Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
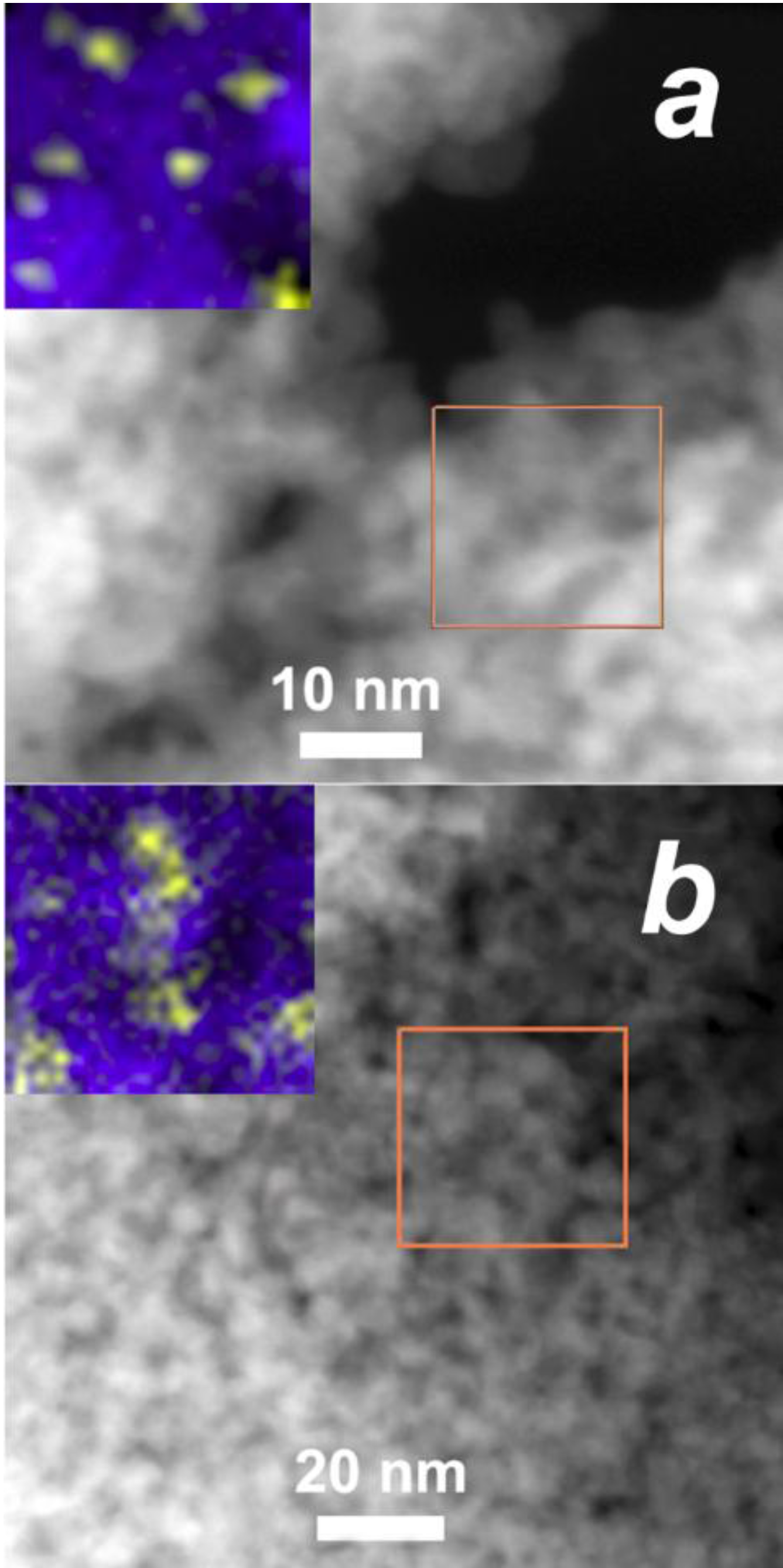
3.1. Materials Composition and Microstructure
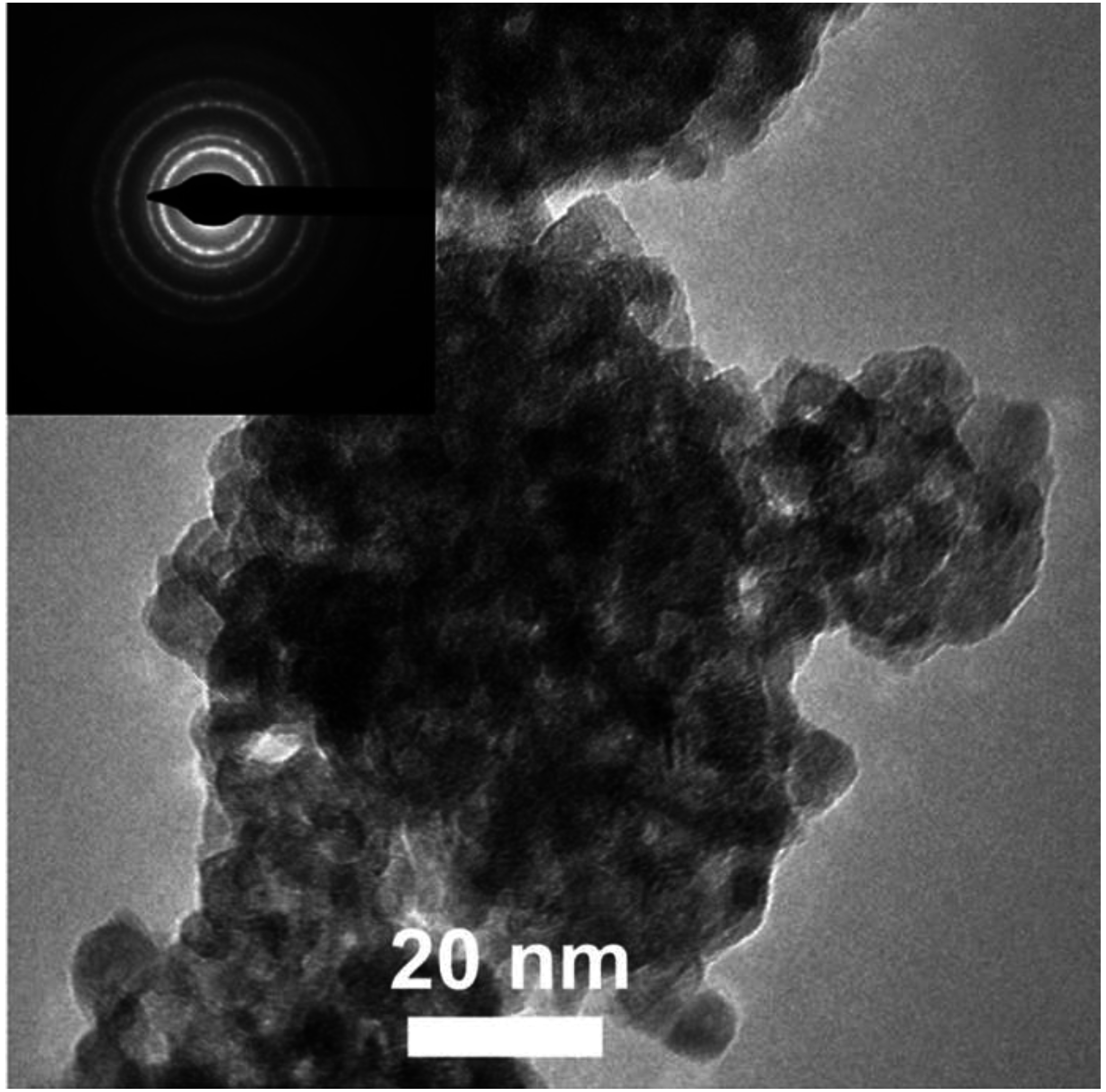
| Sample | Crystalline Phase a | Modifier Content and Composition | Particle Size, nm | BET Area, m2/g | |||
|---|---|---|---|---|---|---|---|
| Total Concentration b, wt.% | Oxidation and Crystalline State c, at.% from Total Modifier Concentration | SnO2 | Modifier e | ||||
| (dXRD)a | (dTEM)d | ||||||
| SnO2 | SnO2 | 3–6 | 2–8 | 95–100 | |||
| SnO2/PdOx | 0.94 ± 0.05 | 70 at.%––PdO (amorphous)
25 at.%––Pd0 5 at.%––Pd3+ | 3–5 | 1–3 | 90–95 | ||
| SnO2/RuOy | 0.81 ± 0.05 | 80 at.%––RuO2 (structured)
20 at.%––Ru3+ | 3–5 | 2–5 | 90–95 | ||
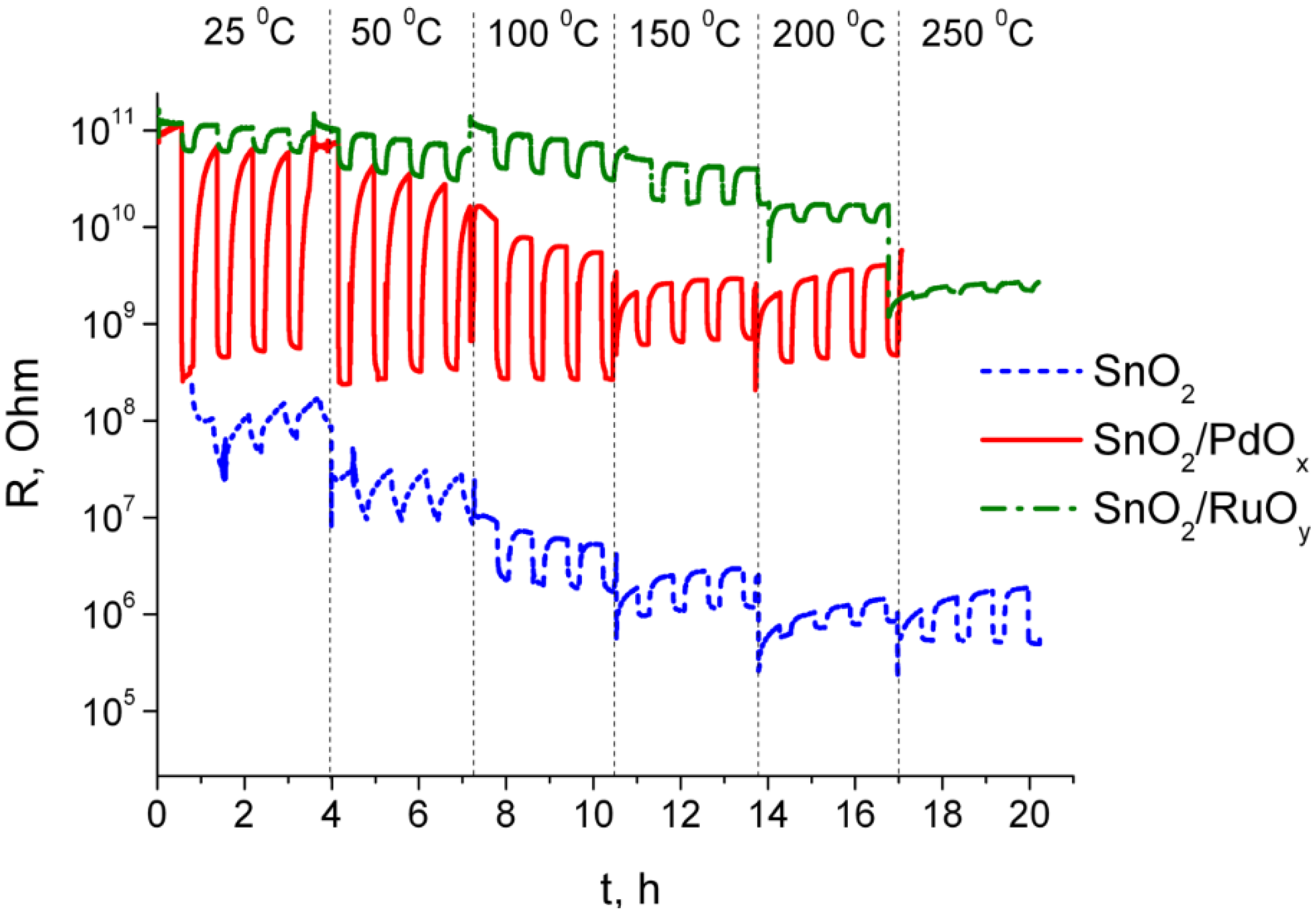
3.2. Sensing Behavior to Individual CO and NH3 Gases
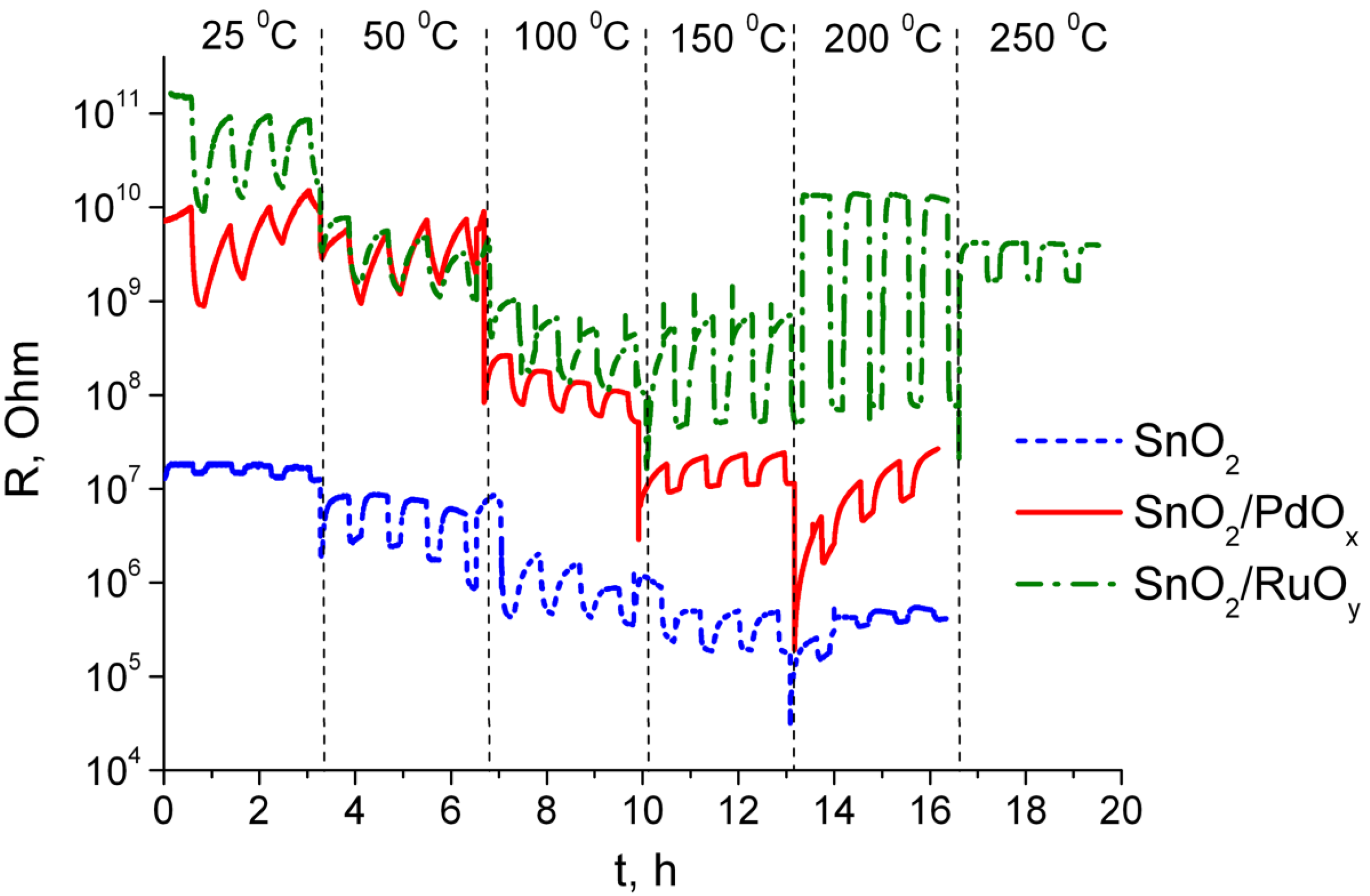
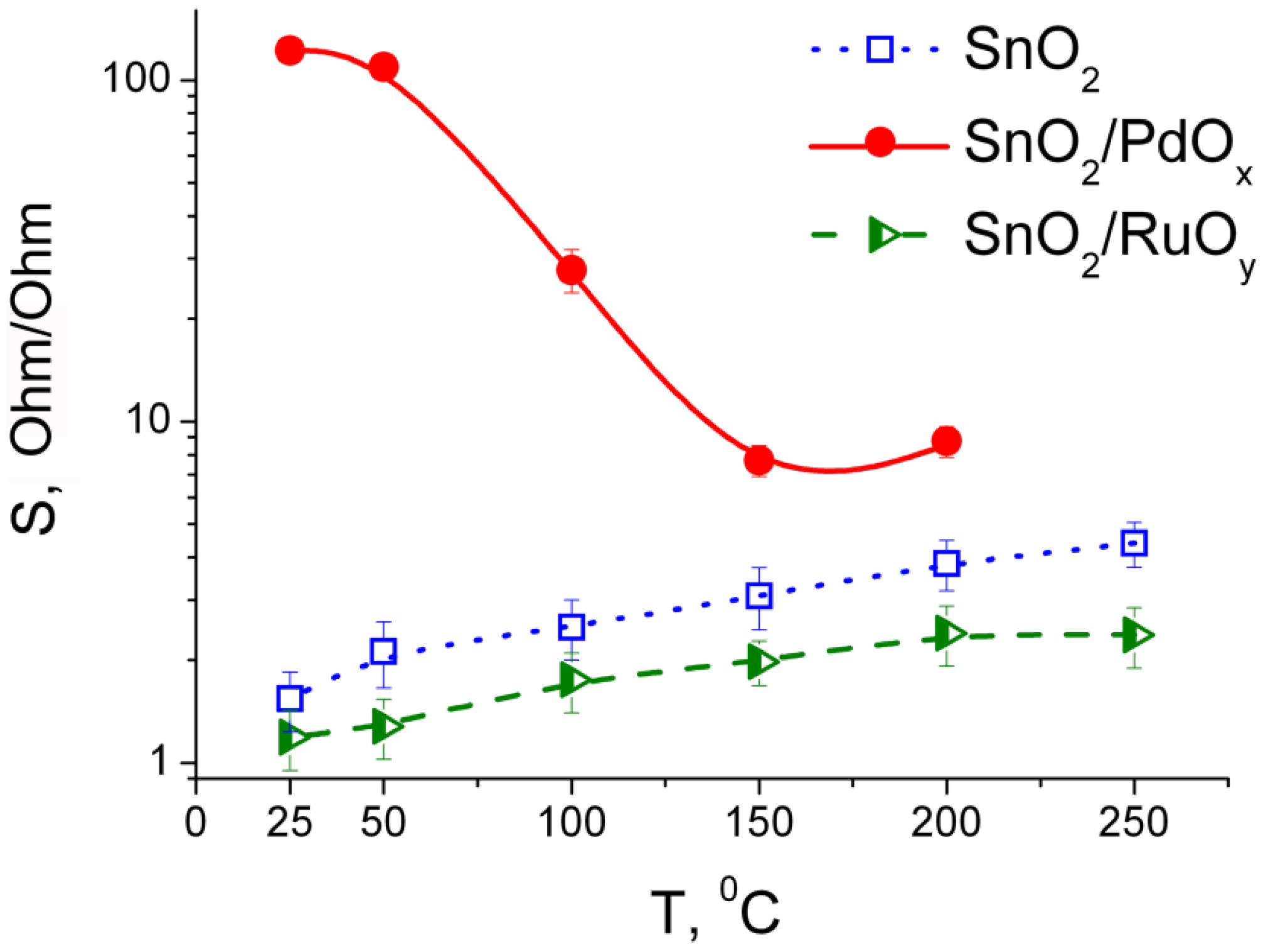
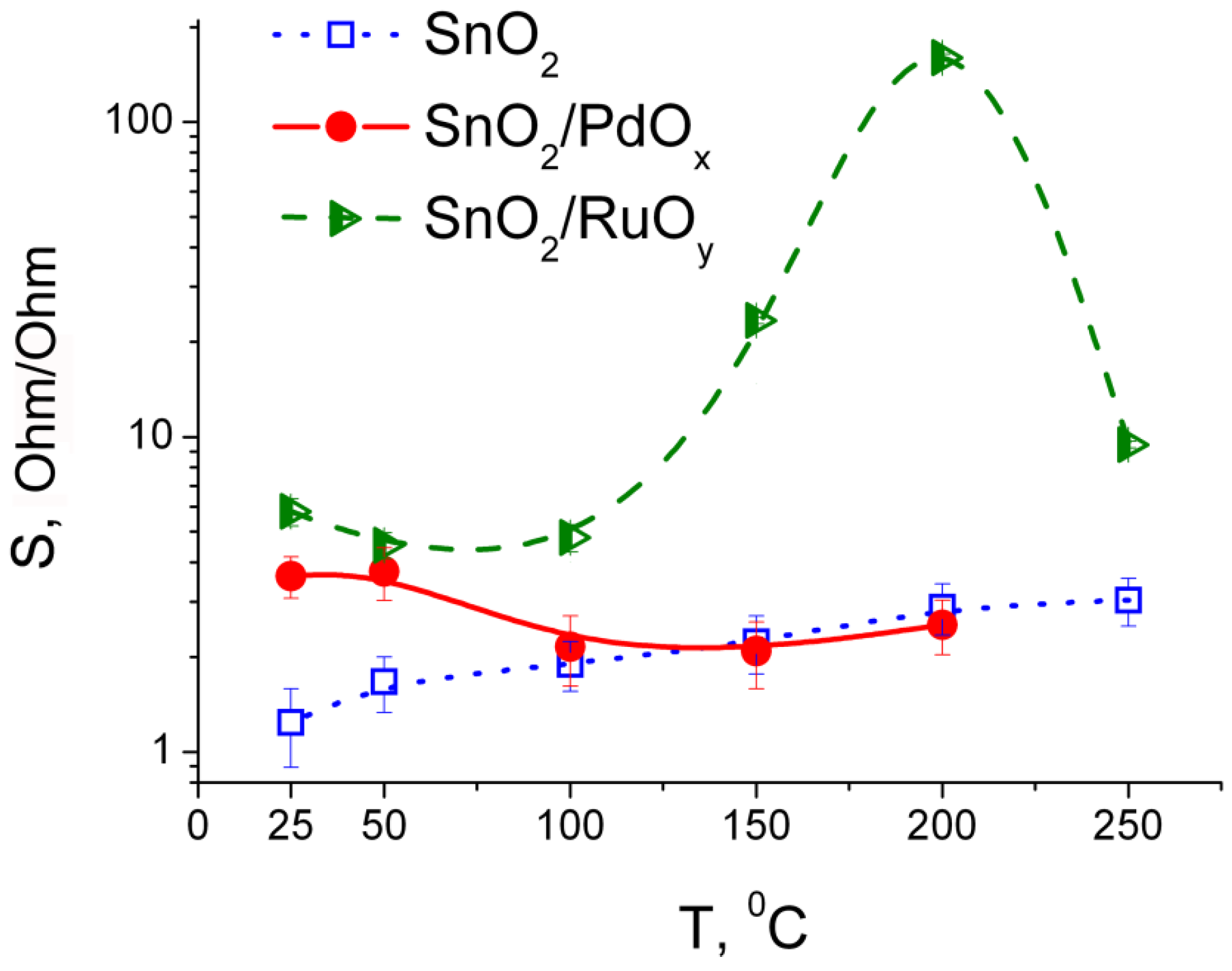
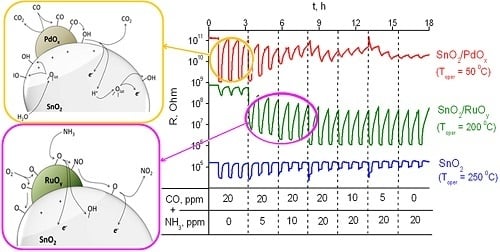
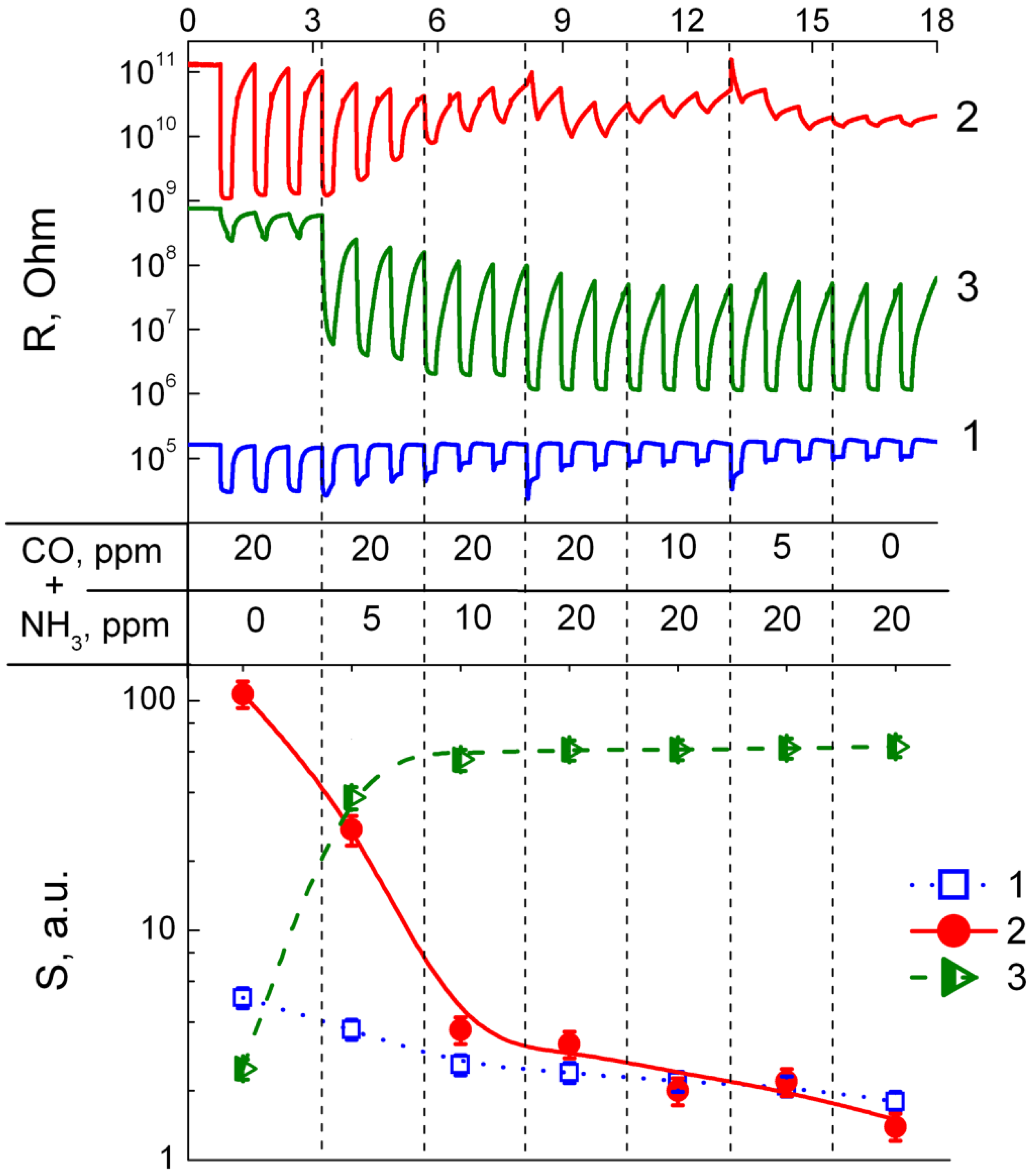
3.3. Sensing Behavior to CO + NH3 Gases Mixtures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: state of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Samotaev, N.N.; Vasiliev, A.A.; Podlepetsky, B.I.; Sokolov, A.V.; Pisliakov, A.V. The mechanism of the formation of selective response of semiconductor gas sensor in mixture of CH4/H2/CO with air. Sens. Actuators B Chem. 2007, 127, 242–247. [Google Scholar] [CrossRef]
- Frank, K.; Kohler, H.; Guth, U. Influence of the measurement conditions on the sensitivity of SnO2 gas sensors operated thermo-cyclically. Sens. Actuators B Chem. 2009, 141, 361–369. [Google Scholar] [CrossRef]
- Ederth, J.; Smulko, J.M.; Kish, L.B.; Heszler, P.; Granqvist, C.G. Comparison of classical and fluctuation-enhanced gas sensing with PdxWO3 nanoparticle films. Sens. Actuators B Chem. 2006, 113, 310–315. [Google Scholar] [CrossRef]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; Weinheim: Wiley, Weinheim, Germany, 2003. [Google Scholar]
- Seifert, R.; Keller, H.; Matthes, J. A review on innovative procedures for the analysis of data from gas sensor systems and networks. Sens. Transducers 2015, 184, 1–10. [Google Scholar]
- Jerger, A.; Kohler, H.; Becker, F.; Keller, H.B.; Seifert, R. New applications of tin oxide gas sensors II. Intelligent sensor system for reliable monitoring of ammonia leakages. Sens. Actuators B Chem. 2002, 81, 301–307. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gaskov, A.M. Chemical modification of nanocrystalline metal oxides: Effect of the real structure and surface chemistry on the sensor properties. Rus. Chem. Bull. 2008, 57, 1106–1125. [Google Scholar] [CrossRef]
- Cabot, A.; Arbiol, J.; Morante, J.R.; Weimar, U.; Barsan, N.; Gopel, W. Analysis of the noble metal catalytic additives introduced by impregnation of as obtained SnO2 sol–gel nanocrystals for gas sensors. Sens. Actuators B Chem. 2000, 70, 87–100. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Aruna, I.; Kruis, F.E.; Kundu, S.; Muhler, M.; Theissmann, R.; Spasova, M. CO ppb sensors based on monodispersed SnOx: Pd mixed nanoparticle layers: Insight into dual conductance response. J. Appl. Phys. 2009. [Google Scholar] [CrossRef]
- Kim, B.; Lu, Y.J.; Hannon, A.; Meyyappan, M.; Li, J. Low temperature Pd/SnO2 sensor for carbon monoxide detection. Sens. Actuators B Chem. 2013, 177, 770–775. [Google Scholar] [CrossRef]
- Manjula, P.; Arunkumar, S.; Manorama, S.V. Au/SnO2 an excellent material for room temperature carbon monoxide sensing. Sens. Actuators B Chem. 2011, 152, 168–175. [Google Scholar] [CrossRef]
- Hubner, M.; Koziej, D.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. An Au clusters related spill-over sensitization mechanism in SnO2-based gas sensors identified by operando HERFD-XAS, work function changes, DC resistance and catalytic conversion studies. Phys. Chem. Chem. Phys. 2012, 14, 13249–13254. [Google Scholar] [CrossRef] [PubMed]
- Ramgir, N.S.; Hwang, Y.K.; Jhung, S.H.; Mulla, I.S.; Chang, J.-S. Effect of Pt concentration on the physicochemical properties and CO sensing activity of mesostructured SnO2. Sens. Actuators B Chem. 2006, 114, 275–282. [Google Scholar] [CrossRef]
- Vasiliev, R.B.; Rumyantseva, M.N.; Yakovlev, N.V.; Gaskov, A.M. CuO/SnO2 thin film heterostructures as chemical sensors to H2S. Sens. Actuators B Chem. 1998, 50, 186–193. [Google Scholar] [CrossRef]
- Wagh, M.S.; Jain, G.H.; Patil, D.R.; Patil, S.A.; Patil, L.A. Modified zinc oxide thick film resistors as NH3 gas sensor. Sens. Actuators B Chem. 2006, 115, 128–133. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pandey, P.; Mishra, V.N.; Dwivedi, R. Sensing mechanism of Pd-doped SnO2 sensor for LPG detection. Solid State Sci. 2009, 11, 1602–1605. [Google Scholar] [CrossRef]
- Mishra, V.N.; Agarwal, R.P. Sensitivity, response and recovery time of SnO2 based thick-film sensor array for H2, CO, CH4 and LPG. Microelectron. J. 1998, 29, 861–874. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter 2003, 15, 813–839. [Google Scholar] [CrossRef]
- Menini, P.; Parret, F.; Guerrero, M.; Soulantica, K.; Erades, L.; Maisonnat, A.; Chaudret, B. CO response of a nanostructured SnO2 gas sensor doped with palladium and platinum. Sens. Actuators B Chem. 2004, 103, 111–114. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Yashina, L.V.; Gaskov, A.M. Role of surface hydroxyl groups in promoting room temperature CO sensing by Pd-modified nanocrystalline SnO2. J. Solid State Chem. 2010, 183, 2389–2399. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Krivetskiy, V.; Yashina, L.V.; Rumyantseva, M.; Konstantinova, E.A.; Ponzoni, A.; Comini, E.; Abakumov, A.; Gaskov, A.M. Catalytic impact of RuOx clusters to high ammonia sensitivity of tin dioxide. Sens. Actuators B Chem. 2012, 175, 186–193. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Konstantinova, E.A.; Grishina, D.A.; Deygen, D.M. CO and NH3 sensor properties and paramagnetic centers of nanocrystalline SnO2 modified by Pd and Ru. Thin Solid Films 2011, 520, 904–908. [Google Scholar] [CrossRef]
- Frolov, D.D.; Kotovshchikov, Y.N.; Morozov, I.V.; Boltalin, A.I.; Fedorova, A.A.; Marikutsa, A.V.; Rumyantseva, M.N.; Gaskov, A.M.; Sadovskaya, E.M.; Abakumov, A.M. Oxygen exchange on nanocrystalline tin dioxide modified by palladium. J. Solid State Chem. 2012, 186, 1–8. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Frolov, D.D.; Morozov, I.V.; Boltalin, A.I.; Fedorova, A.A.; Petukhov, I.; Yashina, L.V.; Konstantinova, E.A.; Sadovskaya, E.M.; et al. Role of PdOx and RuOy clusters in oxygen exchange between nanocrystalline tin dioxide and the gas phase. J. Phys. Chem. C 2013, 117, 23858–23867. [Google Scholar] [CrossRef]
- Marikutsa, A.V.; Rumyantseva, M.N.; Konstantinova, E.A.; Shatalova, T.B.; Gaskov, A.M. Active sites on nanocrystalline tin dioxide surface: effect of palladium and ruthenium oxides clusters. J. Phys. Chem. C 2014, 118, 21541–21549. [Google Scholar] [CrossRef]
- Frank, K.; Magapu, V.; Schindler, V.; Kohler, H.; Keller, H.B.; Seifert, R. Chemical analysis with tin oxide gas sensors: Choice of additives, method of operation and analysis of numerical signal. Sens. Lett. 2008, 6, 908–911. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures. Chemosensors 2015, 3, 241-252. https://doi.org/10.3390/chemosensors3040241
Marikutsa A, Rumyantseva M, Gaskov A. Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures. Chemosensors. 2015; 3(4):241-252. https://doi.org/10.3390/chemosensors3040241
Chicago/Turabian StyleMarikutsa, Artem, Marina Rumyantseva, and Alexander Gaskov. 2015. "Selectivity of Catalytically Modified Tin Dioxide to CO and NH3 Gas Mixtures" Chemosensors 3, no. 4: 241-252. https://doi.org/10.3390/chemosensors3040241