcomes of stereotactic body radiotherapy with intensity-modulated radiotherapy for patients with locally advanced unresectable pancreatic cancer. Eur. J. Gastroenterol. Hepatol. 2015, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Ling, D.C.; Wegner, R.E.; Flickinger, J.C.; Heron, D.E.; Zeh, H.; Moser, A.J.; Burton, S.A. Stereotactic body radiotherapy in the treatment of Pancreatic Adenocarcinoma in elderly patients. Radiat. Oncol. 2013, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Roed, H.; Sengelov, L.; Traberg, A.; Ohlhuis, L.; Pedersen, J.; Nellemann, H.; Berthelsen, A.K.; Eberholst, F.; Engelholm, S.A.; et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother. Oncol. 2005, 76, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Einstein, D.; Ibarra, R.A.; Yao, M.; Kunos, C.; Ellis, R.; Brindle, J.; Singh, D.; Hardacre, J.; Zhang, Y.; et al. Stereotactic Body Radiation Therapy for Nonresectable Tumors of the Pancreas. J. Surg. Res. 2012, 174, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Didolkar, M.S.; Coleman, C.W.; Brenner, M.J.; Chu, K.U.; Olexa, N.; Stanwyck, E.; Yu, A.; Neerchal, N.; Rabinowitz, S. Image-Guided Stereotactic Radiosurgery for Locally Advanced Pancreatic Adenocarcinoma Results of First 85 Patients. J. Gastrointest. Surg. 2010, 14, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Steve, J.; Krasinskas, A.M.; Zureikat, A.H.; Lembersky, B.C.; Gibson, M.K.; Stoller, R.G.; Zeh, H.J.; Bahary, N. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J. Surg. Oncol. 2013, 108, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Comito, T.; Ghidini, A.; Torri, V.; Scorsetti, M.; Barni, S. Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int. J. Radiat. Oncol. 2017, 97, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S.; et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A.; et al. Single-Fraction Stereotactic Body Radiation Therapy and Sequential Gemcitabine for the Treatment of Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. 2011, 81, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Koong, A.C.; Le, Q.T.; Ho, A.; Fong, B.; Fisher, G.; Cho, C.; Ford, J.; Poen, J.; Gibbs, I.C.; Mehta, V.K.; et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. 2004, 58, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.T.; Schellenberg, D.; Shen, J.; Kim, J.; Goodman, K.A.; Fisher, G.A.; Ford, J.M.; Desser, T.; Quon, A.; Koong, A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009, 115, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Jain, S.; Goldstein, M.; Miksad, R.; Pleskow, D.; Sawhney, M.; Brennan, D.; Callery, M.; Vollmer, C. Stereotactic Body Radiotherapy and Gemcitabine for Locally Advanced Pancreatic Cancer. Int. J. Radiat. Oncol. 2010, 78, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.; Comito, T.; Alongi, F.; Navarria, P.; Iftode, C.; Mancosu, P.; Reggiori, G.; Clerici, E.; Rimassa, L.; Zerbi, A.; et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat. Oncol. 2013, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.K.; Collins, S.P.; Slack, R.; Tse, G.; Charabaty, A.; Ley, L.; Berzcel, L.; Lei, S.; Suy, S.; Haddad, N.; et al. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat. Oncol. 2013, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Dunn, J.A.; Almond, J.; Beger, H.G.; Pederzoli, P.; Bassi, C.; Dervenis, C.; Fernandez-Cruz, L.; Lacaine, F.; et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann. Surg. 2001, 234, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, T.J.; Seo, Y.; Willis, J.; Stellato, T.A.; Siegel, C.T.; Harpp, D.; Willson, J.K.; Gibbons, J.; Sanabria, J.R.; Hardacre, J.M.; et al. The Impact of Resection Margin Status and Postoperative CA19-9 Levels on Survival and Patterns of Recurrence after Postoperative High-Dose Radiotherapy With 5-FU—Based Concurrent Chemotherapy for Resectable Pancreatic Cancer. Am. J. Clin. Oncol. 2008, 31, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Johns, A.L.; Merrett, N.D.; Gill, A.J.; Colvin, E.K.; Scarlett, C.J.; Nguyen, N.Q.; Leong, R.W.L.; Cosman, P.H.; Kelly, M.I.; et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J. Clin. Oncol. 2009, 27, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.; Smith, R.A.; Whelan, P.; Sutton, R.; Raraty, M.; Neoptolemos, J.P.; Ghaneh, P. Classification of R1 resections for pancreatic cancer: The prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology 2009, 55, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mellon, E.A.; Hoffe, S.E.; Springett, G.M.; Frakes, J.M.; Strom, T.J.; Hodul, P.J.; Malafa, M.P.; Chuong, M.D.; Shridhar, R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. (Madr) 2015, 54, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Huguet, F.; van Laethem, J.-L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs. Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled after 4 Months of Gemcitabine with or without Erlotinib. JAMA 2016, 315, 1844. [Google Scholar] [CrossRef] [PubMed]
- Klinkenbijl, J.H.; Jeekel, J.; Sahmoud, T.; van Pel, R.; Couvreur, M.L.; Veenhof, C.H.; Arnaud, J.P.; Gonzalez, D.G.; de Wit, L.T.; Hennipman, A.; et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 1999, 230, 776. [Google Scholar] [CrossRef] [PubMed]
- Kalser, M.H.; Ellenberg, S.S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. 1985, 120, 899–903. [Google Scholar] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet (Lond. Engl.) 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Regine, W.F.; Winter, K.A.; Abrams, R.A.; Safran, H.; Hoffman, J.P.; Konski, A.; Benson, A.B.; Macdonald, J.S.; Kudrimoti, M.R.; Fromm, M.L.; et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA 2008, 299, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Palmer, D.; Jackson, R.; Cox, T.; Neoptolemos, J.P.; Ghaneh, P.; Rawcliffe, C.L.; Bassi, C.; Stocken, D.D.; Cunningham, D.; et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 2014, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Hamm, J.; Dancey, J.; Eisenberg, P.D.; Dagenais, M.; Fields, A.; Hagan, K.; Greenberg, B.; Colwell, B.; Zee, B.; et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2003, 21, 3296–3302. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Furuse, J.; Boku, N.; Okusaka, T.; Ikeda, M.; Ohkawa, S.; Fukutomi, A.; Hamamoto, Y.; Nakamura, K.; Fukuda, H.; et al. Phase II study of gemcitabine chemotherapy alone for locally advanced pancreatic carcinoma: JCOG0506. Jpn. J. Clin. Oncol. 2010, 40, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence Rates. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Comito, T.; Cozzi, L.; Clerici, E.; Tozzi, A.; Franzese, C.; Navarria, P.; Fogliata, A.; Tomatis, S.; D’Agostino, G.; et al. The challenge of inoperable hepatocellular carcinoma (HCC): Results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J. Cancer Res. Clin. Oncol. 2015, 141, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Lasley, F.D.; Mannina, E.M.; Johnson, C.S.; Perkins, S.M.; Althouse, S.; Maluccio, M.; Kwo, P.; Cardenes, H. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract. Radiat. Oncol. 2015, 5, e443–e449. [Google Scholar] [CrossRef] [PubMed]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.S.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-K.; Kim, M.-S.; Cho, C.K.; Yang, K.M.; Yoo, H.J.; Kim, J.H.; Bae, S.H.; Jung, D.H.; Kim, K.B.; Lee, D.H.; et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer 2012, 118, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Méndez Romero, A.; Wunderink, W.; Hussain, S.M.; de Pooter, J.A.; Heijmen, B.J.M.; Nowak, P.C.J.M.; Nuyttens, J.J.; Brandwijk, R.P.; Verhoef, C.; Ijzermans, J.N.M.; et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. (Madr) 2006, 45, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.; Turley, F.; Redden, D.T.; Saddekni, S.; Aal, A.K.A.; Keene, K.; Yang, E.; Zarzour, J.; Bolus, D.; Smith, J.K.; et al. Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of ≥3 cm. HPB 2015, 17, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Dawson, L.A. Hepatocellular Carcinoma Radiation Therapy: Review of Evidence and Future Opportunities. Int. J. Radiat. Oncol. 2013, 87, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Popp, I.; Grosu, A.L.; Niedermann, G.; Duda, D.G. Immune modulation by hypofractionated stereotactic radiation therapy: Therapeutic implications. Radiother. Oncol. 2016, 120, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Combining Radiotherapy and Cancer Immunotherapy: A Paradigm Shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Demaria, S.; Formenti, S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J. Immunother. Cancer 2016, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Order, S.E. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer 1977, 39, 737–743. [Google Scholar] [CrossRef]
- Uh, S.; Lee, S.M.; Kim, H.T.; Chung, Y.; Kim, Y.H.; Park, C.; Huh, S.J.; Lee, H.B. The effect of radiation therapy on immune function in patients with squamous cell lung carcinoma. Chest 1994, 105, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Tran, P.T.; Lim, M.; Drake, C.G.; Deweese, T.L. Stereotactic radiation therapy combined with immunotherapy: Augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park) 2015, 29, 331–340. [Google Scholar] [PubMed]
- Kim, H.J.; Park, S.; Kim, K.J.; Seong, J. The Clinical Implications of Soluble PD-L1 in Hepatocellular Carcinoma Patients Treated With Radiation Therapy. Int. J. Radiat. Oncol. 2017, 99, S89. [Google Scholar] [CrossRef]
- Patel, K.R.; Shoukat, S.; Oliver, D.E.; Chowdhary, M.; Rizzo, M.; Lawson, D.H.; Khosa, F.; Liu, Y.; Khan, M.K. Ipilimumab and Stereotactic Radiosurgery vs. Stereotactic Radiosurgery Alone for Newly Diagnosed Melanoma Brain Metastases. Am. J. Clin. Oncol. 2017, 40, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic Radiosurgery for Melanoma Brain Metastases in Patients Receiving Ipilimumab: Safety Profile and Efficacy of Combined Treatment. Int. J. Radiat. Oncol. 2015, 92, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Narang, A.; Thompson, E.; Anders, R.; Waters, K.; Poling, J.; Rosati, L.M.; Huang, C.Y.; Tran, P.T.; Herman, J.M.; et al. Characterizing Tumor Infiltrating Lymphocytes Following Neoadjuvant Chemotherapy and Radiation in Pancreatic Adenocarcinoma. Int. J. Radiat. Oncol. 2017, 99, S91–S92. [Google Scholar] [CrossRef]
- Foley, K.; Kim, V.; Jaffee, E.; Zheng, L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016, 381, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchelebi, L.; Zaorsky, N.; Mackley, H. Stereotactic Body Radiation Therapy in the Management of Upper GI Malignancies. Biomedicines 2018, 6, 7. https://doi.org/10.3390/biomedicines6010007
Tchelebi L, Zaorsky N, Mackley H. Stereotactic Body Radiation Therapy in the Management of Upper GI Malignancies. Biomedicines. 2018; 6(1):7. https://doi.org/10.3390/biomedicines6010007
Chicago/Turabian StyleTchelebi, Leila, Nicholas Zaorsky, and Heath Mackley. 2018. "Stereotactic Body Radiation Therapy in the Management of Upper GI Malignancies" Biomedicines 6, no. 1: 7. https://doi.org/10.3390/biomedicines6010007