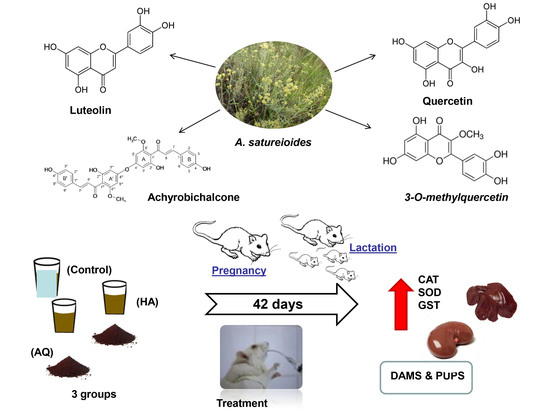
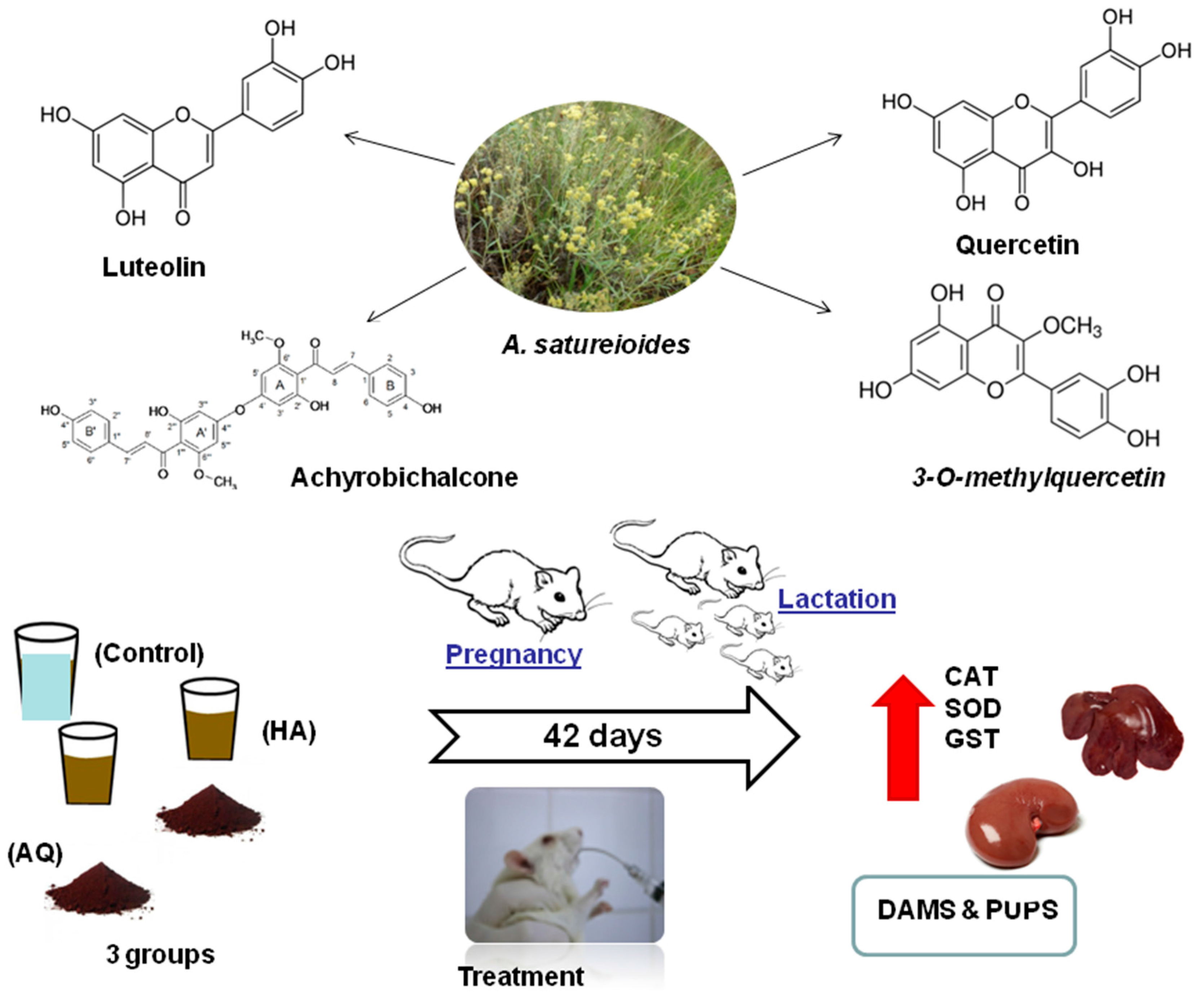
Supplementation with Achyrocline satureioides Inflorescence Extracts to Pregnant and Breastfeeding Rats Induces Tissue-Specific Changes in Enzymatic Activity and Lower Neonatal Survival
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material
2.2. Chemicals
2.3. Preparation of AS Extracts
2.4. Flavonoid Content Determination
2.5. Animal Model and Experimental Design
2.6. Antioxidant Enzymes and Glutathione S-Transferase (GST)
2.7. Oxidative Damage Markers
2.8. Statistical Analysis
3. Results
3.1. AS Extract Composition
3.2. Reproductive, Maternal, and Litter Data in Pups
3.3. Maternal Oxidative Parameters
3.4. Pups’ Oxidative Parameters
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, A.C.F.; Folmer, V.; Rosa, H.D.; Costa, M.T.; Boligon, A.A.; Fávero, R.P.; Roos, D.H.; Puntel, G.P. In vitro and in silico antioxidant and toxicological activities of Achyrocline satureioides. J. Ethnopharmacol. 2016, 194, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Retta, D.; Dellacassa, E.; Villamil, J.; Suárez, S.; Bandoni, A.L. Marcela, a promising medicinal and aromatic plant from Latin America: A review. Ind. Crops Prod. 2012, 38, 27–38. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Schenkel, E.P.; Bauer, L.; Langeloh, A. Pharmacological investigations on Achyrocline satureioides (Lam.) DC., compositae. J. Ethnopharmacol. 1988, 22, 281–293. [Google Scholar]
- Arredondo, M.F.; Blasina, F.; Echeverry, C.; Morquio, A.; Ferreira, M.; Abin-Carriquiry, J.A.; Lafon, L.; Dajas, F. Cytoprotection by Achyrocline satureioides (Lam) D.C. and some of its main flavonoids against oxidative stress. J. Ethnopharmacol. 2004, 91, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Bombelli, R.; Carcano, E.; Luini, A.; Marino, F.; Crema, F.; Dajas, F.; Lecchini, S. Immunomodulatory properties of Achyrocline satureioides (Lam.) D.C. infusion: A study on human leukocytes. J. Ethnopharmacol. 2008, 116, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Morquio, A.; Rivera-Megret, F.; Dajas, F. Photoprotection by topical application of Achyrocline satureioides (‘Marcela’). Phytother. Res. 2005, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Sabini, M.C.; Cariddi, L.N.; Escobar, F.M.; Mañas, F.; Comini, L.; Reinoso, E.; Sutil, S.B.; Acosta, A.C.; Núñez Montoya, S.; Contigiani, M.S.; et al. Evaluation of the cytotoxicity, genotoxicity and apoptotic induction of an aqueous extract of Achyrocline satureioides (Lam.) DC. Food Chem. Toxicol. 2013, 60, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Carini, J.P.; Klamt, F.; Bassani, V.L. Flavonoids from Achyrocline. Satureioides: Promising biomolecules for anticancer therapy. RSC Adv. 2013, 4, 3131–3144. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and other reactive species in disease. eLS 2005, 2005. [Google Scholar] [CrossRef]
- Barenys, M.; Masjosthusmann, S.; Fritsche, E. Is intake of flavonoid-based food supplements during pregnancy safe for the developing child–A literature review. Curr. Drug Targets 2017, 18, 196–231. [Google Scholar] [CrossRef] [PubMed]
- Lindzon, G.; Sadry, S.; Sharp, J. Toronto Notes for Medical Students, 27th ed.; Type & Graphics Inc.: North York, ON, Canada, 2011. [Google Scholar]
- John, L.J.; Shantakumari, N. Herbal medicines use during pregnancy: A review from the middle east. Oman Med. J. 2015, 30, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar]
- Baby, B.; Antony, P.; Halabi, W.; Homedi, Z.; Vijayan, R. Structural insights into the polypharmacological activity of quercetin on serine/threonine kinases. Drug Des. Dev. Ther. 2016, 10, 3109–3123. [Google Scholar]
- Castelli, S.; Coletta, A.; D’Annessa, I.; Fiorani, P.; Tesauro, C.; Desideri, A. Interaction between natural compounds and human topoisomerase I. Biol. Chem. 2012, 393, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Prado, I.M.; Helena, A.F.; Uyemura, S.A.; Santos, A.C.; Curti, C. The interaction of flavonoids with mitochondria: Effects on energetic processes. Chem.-Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Breimer, L.H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: The role of DNA base damage. Mol. Carcinog. 1990, 3, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Baierle, M.; Charão, M.F.; Bubols, G.B.; Gravina, F.S.; Zielinsky, P.; Arbo, M.D.; Cristina Garcia, S. Polyphenol-rich food general and on pregnancy effects: A review. Drug Chem. Toxicol. 2016, 545, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeidan-Chulian, F.; Gelain, D.P.; Kolling, E.A.; Rybarczyk-Filho, J.L.; Ambrosi, P.; Resende Terra, S.; Pires, A.S.; Da Rocha, J.B.T.; Behr, G.A.; Fonseca Moreira, J.C. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxid. Med. Cell Longev. 2013, 152, 67–78. [Google Scholar] [CrossRef]
- Schrö Der-Van Der Elst, J.P.; van Der Heide, D.; Rokos, H.; Morreale De Escobar, G.; Kö Hrle, J. Synthetic flavonoids cross the placenta in the rat and are found in fetal brain. APS 1998, 49209, 253–256. [Google Scholar]
- Vanhees, K.; Godschalk, R.W.; Sanders, A.; van Waalwijk van Doorn-Khosrovani, S.B.; van Schooten, F.J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 2011, 290, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Carini, J.P.; Leitão, G.G.; Schneider, P.H.; Santos, C.C.; Costa, F.N.; Holzschuh, M.H.; Klamt, F.; Bassani, V.L. Isolation of achyrobichalcone from Achyrocline satureioides by high-speed countercurrent chromatography. Curr. Pharm. Biotechnol. 2015, 16, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bidone, J.; Bica, V.C.; Petrovick, P.R.; Simões, C.M.O.; Koester, L.S.; Bassani, V.L.; Teixeira, H.F. Simultaneous quantification of flavonoids from Achyrocline satureioides by a polar-reversed phase lc method-application to skin permeation/retention studies. Pharmazie 2014, 69, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, K.; Tanno, A. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H.; Cramer, W.A.; Vonjagow, G. The folin by oliver. Anal. Biochem. 1994, 217, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase the role of superoxide anion in the epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Mannervik, B.; Alin, P.; Guthenberg, C.; Jensson, H.; Tahir, M.K.; Warholm, M.; Jörnvall, H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: Correlation between structural data and enzymatic properties. Proc. Natl. Acad. Sci. USA 1985, 82, 7202–7206. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, H.B. Assays for differentiation of glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Dankovic, D.A.; Naumann, B.D.; Maier, A.; Dourson, M.L.; Levy, L.S. The scientific basis of uncertainty factors used in setting occupational exposure limits. J. Occup. Environ. Hyg. 2015, 12, S55–S68. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Williamson, G. Biomarkers for exposure to dietary flavonoids: A review of the current evidence for identification of quercetin glycosides in plasma. Br. J. Nutr. 2001, 86, S105–S110. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, A.L.; Herring, B.J.; Lockard, J.E.; Miller, B.M.; Kim, H.; Hood, R.D.; Bailey, M.M. Exposure to green tea extract alters the incidence of specific cyclophosphamide-induced malformations. Birth Defects Res. 2012, 95, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, C.E.; Morrone, M.D.S.; Weber, M.H.; Lorenzi, R.; Behr, G.A.; Moreira, J.C.F. The effects of vitamin A supplementation to rats during gestation and lactation upon redox parameters: Increased oxidative stress and redox modulation in mothers and their offspring. Food Chem. Toxicol. 2011, 49, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [PubMed]
- Sahu, S.C.; Gray, G.C. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996, 104, 193–196. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen toxicity: A radical explanation. J. Exp. Biol. 1098, 201, 1203–1209. [Google Scholar] [CrossRef]
- Bannister, J.V.; Calabarese, L. Assays for SOD. Methods Biochem. 1987, 32, 279–312. [Google Scholar]
- Kadarian, C.; Broussalis, A.M.; Miño, J.; Lopez, P.; Gorzalczany, S.; Ferraro, G.; Acevedo, C. Hepatoprotective activity of Achyrocline satureioides (Lam) D.C. Pharmacol. Res. 2002, 45, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, B.J. Introduction to the special section on new ideas about cerebellar function. Behav. Neurosci. 2016, 130, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.S.; Noonan, M.P.; Boorman, E.D.; Walton, M.E.; Behrens, T.E. Frontal cortex and reward-guided learning and decision-making. Neuron 2011, 70, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Frank, L.M. New experiences enhance coordinated neural activity in the hippocampus. Neuron 2008, 57, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Morgane, P.J.; Austinlafrance, E.; Bronzino, J.; Tonkiss, J.; Diazcintra, S.; Cintra, L.; Kemper, T.; Galler, J.R. Prenatal malnutrition and development of the brain. Neurosci. Biobehav. Rev. 1993, 17, 91–128. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Coussio, J.; Ciccia, G. Antioxidant and free radical scavenging effects in extracts of the medicinal herb Achyrocline satureioides (Lam.) DC. (“marcela”). Braz. J. Med. Biol. Res. 1998, 31, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; de Bock, L.; Godschalk, R.W.L.; van Schooten, F.J.; van Waalwijk van Doorn-Khosrovani, S.B. Prenatal exposure to flavonoids: Implication for cancer risk. Toxicol. Sci. 2011, 120, 59–67. [Google Scholar] [CrossRef] [PubMed]

| Samples | Quercetin (g/100 g Extract) | 3-O-methylquercetin (g/100 g Extract) | Luteolin (g/100 g Extract) | Achyrobichalcone (g/100 g Extract) | Total Flavonoid (g/100 g Extract) |
|---|---|---|---|---|---|
| Freeze-dried hydroalcoholic | 2.77 ± 0.6 b | 6.23 ± 0.5 a | 1.80 ± 0.01 a | 1.60 ± 0.8 a | 12.4 a |
| Aqueous extract | 1.68 ± 0.3 b | 2.70 ± 0.1 a | 0.50 ± 0.02 b | 0.60 ± 0.2 b | 5.6 b |
| Achyrocline Satureioides (mg·kg−1·day−1) | |||
|---|---|---|---|
| Reproductive Parameters | Control (Water) | Aqueous (AQ) Extract (47) | Hydroalcoholic (HA) Extract (35) |
| Gestation weight gain (%) | 15.8 ± 5.1 | 13.8 ± 7.1 | 14.8 ± 4.4 |
| Lactation weight gain (%) | 13.8 ± 6.1 | 11.5 ± 2.1 | 11.8 ± 2.4 |
| Gestation length (days) | 21 ± 1 | 23 ± 1 | 22 ± 1 |
| No. of implantations | 10 ± 4.1 | 8.5 ± 5.1 | 11.8 ± 2.4 |
| Delivery index (%) | 98 ± 5.1 | 73 ± 6.1 | 80 ± 5.5 |
| Days before eye opening | 13 ± 1 | 10 ± 2 | 9 ± 1* |
| No. of pups | 24 ± 1 | 11 ± 6.1 * | 20 ± 2.1 *,# |
| Viability index (%) | |||
| Day 0 | 98.3 ± 1.6 | 70.3 ± 1.3 * | 90.4 ± 3.6 |
| Day 7 | 99.3 ± 2.1 | 88.2 ± 2.3 * | 98.4 ± 2.9 |
| Day 14 | 88.3 ± 3.1 | 85.3 ± 1.3 | 87.4 ± 3.3 |
| Day 21 | 98.5 ± 1.6 | 97.3 ± 5.3 | 90.4 ± 5.6 |
| Day 42 | 98.3 ± 5.6 | 96.3 ± 3.5 | 98.4 ± 3.7 |
| Pup weight (g) | |||
| Day 5 | 9.8 ± 1.1 | 8.2 ± 1.3 | 8.4 ± 1.9 |
| Day 14 | 18.3 ± 2.2 | 15.3 ± 1.0 | 17.4 ± 2.4 |
| Day 21 | 30.5 ± 5.2 | 29.3 ± 2.3 | 30.4 ± 2.6 |
| Day 42 | 48.3 ± 3.1 | 44.3 ± 2.3 | 42.4 ± 3.7 |
| Achyrocline Satureioides (mg·kg−1·day−1) | |||
|---|---|---|---|
| Biochemical Parameters | Control (Water) | AQ (47) | HA (35) |
| Liver | |||
| TBARS level (nmol MDA/mg protein) | 7.21 ± 1.4 | 7.54 ± 1.1 | 6.11 ± 1.1 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.52 ± 0.5 | 1.66 ± 0.5 | 1.71 ± 0.3 |
| Total thiol content (mmol SH/mg protein) | 52.6 ± 5.2 | 53.61 ± 2.8 | 55.51 ± 5.2 |
| TRAP (under curve area) | 354.3 ± 18.5 | 357.76 ± 61.1 | 338.7 ± 27.7 |
| GST activity (U GST/mg protein) | 0.217 ± 0.5 | 0.261 ± 0.3 * | 0.278 ± 0.2 * |
| CAT activity (U CAT/mg protein) | 42.6 ± 1.8 | 47.62 ± 2.5 * | 49.79 ± 1.7 * |
| SOD activity (U SOD/mg protein) | 32.76 ± 9.4 | 38.32 ± 1.5 * | 39.09 ± 2.5 * |
| SOD/CAT ratio (arbitrary units) | 0.76 ± 0.3 | 0.80 ± 0.5 * | 0.78 ± 0.9 * |
| Kidney | |||
| TBARS level (nmol MDA/mg protein) | 5.34 ± 1.8 | 3.28 ± 1.6 | 4.3846 ± 1.5 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.77 ± 0.2 | 1.73 ± 0.5 | 1.38 ± 0.3 |
| Total thiol content (mmol SH/mg protein) | 44.96 ± 5.9 | 45.53 ± 6.2 | 47.34 ± 5.3 |
| TRAP (under curve area) | 176.3 ± 27.8 | 224.3 ± 22.7 | 178.8 ± 21.79 |
| CAT activity (U CAT/mg protein) | 31.87 ± 4.1 | 37.48 ± 3.1 * | 36.43 ± 3.4 * |
| SOD activity (U SOD/mg protein) | 18.16 ± 2.5 | 19.81 ± 6.5 * | 20.52 ± 1.2 * |
| GST activity (U GST/mg protein) | 0.217 ± 0.5 | 0.251 ± 0.3 * | 0.248 ± 0.2 * |
| SOD/CAT ratio (arbitrary units) | 0.56 ± 0.6 | 0.52 ± 0.7 * | 0.56 ± 0.7 |
| Heart | |||
| TBARS level (nmol MDA/mg protein) | 2.0379 ± 0.2 | 2.2703 ± 0.5 | 2.1881 ± 0.6 |
| Carbonyl level (nmol carbonyl/mg protein) | 2.0249 ± 0.3 | 1.9941 ± 0.2 | 1.6428 ± 0.4 |
| Total thiol content (mmol SH/mg protein) | 35.01 ± 5.8 | 36.91 ± 8.5 | 43.95 ± 7.4 |
| TRAP (under curve area) | 488.45 ± 48.0 | 401.53 ± 22.55 | 407.7 ± 60.39 |
| CAT activity (U CAT/mg protein) | 6.42 ± 0.5 | 6.43 ± 1.6 | 6.3 ± 2.0 |
| SOD activity (U SOD/mg protein) | 5.25 ± 1.2 | 6.75 ± 2.7 | 6.5 ± 1.7 |
| GST activity (U GST/mg protein) | 0.217 ± 0.8 | 0.251 ± 0.2 * | 0.248 ± 0.3 * |
| SOD/CAT ratio (arbitrary units) | 0.81 ± 0.8 | 1.04 ± 0.8 * | 1.03 ± 0.8 * |
| Cerebellum | |||
| TBARS level (nmol MDA/mg protein) | 6.81 ± 5.4 | 6.54 ± 1.6 | 6.01 ± 1.1 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.2 ± 0.3 | 1.6 ± 0.9 | 1.0 ± 0.7 |
| Total thiol content (mmol SH/mg protein) | 50.6 ± 2.2 | 52.61 ± 8.8 | 49.51 ± 7.2 |
| CAT activity (U CAT/mg protein) | 4.66 ± 1.8 | 4.62 ± 3.5 | 6.79 ± 1.7 |
| SOD activity (U SOD/mg protein) | 6.76 ± 1.4 | 10.32 ± 4 * | 11.09 ± 2.5 |
| SOD/CAT ratio (arbitrary units) | 1.45 ± 1.3 | 2.23 ± 0.8 * | 1.63 ± 0.9 |
| Hippocampus | |||
| TBARS level (nmol MDA/mg protein) | 5.38 ± 1.8 | 3.08 ± 1.6 | 4.46 ± 1.5 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.77 ± 0.2 | 1.76 ± 0.5 | 1.28 ± 0.3 |
| Total thiol content (mmol SH/mg protein) | 44.96 ± 5.9 | 45.53 ± 6.2 | 47.34 ± 5.3 |
| CAT activity (U CAT/mg protein) | 11.87 ± 4.1 | 12.48 ± 2.1 | 10.43 ± 3.4 |
| SOD activity (U SOD/mg protein) | 8.16 ± 2.5 | 5.81 ± 1.5 * | 5.52 ± 3.2 * |
| SOD/CAT ratio (arbitrary units) | 0.68 ± 0.1 | 0.46 ± 0.1 * | 0.52 ± 0.2 |
| Cortex | |||
| TBARS level (nmol MDA/mg protein) | 2.0 ± 0.2 | 2.03 ± 0.5 | 2.81 ± 0.6 |
| Carbonyl level (nmol carbonyl/mg protein) | 2.49 ± 0.3 | 1.99 ± 0.2 | 1.68 ± 0.4 |
| Total thiol content (mmol SH/mg protein) | 35.01 ± 5.8 | 36.91 ± 8.5 | 43.95 ± 7.4 |
| CAT activity (U CAT/mg protein) | 6.42 ± 0.5 | 6.43 ± 1.6 | 6.51 ± 0.2 |
| SOD activity (U SOD/mg protein) | 5.25 ± 1.2 | 6.75 ± 2.7 | 6.31 ± 0.8 |
| SOD/CAT ratio (arbitrary units) | 0.81 ± 1.8 | 1.04 ± 2.8 | 0.96 ± 1 |
| Achyrocline satureioides (AS, mg·kg−1·day−1) | |||
|---|---|---|---|
| Control (Water) | AQ (47) | HA (35) | |
| No. of Pups Examined | 24 | 11 | 20 |
| Liver | |||
| TBARS level (nmol MDA/mg protein) | 6.81 ± 1.4 | 6.54 ± 1.6 | 6.01 ± 1.1 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.62 ± 0.3 | 1.66 ± 0.8 | 1.80 ± 0.7 |
| Total thiol content (mmol SH/mg protein) | 55.6 ± 2.2 | 58.61 ± 8.8 | 53.51 ± 6.2 |
| TRAP (under curve area) | 324.3 ± 55.25 | 317.76 ± 29.9 | 318.37 ± 7.1 |
| CAT activity (U CAT/mg protein) | 42.66 ± 1.8 | 54.62 ± 3.5 * | 56.79 ± 1.7 * |
| SOD activity (U SOD/mg protein) | 36.76 ± 1.4 | 40.32 ± 1.5 * | 46.09 ± 2.5 * |
| SOD/CAT ratio (arbitrary units) | |||
| GST activity (U GST/mg protein) | 0.217 ± 0.5 | 0.251 ± 0.3 * | 0.248 ± 0.2 * |
| Kidney | |||
| TBARS level (nmol MDA/mg protein) | 5.38 ± 1.8 | 3.08 ± 1.6 | 4.46 ± 1.5 |
| Carbonyl level (nmol carbonyl/mg protein) | 1.77 ± 0.2 | 1.76 ± 0.5 | 1.28 ± 0.3 |
| Total thiol content (mmol SH/mg protein) | 44.96 ± 5.9 | 45.53 ± 6.2 | 47.34 ± 5.3 |
| TRAP (under curve area) | 176.6 ± 27.82 | 124.33 ± 32.76 | 128.78 ± 21.79 |
| CAT activity (U CAT/mg protein) | 31.87 ± 4.1 | 37.48 ± 3.1 | 36.43 ± 3.4 |
| SOD activity (U SOD/mg protein) | 18.16 ± 2.5 | 25.81 ± 1.5 * | 25.52 ± 3.2 * |
| SOD/CAT ratio (arbitrary units) | 0.56 ± 0.5 | 0.68 ± 0.2 | 0.70 ± 1.0 |
| GST activity (U GST/mg protein) | 0.05 ± 0.7 | 0.08 ± 0.5 | 0.09 ± 0.6 |
| Heart | |||
| TBARS level (nmol MDA/mg protein) | 2.0 ± 0.2 | 2.03 ± 0.5 | 2.81 ± 0.6 |
| Carbonyl level (nmol carbonyl/mg protein) | 2.49 ± 0.3 | 1.99 ± 0.2 | 1.68 ± 0.4 |
| Total thiol content (mmol SH/mg protein) | 35.01 ± 5.8 | 36.91 ± 8.5 | 43.95 ± 7.4 |
| TRAP (under curve area) | 488.5 ± 48.57 | 401.5 ± 22.57 | 407.89 ± 60.4 |
| CAT activity (U CAT/mg protein) | 6.42 ± 0.5 | 6.43 ± 1.6 | 6.5 ± 1.3 |
| SOD activity (U SOD/mg protein) | 5.25 ± 1.2 | 6.75 ± 2.7 | 6.3 ± 2.3 |
| SOD/CAT ratio (arbitrary units) | 0.81 ± 1.7 | 1.04 ± 1.2 | 0.96 ± 2.9 |
| GST activity (U GST/mg protein) | 0.06 ± 0.7 | 0.05 ± 0.7 | 0.06 ± 0.5 |
| Cerebellum | |||
| TBARS level (nmol MDA/mg protein) | 6.81 ± 1.4 | 6.54 ± 1.6 | 6.01 ± 1.1 |
| Carbonyl (nmol carbonyl/mg protein) | 1.62 ± 0.3 | 1.66 ± 0.8 | 1.80 ± 0.7 |
| Total thiol (mmol SH/mg protein) | 55.6 ± 2.2 | 58.61 ± 8.8 | 53.51 ± 6.2 |
| CAT activity (U CAT/mg protein) | 2.66 ± 1.8 | 4.2 ± 3.5 | 6.79 ± 1.7 |
| SOD activity (U SOD/mg protein) | 36.76 ± 1.4 | 40.2 ± 1.5 | 46.09 ± 2.5 |
| SOD/CAT ratio (arbitrary units) | 0.86 ± 1.8 | 0.74 ± 1.2 | 0.81 ± 1.6 |
| Hippocampus | |||
| TBARS level (nmol MDA/mg protein) | 3.38 ± 1.8 | 3.08 ± 1.6 | 4.46 ± 1.5 |
| Carbonyl (nmol carbonyl/mg protein) | 1.77 ± 0.2 | 1.76 ± 0.5 | 1.28 ± 0.3 |
| Total thiol (mmol SH/mg protein) | 44.96 ± 5.9 | 45.53 ± 6.2 | 47.34 ± 5.3 |
| CAT activity (U CAT/mg protein) | 4.87 ± 4.1 | 7.48 ± 5.1 | 6.43 ± 3.4 |
| SOD activity (U SOD/mg protein) | 5.16 ± 2.5 | 5.81 ± 5.5 | 5.52 ± 3.2 |
| SOD/CAT ratio (arbitrary units) | 1.05 ± 1.5 | 0.77 ± 2.3 | 0.85 ± 0.5 |
| Cortex | |||
| TBARS level (nmol MDA/mg protein) | 2.0 ± 0.2 | 2.03 ± 0.5 | 2.81 ± 0.6 |
| Carbonyl (nmol carbonyl/mg protein) | 2.49 ± 0.3 | 1.99 ± 0.2 | 1.68 ± 0.4 |
| Total thiol (mmol SH/mg protein) | 15.01 ± 5.8 | 16.91 ± 8.5 | 13.95 ± 7.4 |
| CAT activity (U CAT/mg protein) | 3.2 ± 0.5 | 3.43 ± 1.6 | 3.5 ± 1 |
| SOD activity (U SOD/mg protein) | 3.25 ± 1.2 | 3.75 ± 2.7 | 3.31 ± 2 |
| SOD/CAT ratio (arbitrary units) | 1.01± 3.5 | 1.09 ± 2.2 | 0.94 ±3.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moresco, K.S.; Silveira, A.K.; Schnorr, C.E.; Zeidán-Chuliá, F.; Bortolin, R.C.; Bittencourt, L.D.S.; Mingori, M.; Heimfarth, L.; Rabelo, T.K.; Morrone, M.D.S.; et al. Supplementation with Achyrocline satureioides Inflorescence Extracts to Pregnant and Breastfeeding Rats Induces Tissue-Specific Changes in Enzymatic Activity and Lower Neonatal Survival. Biomedicines 2017, 5, 53. https://doi.org/10.3390/biomedicines5030053
Moresco KS, Silveira AK, Schnorr CE, Zeidán-Chuliá F, Bortolin RC, Bittencourt LDS, Mingori M, Heimfarth L, Rabelo TK, Morrone MDS, et al. Supplementation with Achyrocline satureioides Inflorescence Extracts to Pregnant and Breastfeeding Rats Induces Tissue-Specific Changes in Enzymatic Activity and Lower Neonatal Survival. Biomedicines. 2017; 5(3):53. https://doi.org/10.3390/biomedicines5030053
Chicago/Turabian StyleMoresco, Karla Suzana, Alexandre Kleber Silveira, Carlos Eduardo Schnorr, Fares Zeidán-Chuliá, Rafael Calixto Bortolin, Leonardo Da Silva Bittencourt, Moara Mingori, Luana Heimfarth, Thallita Kelly Rabelo, Maurilio Da Silva Morrone, and et al. 2017. "Supplementation with Achyrocline satureioides Inflorescence Extracts to Pregnant and Breastfeeding Rats Induces Tissue-Specific Changes in Enzymatic Activity and Lower Neonatal Survival" Biomedicines 5, no. 3: 53. https://doi.org/10.3390/biomedicines5030053