A Low-Cost Label-Free AFB1 Impedimetric Immunosensor Based on Functionalized CD-Trodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus and Electrochemical Cell
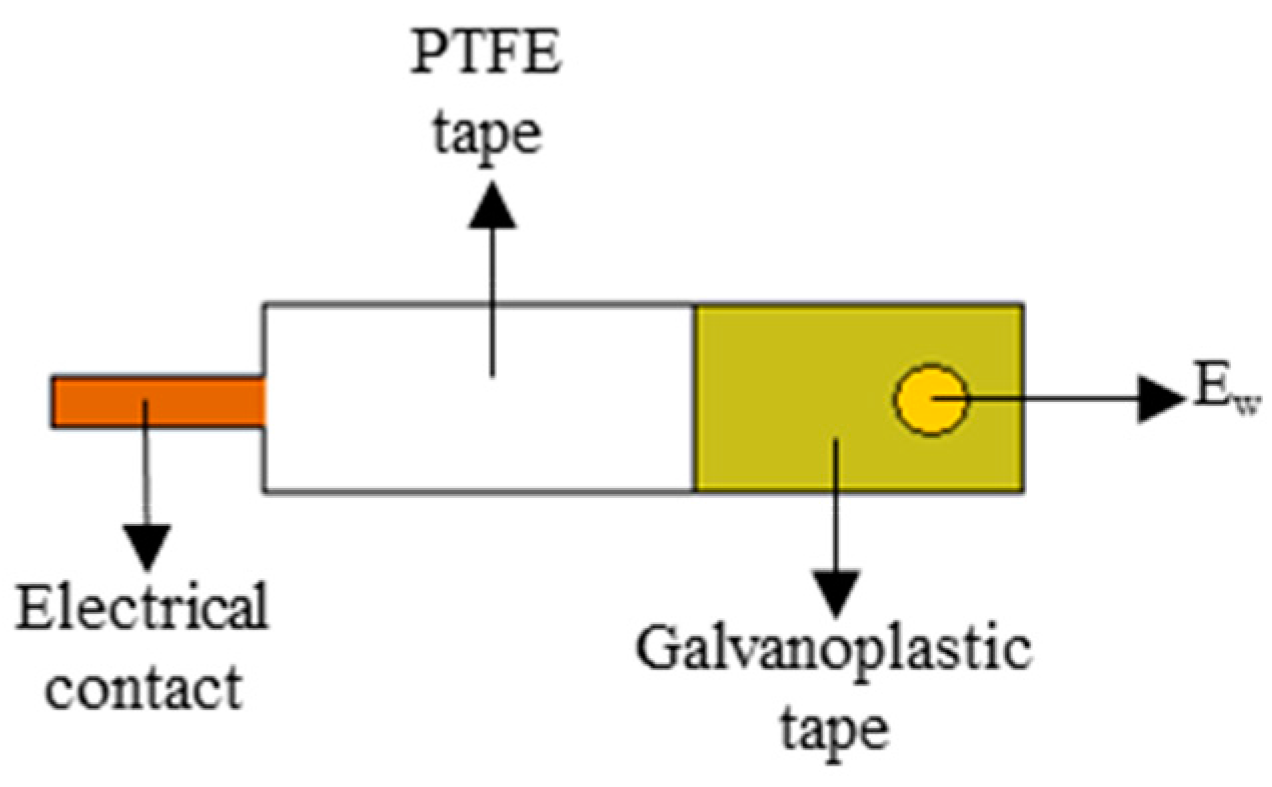
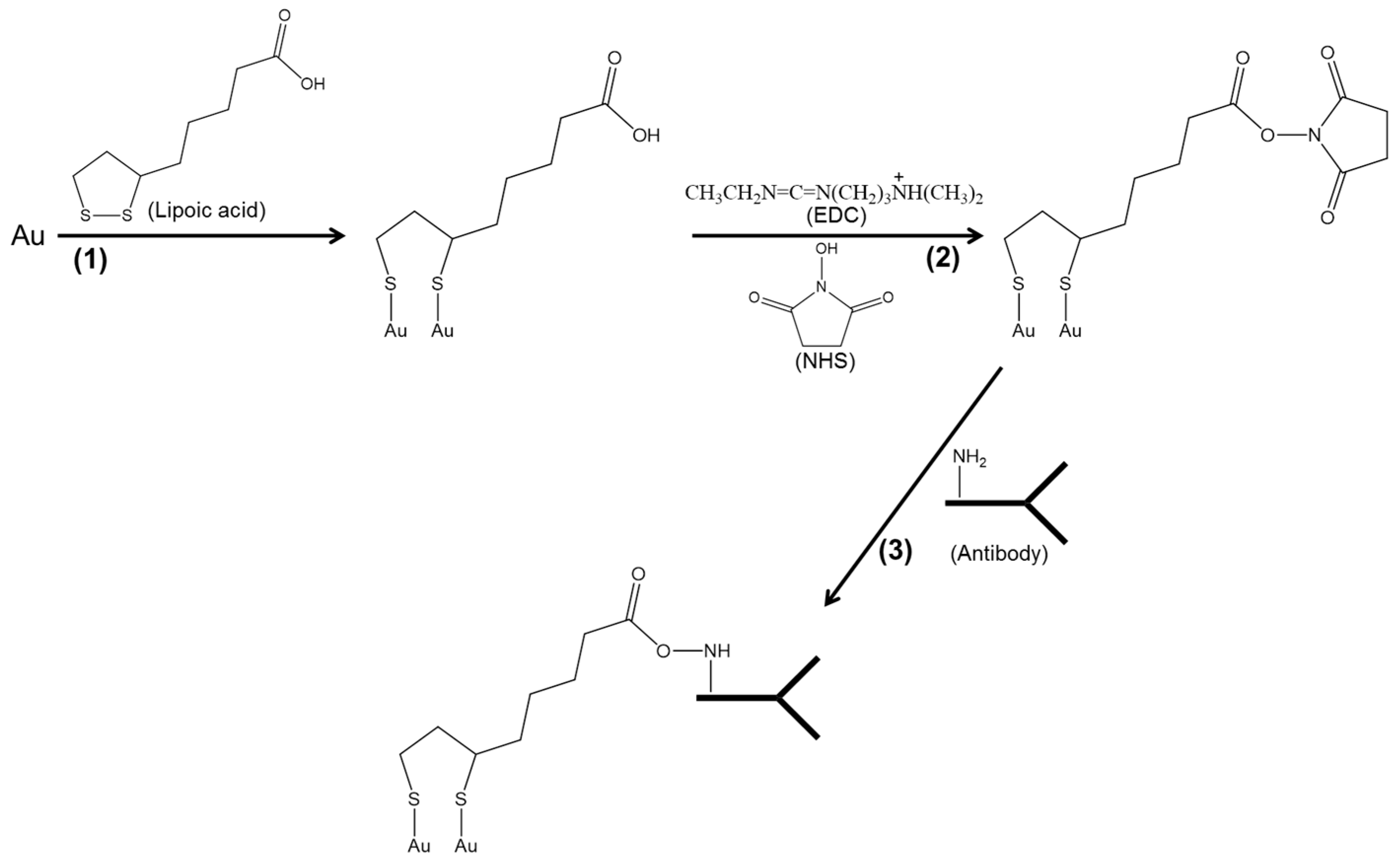
2.3. Construction of Gold Electrode (AuCD-Trode)
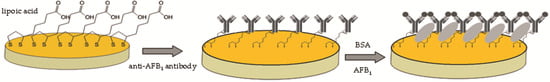
2.4. Immunosensor Development
2.5. Electrochemical Measurements
2.6. Extraction of AFB1 from Peanut Samples
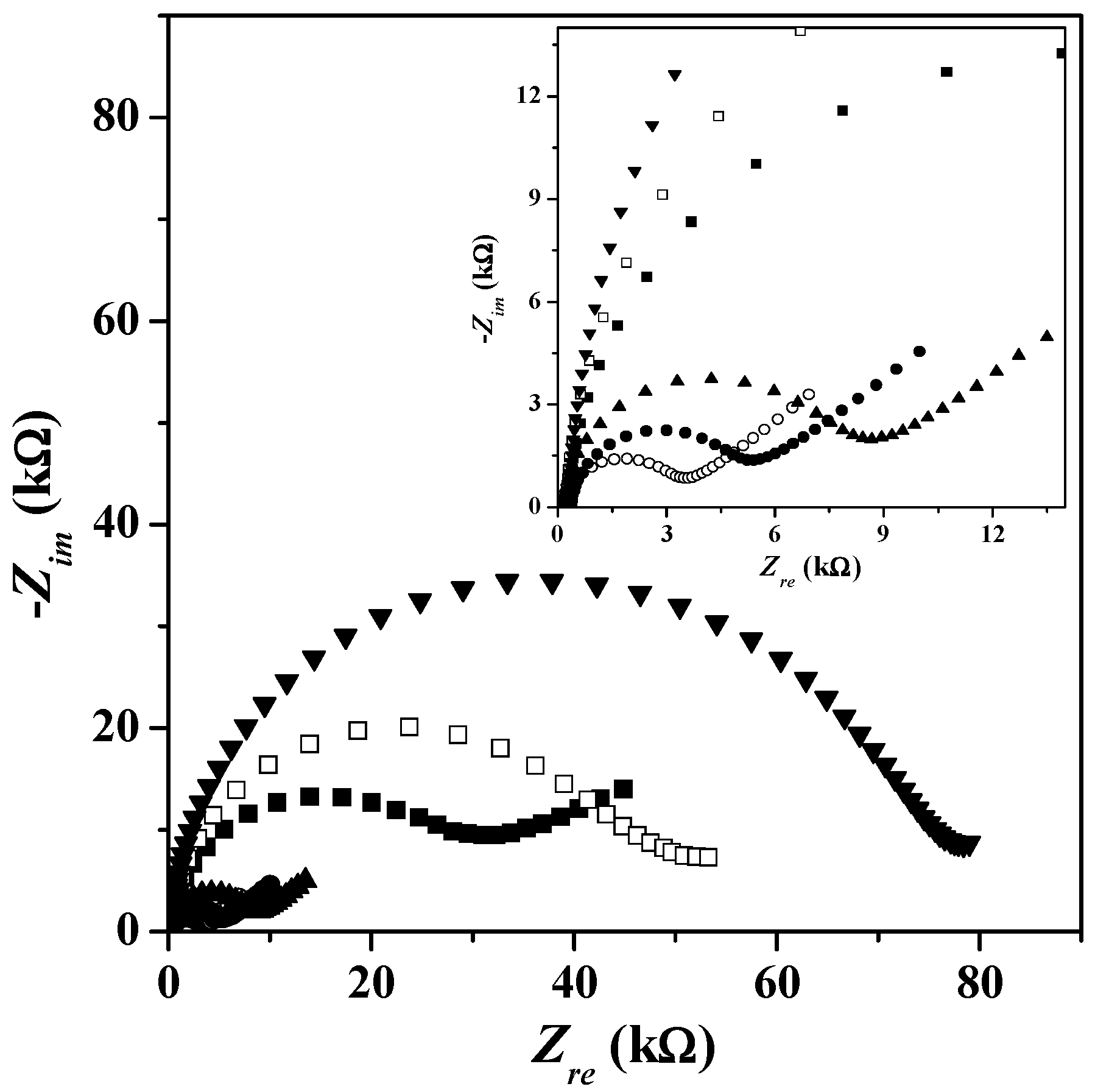
3. Results and Discussion
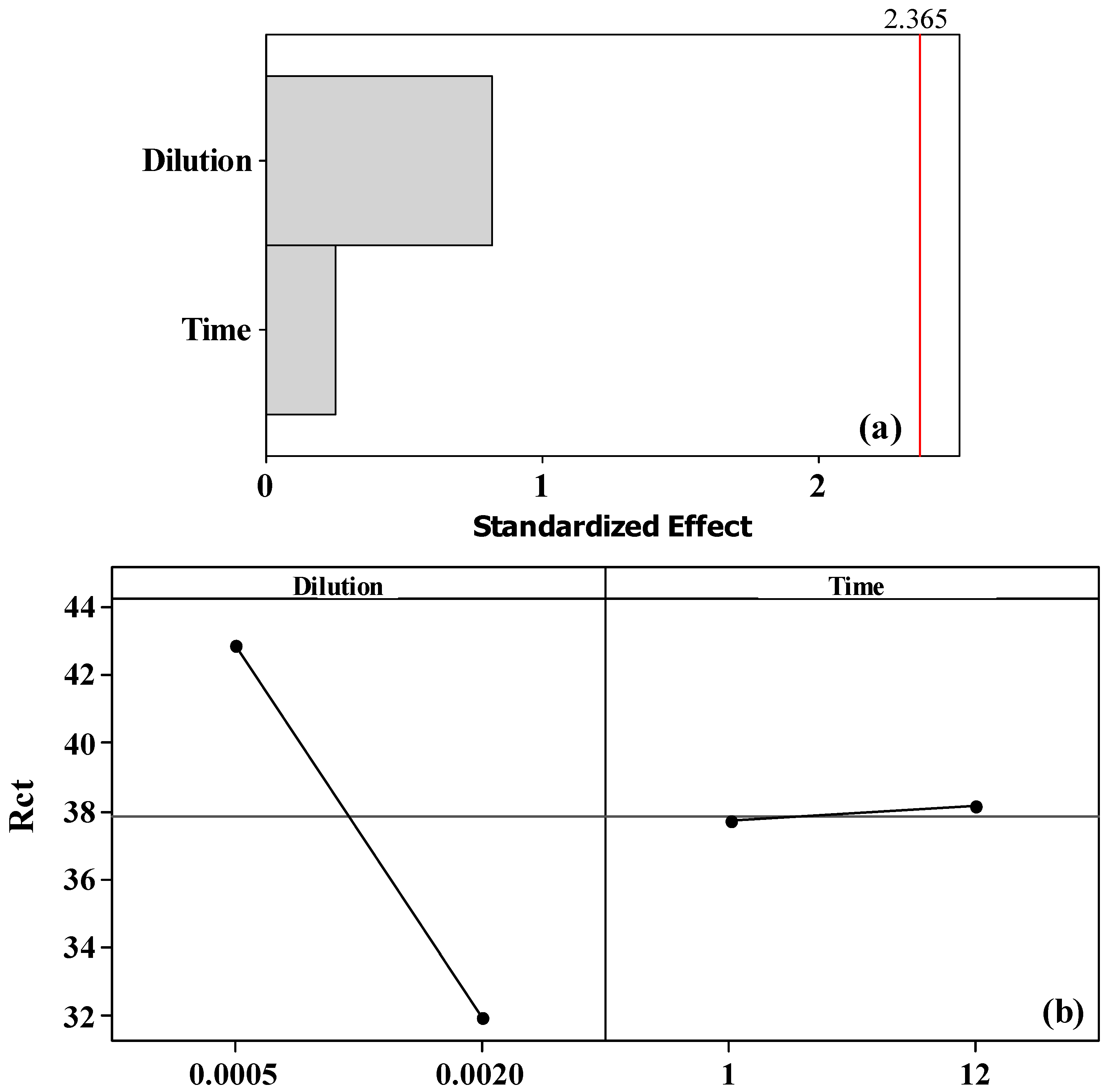
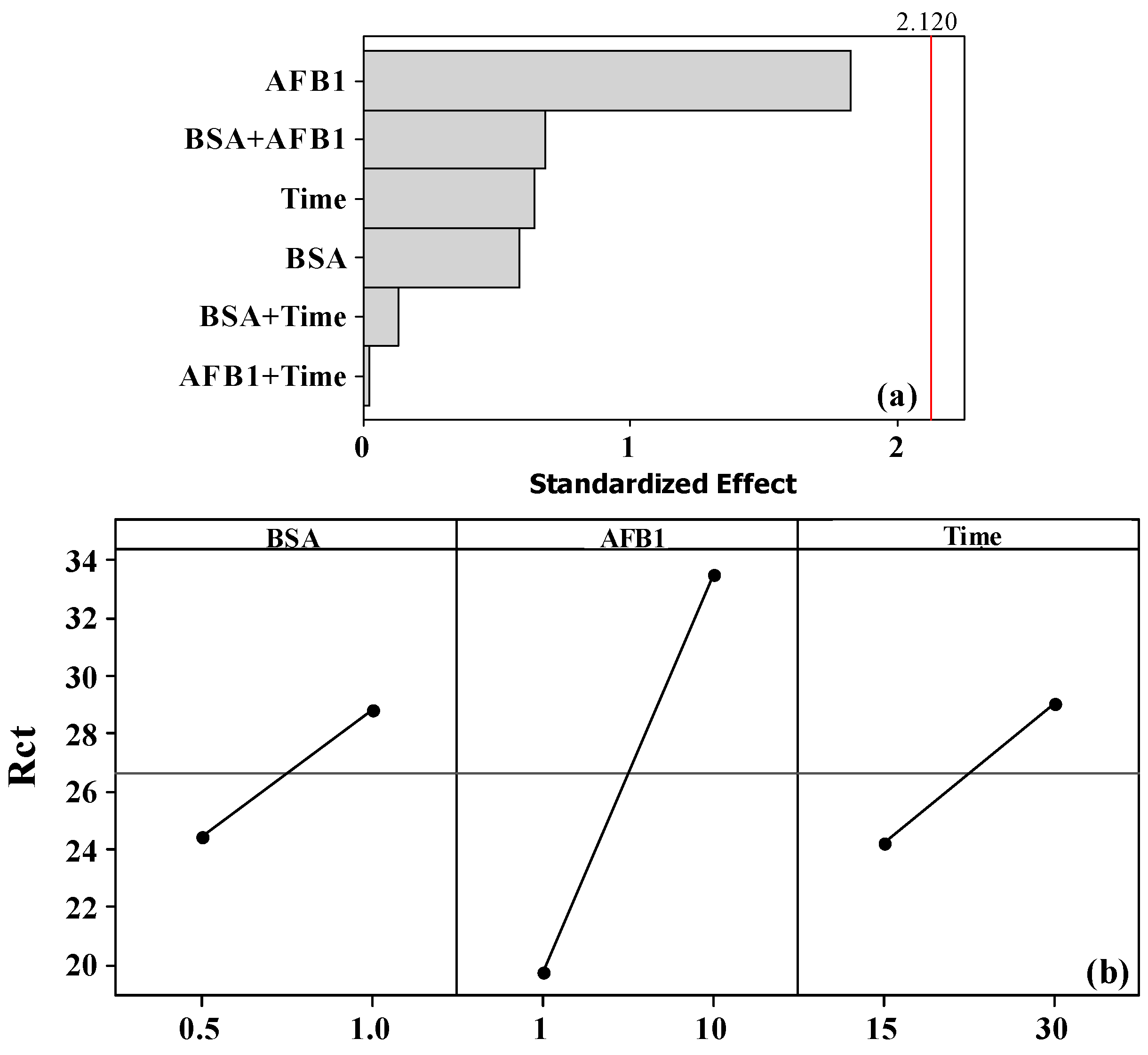
3.1. Chemometric Study of Antibody, BSA, and AFB1 Concentrations and Incubation Time
3.2. Analytical Curve
3.3. Application in Peanut Samples
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AFB1 | Aflatoxin B1 |
| CD-trode | Electrode obtained from recordable compact disk |
| EDC | 1-Ethyl-3-(3-(dimethylamino)-propyl)carbodiimide |
| NHS | N-hydroxy succinimide |
| SAM | Self-assembled monolayer |
References
- Nayak, S.; Sashidhar, R.B. Metabolic intervention of aflatoxin B1 toxicity by curcumin. J. Ethnopharmacol. 2010, 127, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, Y.; Mo, Z.; Cheng, W.; Yang, S.; Wang, X.; Chen, F. Rapid detection of aflatoxin B1 on membrane by dot-immunogold filtration assay. Talanta 2010, 81, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Hashemi, J.; Kram, G.A.; Alizadeh, N. Enhanced synchronous spectrofluorimetric determination of aflatoxin B1 in pistachio samples using multivariate analysis. Anal. Chim. Acta 2007, 582, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-González, M.P.; Mattusch, J.; Wennrich, R. Stability and determination of aflatoxins by high-performance liquid chromatography with amperometric detection. J. Chromatogr. A 1998, 828, 439–444. [Google Scholar] [CrossRef]
- Khayoon, W.S.; Saad, B.; Yan, C.B.; Hashim, N.H.; Ali, A.S.M.; Salleh, M.I.; Salleh, B. Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chem. 2010, 118, 882–886. [Google Scholar] [CrossRef]
- Gimeno, A.; Martins, M.L. Rapid thin layer chromatographic determination of patulin, citrinin, and aflatoxin in apples and pears, and their juices and jams. J. Assoc. Off. Anal. Chem. 1983, 66, 85–91. [Google Scholar] [PubMed]
- Xiulan, S.; Xiaolian, Z.; Jian, T.; Xiaohong, G.; Jun, Z.; Chu, F.S. Development of an immunochromatographic assay for detection of aflatoxin B1 in foods. Food Control 2006, 17, 256–262. [Google Scholar] [CrossRef]
- Ansari, A.A.; Kaushik, A.; Solanki, P.R.; Malhotra, B.D. Nanostructured zinc oxide platform for mycotoxin detection. Bioelectrochemistry 2010, 77, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Moccelini, S.K.; Fernandes, S.C.; Vieira, I.C. Bean sprout peroxidase biosensor based on l-cysteine self-assembled monolayer for the determination of dopamine. Sens. Actuators B 2008, 133, 364–369. [Google Scholar] [CrossRef]
- Asav, E.; Akyilmaz, E. Preparation and optimization of a bienzymic biosensor based on self-assembled monolayer modified gold electrode for alcohol and glucose detection. Biosens. Bioelectron. 2010, 25, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Biebuyck, H.A.; Bain, C.D.; Whitesides, G.M. Comparison of organic monolayers on polycrystalline gold spontaneously assembled from solutions containing dialkyl disulfides or alkanethiols. Langmuir 1994, 10, 1825–1831. [Google Scholar] [CrossRef]
- Van der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Wu, X.; Jiang, H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode. Biochem. Eng. J. 2006, 32, 211–217. [Google Scholar] [CrossRef]
- Carlson, M.A.; Bargeron, C.B.; Benson, R.C.; Fraser, A.B.; Phillips, T.E.; Velky, J.T.; Groopman, J.D.; Strickland, P.T.; Ko, H.W. An automated, handheld biosensor for aflatoxin. Biosens. Bioelectron. 2000, 14, 841–848. [Google Scholar] [CrossRef]
- Piermarini, S.; Micheli, L.; Ammida, N.H.S.; Palleschi, G.; Moscone, D. Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1 detection. Biosens. Bioelectron. 2007, 22, 1434–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, A.L.; Qi, Q.A.; Dong, Z.L.; Liang, K.Z. An electrochemical enzyme immunoassay for aflatoxin B1 based on bio-electrocatalytic reaction with room-temperature ionic liquid and nanoparticle-modified electrodes. Sens. Instrum. Food Qual. Saf. 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Owino, J.H.O.; Ignaszak, A.; Al-Ahmed, A.; Baker, P.G.L.; Alemu, H.; Ngila, J.C.; Iwuoha, E.I. Modelling of the impedimetric responses of an aflatoxin B1 immunosensor prepared on an electrosynthetic polyaniline platform. Anal. Bioanal. Chem. 2007, 388, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Zaijun, L.; Zhongyun, W.; Xiulan, S.; Yinjun, F.; Peipei, C. A sensitive and highly stable electrochemical impedance immunosensor based on the formation of silica gel-ionic liquid biocompatible film on the glassy carbon electrode for the determination of aflatoxin B1 in bee pollen. Talanta 2010, 80, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, J.; Shen, G.; Yu, R. A label-free electrochemical impedance immunosensor for the sensitive detection of aflatoxin B1. Anal. Methods 2015, 7, 2354–2359. [Google Scholar] [CrossRef]
- Srivastava, S.; Ali, M.A.; Umrao, S.; Parashar, U.K.; Srivastava, A.; Sumana, G.; Malhotra, B.D.; Pandey, S.S.; Hayase, S. Graphene oxide-based biosensor for food toxin detection. Appl. Biochem. Biotechnol. 2014, 174, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, W.; Xiong, Y.; Xu, Y.; Li, C.M. Multifunctionalized reduced graphene oxide-doped polypyrrole/pyrrolepropylic acid nanocomposite impedimetric immunosensor to ultra-sensitively detect small molecular aflatoxin B1. Biosens. Bioelectron. 2015, 63, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Hu, C.; Wu, H.; Yang, Y.; Huang, C.; Jia, N. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015, 176, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cao, L.; Li, Q.; Ma, K.; Yan, P.; Kirk, D.W. Fabrication and modeling of an ultrasensitive label free impedimetric immunosensor for Aflatoxin B1 based on poly(o-phenylenediamine) modified gold 3D nano electrode ensembles. RSC Adv. 2015, 5, 55209–55217. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Fu, Y.; Xiong, Y.; Li, Y. A portable electrochemical immunosensor for rapid detection of trace aflatoxin B 1 in rice. Anal. Methods 2016, 8, 548–553. [Google Scholar] [CrossRef]
- Angnes, L.; Richter, E.M.; Augelli, M.A.; Kume, G.H. Gold electrodes from recordable CDs. Anal. Chem. 2000, 72, 5503–5506. [Google Scholar] [CrossRef] [PubMed]
- Foguel, M.V.; Santos, G.P.; Ferreira, A.A.P.; Magnani, M.; Mascini, M.; Skladal, P.; Benedetti, A.V.; Yamanaka, H. Comparison of gold CD-R types as electrochemical device and as platform for biosensors. J. Braz. Chem. Soc. 2016, 27, 650–662. [Google Scholar] [CrossRef]
- Zoski, C.G. Preface. In Handbook of Electrochemistry; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Su, X.L.; Li, Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2004, 19, 563–574. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Uliana, C.V.; Tognolli, J.O.; Yamanaka, H. Application of factorial design experiments to the development of a disposable amperometric DNA biosensor. Electroanalysis 2011, 23, 2607–2615. [Google Scholar] [CrossRef]
- Hnaien, M.; Diouani, M.F.; Helali, S.; Hafaid, I.; Hassen, W.M.; Renault, N.J.; Ghram, A.; Abdelghani, A. Immobilization of specific antibody on SAM functionalized gold electrode for rabies virus detection by electrochemical impedance spectroscopy. Biochem. Eng. J. 2008, 39, 443–449. [Google Scholar] [CrossRef]
- Brett, C.; Brett, A. Electrochemistry: Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Ensminger, L.G. The association of official analytical chemists. Clin. Toxicol. 1976, 9, 471. [Google Scholar] [CrossRef]
- Pupim Ferreira, A.A.; Alves, M.J.M.; Barrozo, S.; Yamanaka, H.; Benedetti, A.V. Optimization of incubation time of protein Tc85 in the construction of biosensor: Is the EIS a good tool? J. Electroanal. Chem. 2010, 643, 1–8. [Google Scholar] [CrossRef]

| Matrix | Dynamic Range | LOD 1 | Ref. |
|---|---|---|---|
| Pt electrodes modified with polyaniline and polystyrene sulphonic acid | 0–6 mg·L−1 | 0.1 mg·L−1 | [17] |
| Silica gel–ionic liquid biocompatible film on the glassy carbon electrode | 0.1–10 ng·mL−1 | 0.01 ng·mL−1 | [18] |
| 1,6-hexanedithiol, colloidal Au, and aflatoxin B1—bovine serum albumin conjugate on a gold electrode | 0.08–100 ng·mL−1 | 0.05 ng·mL−1 | [19] |
| Graphene oxide on Au electrode | 0.5–5 ng·mL−1 | 0.23 ng·mL−1 | [20] |
| Graphene/polypyrrole/pyrrolepropylic acid composite film on glassy carbon electrode | 10 fg·mL−1–10 pg·mL−1 | 10 fg·mL−1 | [21] |
| Poly(amidoamine) dendrimers of fourth generation immobilized on gold electrode covered by cystamine | 0.03–3.1 ng·mL−1 | 0.12 ng·mL−1 | [22] * |
| MWCNT 2/ionic liquid composite films on glassy carbon electrode | 0.1–10 ng·mL−1 | 0.03 ng·mL−1 | [23] |
| Poly(ophenylenediamine) electropolymerized film modified gold three-dimensional nanoelectrode ensembles | 0.04–8.0 ng·mL−1 | 0.019 ng·mL−1 | [24] |
| Screen-printed interdigitated microelectrodes modified with 3-Dithiobis-(sulfosuccinimidyl-propionate) and Protein G | 5–20 ng·mL−1 | 5 ng·mL−1 | [25] |
| Sample | Concentration × 10−9/mol·L−1 | Concentration/µg·kg−1 |
|---|---|---|
| A | 13 ± 1 | 65 ± 6 |
| B | 13.3 ± 0.6 | 67 ± 3 |
| C | 5.0 ± 0.3 | 25 ± 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foguel, M.V.; Furlan Giordano, G.; De Sylos, C.M.; Carlos, I.Z.; Pupim Ferreira, A.A.; Benedetti, A.V.; Yamanaka, H. A Low-Cost Label-Free AFB1 Impedimetric Immunosensor Based on Functionalized CD-Trodes. Chemosensors 2016, 4, 17. https://doi.org/10.3390/chemosensors4030017
Foguel MV, Furlan Giordano G, De Sylos CM, Carlos IZ, Pupim Ferreira AA, Benedetti AV, Yamanaka H. A Low-Cost Label-Free AFB1 Impedimetric Immunosensor Based on Functionalized CD-Trodes. Chemosensors. 2016; 4(3):17. https://doi.org/10.3390/chemosensors4030017
Chicago/Turabian StyleFoguel, Marcos Vinicius, Gabriela Furlan Giordano, Célia Maria De Sylos, Iracilda Zeppone Carlos, Antonio Aparecido Pupim Ferreira, Assis Vicente Benedetti, and Hideko Yamanaka. 2016. "A Low-Cost Label-Free AFB1 Impedimetric Immunosensor Based on Functionalized CD-Trodes" Chemosensors 4, no. 3: 17. https://doi.org/10.3390/chemosensors4030017