New Insights into the Pathophysiology and Treatment of Fibromyalgia
Abstract
:1. Introduction
2. Current Pathophysiological Concepts of Chronic Pain and Fibromyalgia
2.1. Medical Concepts
2.2. Psychological Concepts
3. Therapy
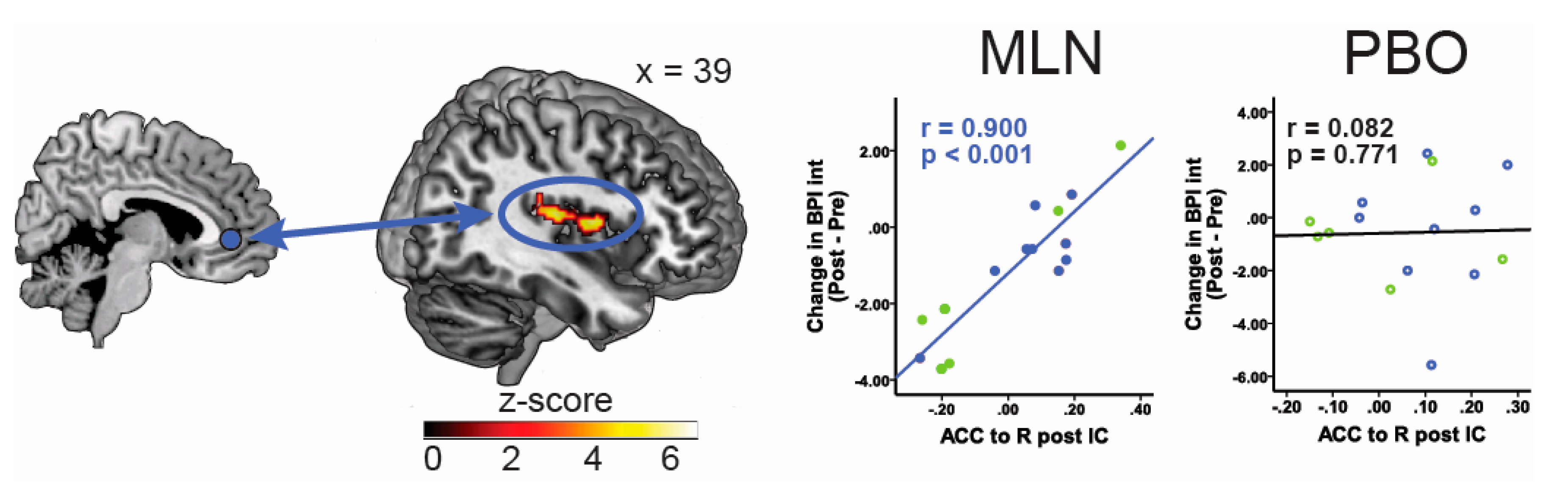
3.1. Pharmacotherapy
3.2. Behavioral Interventions
4. Conclusions and Outlook
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| DMN | Default mode network |
| FM | Fibromyalgia |
| GABA | γ-Amino-butyric-acid |
| IC | Insular cortex |
| MLN | Milnacipran |
| fMRI | Functional magnetic resonance imaging |
| NE | Norepinephrine |
| rs-fc | Resting state functional connectivity |
| US | Unconditioned stimulus |
| CS | Conditioned stimulus |
| CBP | Chronic back pain |
| HCs | Healthy controls |
| IBS | Irritable bowel syndrome |
| EULAR | European League against Rheumatism |
| IPL | Inferior parietal lobule |
| ACC | Anterior cingulate cortex |
| BPI Int | Brief Pain Inventory interference scores |
| NMDA | N-Methyl-D-Aspartat |
References
- Blyth, F.M.; March, L.M.; Brnabic, A.J.M.; Jorm, L.R.; Williamson, M.; Cousins, M.J. Chronic pain in Australia: A prevalence study. Pain 2001, 89, 127–134. [Google Scholar] [CrossRef]
- Harker, J.; Reid, K.J.; Bekkering, G.E.; Kellen, E.; Malgorzata, M.; Riemsma, R.; Worthy, G.; Misso, K.; Kleijnen, J. Epidemiology of chronic pain in Denmark and Sweden. Pain Res. Treat. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Singh, V.; Datta, S.; Cohen, S.P.; Hirsch, J. A Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009, 12, E35–E70. [Google Scholar] [PubMed]
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Brummett, C.M.; Urquhart, A.G.; Hassett, A.L.; Tsodikov, A.; Hallstrom, B.R.; Wood, N.I.; Williams, D.A.; Clauw, D.J. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 2015, 67, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The Prevalence and Characteristics of Fibromyalgia in the General Population. Arthritis Rheumatol. 1995, 38, 19–28. [Google Scholar] [CrossRef]
- Assumpção, A.; Cavalcante, A.; Capela, C.; Al, E. Prevalence of fibromyalgia in a low socioeconomic status population. BMC Musculoskelet. Disord. 2009, 10, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheumatol. 2008, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Hudson, J.I.; Hess, E.V.; Ware, A.E.; Fritz, D.A.; Auchenbach, M.B.; Starck, L.O.; Keck, P.E. Family Study of Fibromyalgia. Arthritis Rheumatol. 2004, 50, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Diatchenko, L.; Nackley, A.G.; Slade, G.D.; Fillingim, R.B.; Maixner, W. Idiopathic pain disorders–pathways of vulnerability. Pain 2006, 123, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.C.; Silman, A.J.; Macfarlane, G.J. Chronic widespread pain in the population: A seven year follow up study. Ann. Rheum. Dis. 2002, 61, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- McBeth, J.; Macfarlane, G.J.; Benjamin, S.; Morris, S.; Silman, A.J.; Beth, J.M.C. The association between tender points, psychological distress, and adverse childhood experiences: A community-based study. Arthritis Rheum. 1999, 42, 1397–1404. [Google Scholar] [CrossRef]
- Gracely, R.H.; Petzke, F.; Wolf, J.M.; Clauw, D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002, 46, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, T.; Gracely, R.; Grant, M.A.; Nachemson, A.; Petzke, F.; Williams, D.; Clauw, D. Evidence of augmented central pain processing in idiopathich chronic low back pain. Arthritis Rheum. 2004, 50, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.B.; Lange, G.; Ciccone, D.S.; Liu, W.C.; Steffener, J.; Natelson, B.H. Functional imaging of pain in patients with primary fibromyalgia. J. Rheumatol. 2004, 31, 364–378. [Google Scholar] [PubMed]
- Jensen, K.B.; Loitoile, R.; Kosek, E.; Petzke, F.; Carville, S.; Fransson, P.; Marcus, H.; Williams, S.C.R.; Choy, E.; Mainguy, Y.; et al. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol. Pain 2012, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Harte, S.E.; Ichesco, E.; Hampson, J.P.; Peltier, S.J.; Schmidt-Wilcke, T.; Clauw, D.J.; Harris, R.E. Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain 2016, 157, 1933. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; LaCount, L.; Park, K.; As-Sanie, S.; Clauw, D.J.; Harris, R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010, 62, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Ichesco, E.; Schmidt-Wilcke, T.; Bhavsar, R.; Clauw, D.J.; Peltier, S.J.; Kim, J.; Napadow, V.; Hampson, J.P.; Kairys, A.E.; Williams, D.A.; et al. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J. Pain 2014, 15, 815–826.e1. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I. Nociceptive processing in the human brain. Curr. Opin. Neurobiol. 2005, 15, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Loggia, M.L.; Cahalan, C.M.; Harris, R.E.; Beissner, F.; Garcia, R.G.; Kim, H.; Barbieri, R.; Wasan, A.D.; Edwards, R.R.; et al. The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015, 67, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Napadow, V.; Harris, R.E. What has functional connectivity and chemical neuroimaging in fibromyalgia taught us about the mechanisms and management of “centralized” pain? Arthritis Res. Ther. 2014, 16, 425. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.J.; Götestam, K.G. Controlling pain reports through operant conditioning: A laboratory demonstration. Percept. Mot. Skills 1985, 60, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, W.E. Behavioral Concepts in Chronic Pain and Illness; Mosby: St. Louis, MO, USA, 1976. [Google Scholar]
- Flor, H.; Knost, B.; Birbaumer, N. The role of operant conditioning in chronic pain: An experimental investigation. Pain 2002, 95, 111–118. [Google Scholar] [CrossRef]
- Knost, B.; Flor, H.; Birbaumer, N.; Schugens, M.M. Learned maintenance of pain: Muscle tension reduces central nervous system processing of painful stimulation in chronic and subchronic pain patients. Psychophysiology 1999, 36, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Elert, J.; Kendall, S.A.; Larsson, B.; Mansson, B.; Gerdle, B. Chronic pain and difficulty in relaxing postural muscles in patients with fibromyalgia and chronic whiplash associated disorders. J. Rheumatol. 2001, 28, 1361–1368. [Google Scholar] [PubMed]
- Hölzl, R.; Kleinbohl, D.; Huse, E. Implicit operant learning of pain sensitization. Pain 2005, 115, 12–20. [Google Scholar]
- Becker, S.; Kleinbo, D.; Klossika, I.; Ho, R. Operant conditioning of enhanced pain sensitivity by heat—Pain titration. Pain 2008, 140, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Kleinböhl, D.; Baus, D.; Hölzl, R. Operant learning of perceptual sensitization and habituation is impaired in fibromyalgia patients with and without irritable bowel syndrome. Pain 2011, 152, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Palomba, D.; Flor, H. Pavlovian conditioning of muscular responses in chronic pain patients: Central and peripheral correlates. Pain 2004, 112, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Jenewein, J.; Moergeli, H.; Sprott, H.; Honegger, D.; Brunner, L.; Ettlin, D.; Grillon, C.; Bloch, K.; Brügger, M.; Schwegler, K.; et al. Fear-learning deficits in subjects with fibromyalgia syndrome? Eur. J. Pain UK 2013, 17, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; Debeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Keck, P.E.; Welge, J.A. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000, 41, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.; Bernardy, K.; Uceyler, N.; Sommer, C.; Häuser, W.; Bernardy, K.; Uçeyler, N.; Sommer, C. Treatment of Fibromyalgia Syndrome with Antidepressants: A Meta-analysis. JAMA J. Am. Med. Assoc. 2009, 301, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Rico-Villademoros, F.; Slim, M.; Calandre, E.P. Amitriptyline for the treatment of fibromyalgia: A comprehensive review. Expert Rev. Neurother. 2015, 15, 1123–1150. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, U.M.; Marteinsdottir, I.; von Knorring, L. Citalopram in patients with fibromyalgia—A randomized, double-blind, placebo-controlled study. Eur. J. Pain 2000, 4, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Norregaard, J.; Volkmann, H.; Danneskiold-Samsoe, B. A randomized controlled trial of citalopram in the treatment of fibromyalgia. Pain 1995, 61, 445–449. [Google Scholar] [CrossRef]
- Arnold, L.M.; Clauw, D.J.; Wohlreich, M.M.; Wang, F.; Ahl, J.; Gaynor, P.J.; Chappell, A.S. Efficacy of duloxetine in patients with fibromyalgia: Pooled analysis of 4 placebo-controlled clinical trials. Prim Care Companion J. Clin. Psychiatry 2009, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Vitton, O.; Gendreau, M.; Gendreau, J.; Kranzler, J.; Rao, S.G. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum. Psychopharmacol. Clin. Exp. 2004, 19, S27–S35. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Moore, R.A.; McQuay, H.J.; Wiffen, P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: A quantitative systematic review. J. Pain Symptom Manag. 2000, 20, 449–458. [Google Scholar] [CrossRef]
- Straube, S.; Derry, S.; Moore, R.A.; McQuay, H.J. Pregabalin in fibromyalgia: Meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology 2010, 49, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Hauser, W.; Bernardy, K.; Uceyler, N.; Sommer, C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin—A meta-analysis of randomized controlled trials. Pain 2009, 145, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Clauw, D.J. Pharmacotherapy in fibromyalgia (FM)—Implications for the underlying pathophysiology. Pharmacol. Ther. 2010, 127, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Clauw, D.J. Fibromyalgia: From pathophysiology to therapy. Nat. Rev. Rheumatol. 2011, 7, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Petzke, F.; Carville, S.; Choy, E.; Fransson, P.; Gracely, R.H.; Vitton, O.; Marcus, H.; Williams, S.C.R.; Ingvar, M.; et al. Segregating the cerebral mechanisms of antidepressants and placebo in fibromyalgia. J. Pain 2014, 15, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Ichesco, E.; Hampson, J.P.; Kairys, A.; Peltier, S.; Harte, S.; Clauw, D.J.; Harris, R.E. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014, 6, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Napadow, V.; Huggins, J.P.; Pauer, L.; Kim, J.; Hampson, J.; Sundgren, P.C.; Foerster, B.; Petrou, M.; Schmidt-Wilcke, T.; Clauw, D.J. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013, 119, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Gromnica-Ihle, E.; Flor, H. Operant Behavioral Treatment of Fibromyalgia: A Controlled Study. Arthritis Rheum. Arthritis Care Res. 2003, 49, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Flor, H.; Turk, D.C. Psychological pain treatment in fibromyalgia syndrome: Efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res. Ther. 2006, 8, R121. [Google Scholar] [CrossRef] [PubMed]
- Diers, M.; Yilmaz, P.; Rance, M.; Thieme, K.; Gracely, R.H.; Rolko, C.; Schley, M.T.; Kiessling, U.; Wang, H.; Flor, H. Treatment-related changes in brain activation in patients with fibromyalgia syndrome. Exp. Brain Res. 2012, 218, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Hassett, A.L.; Williams, D.A. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract. Res. Clin. Rheumatol. 2011, 25, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.M.; Papas, R.K.; Chatkoff, D.K.; Kerns, R.D. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Kosek, E.; Wicksell, R.; Kemani, M.; Olsson, G.; Merle, J.V.; Kadetoff, D.; Ingvar, M. Cognitive Behavioral Therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgeia. Pain 2012, 153, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Kim, J.; Cahalan, C.M.; Loggia, M.L.; Franceschelli, O.; Berna, C.; Schur, P.; Napadow, V.; Edwards, R.R. Effects of Cognitive-behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin. J. Pain 2017, 33, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Flor, H. Extinction of pain memories: Importance for the treatment of chronic pain. In Current Topics in Pain, Proceedings of the 12th World Congress Pain, Glasgow, UK, 17–22 August 2008; IASP Press: Seattle, WA, USA, 2009; pp. 221–244. [Google Scholar]
- Thieme, K.; Gracely, R.H. Are psychological treatments effective for fibromyalgia pain? Curr. Rheumatol. Rep. 2009, 11, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Turk, D.C. Cognitive-behavioral and operant-behavioral therapy for people with fibromyalgia. Reumatismo 2012, 64, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ang, D.C.; Jensen, M.P.; Steiner, J.L.; Hilligoss, J.; Gracely, R.H.; Saha, C. Combining cognitive-behavioral therapy and milnacipran for fibromyalgia: A feasibility randomized-controlled trial. Clin. J. Pain 2013, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Rothbaum, B.O.; Tannenbaum, L.; Anderson, P. Cognitive Enhancers as Adjuncts to Psychotherapy. Arch. Gen. Psychiatry 2004, 61, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Meuret, A.E.; Smits, J.A.J.; Simon, N.M.; Pollack, M.H.; Eisenmenger, K.; Shiekh, M.; Otto, M.W. Augmentation of Exposure Therapy with d-Cycloserine for Social Anxiety Disorder. Arch. Gen. Psychiatry 2006, 63, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Wotjak, C.T. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev. Med. Chem. 2005, 5, 659–670. [Google Scholar] [CrossRef] [PubMed]
| Name | Substance Class | Mechanism of Action | Recommended Dosage | Grade of Recommendation * |
|---|---|---|---|---|
| Amitriptyline | Tricyclic antidepressant | Inhibition of the presynaptic serotonin and norepinephrine transporter; 5-HT2A, 5-HT2C, 5-HT6, 5-HT7 receptor antagonism | 10–50 mg/day | weak for |
| Cyclobenzaprine | Muscle relaxant, tricyclic antidepressant derivative | Inhibition of the presynaptic serotonin and norepinephrine transporter; 5-HT2A receptor antagonism | 10–40 mg/day | weak for |
| Duloxetine | Antidepressant, serotonin and norepinephrine reuptake inhibitor | Selective serotonin and norepinephrine reuptake inhibition | 20–120 mg/day | weak for |
| Milnacipran | Antidepressant, serotonin and norepinephrine reuptake inhibitor | Selective serotonin and norepinephrine reuptake inhibition | 100–200 mg/day | weak for |
| Pregabalin | Anticonvulsant | Modulation of the α2δ subunit of a presynaptic calcium channel | 300–450 mg/day | weak for |
| Gabapentin | Anticonvulsant | Modulation of the α2δ subunit of a presynaptic calcium channel; increased GABA turn over | 1200 mg/day | weak for |
| Tramadol | Weak opioid | Weak μ-receptor agonism, norepinephrine reuptake inhibition | 150 mg/day | weak for |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt-Wilcke, T.; Diers, M. New Insights into the Pathophysiology and Treatment of Fibromyalgia. Biomedicines 2017, 5, 22. https://doi.org/10.3390/biomedicines5020022
Schmidt-Wilcke T, Diers M. New Insights into the Pathophysiology and Treatment of Fibromyalgia. Biomedicines. 2017; 5(2):22. https://doi.org/10.3390/biomedicines5020022
Chicago/Turabian StyleSchmidt-Wilcke, Tobias, and Martin Diers. 2017. "New Insights into the Pathophysiology and Treatment of Fibromyalgia" Biomedicines 5, no. 2: 22. https://doi.org/10.3390/biomedicines5020022





