Development of Solid-State Electrochemiluminescence (ECL) Sensor Based on Ru(bpy)32+-Encapsulated Silica Nanoparticles for the Detection of Biogenic Polyamines
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Instrumentation and Methods
2.3. Nanoparticles Synthesis
2.4. Nanoparticles Surface Modification
2.5. Immobilization
3. Results and Discussion
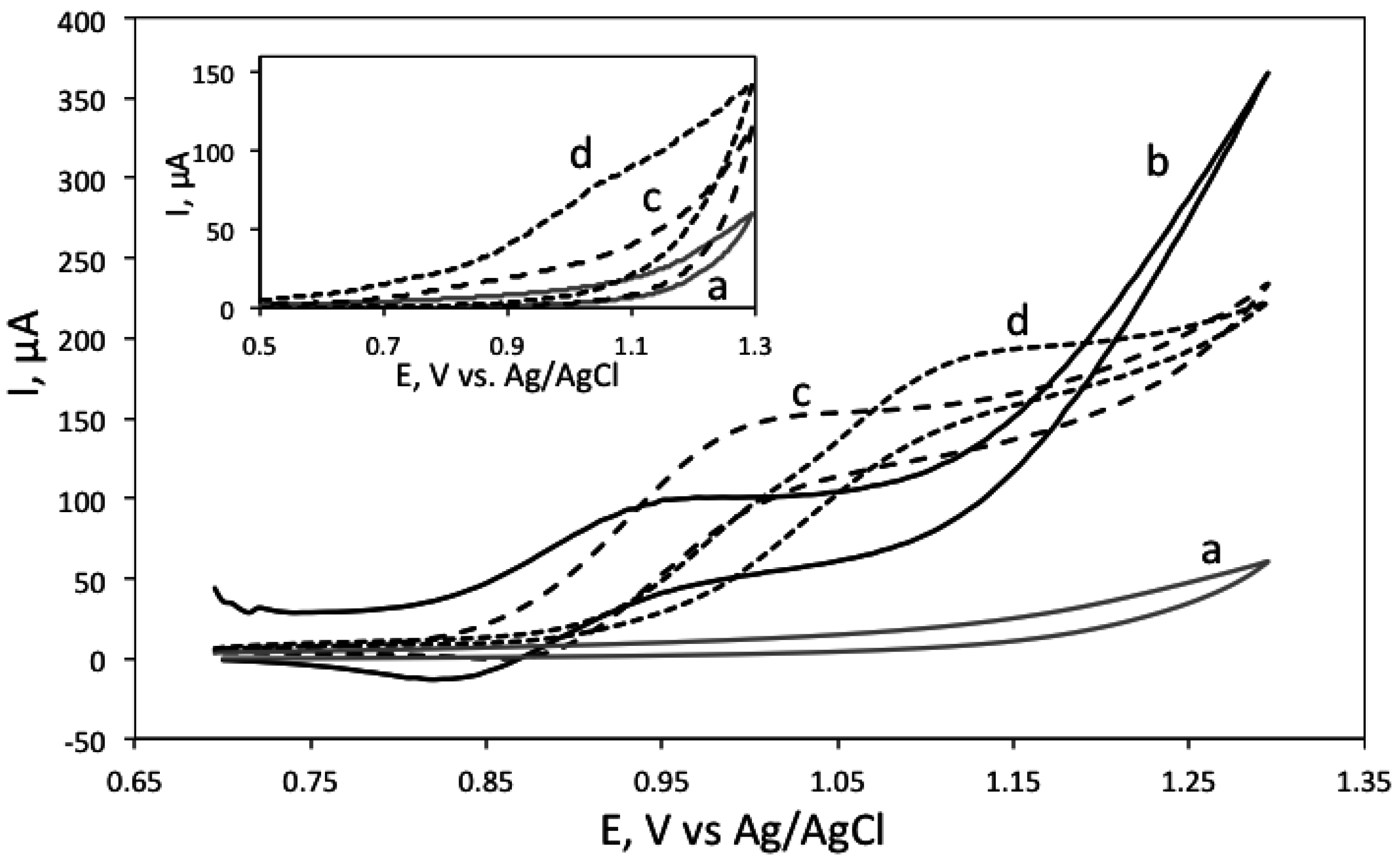
3.1. Electrochemical and ECL Behaviour of Biogenic Amines
| Biogenic Amine | S/N at pH 7.5 | S/N pH 9.2 |
|---|---|---|
| Spermine | 320 | 85 |
| Spermidine | 355 | 91 |
| Putrescine | 15 | 25 |
| Cadaverine | 5 | 18 |
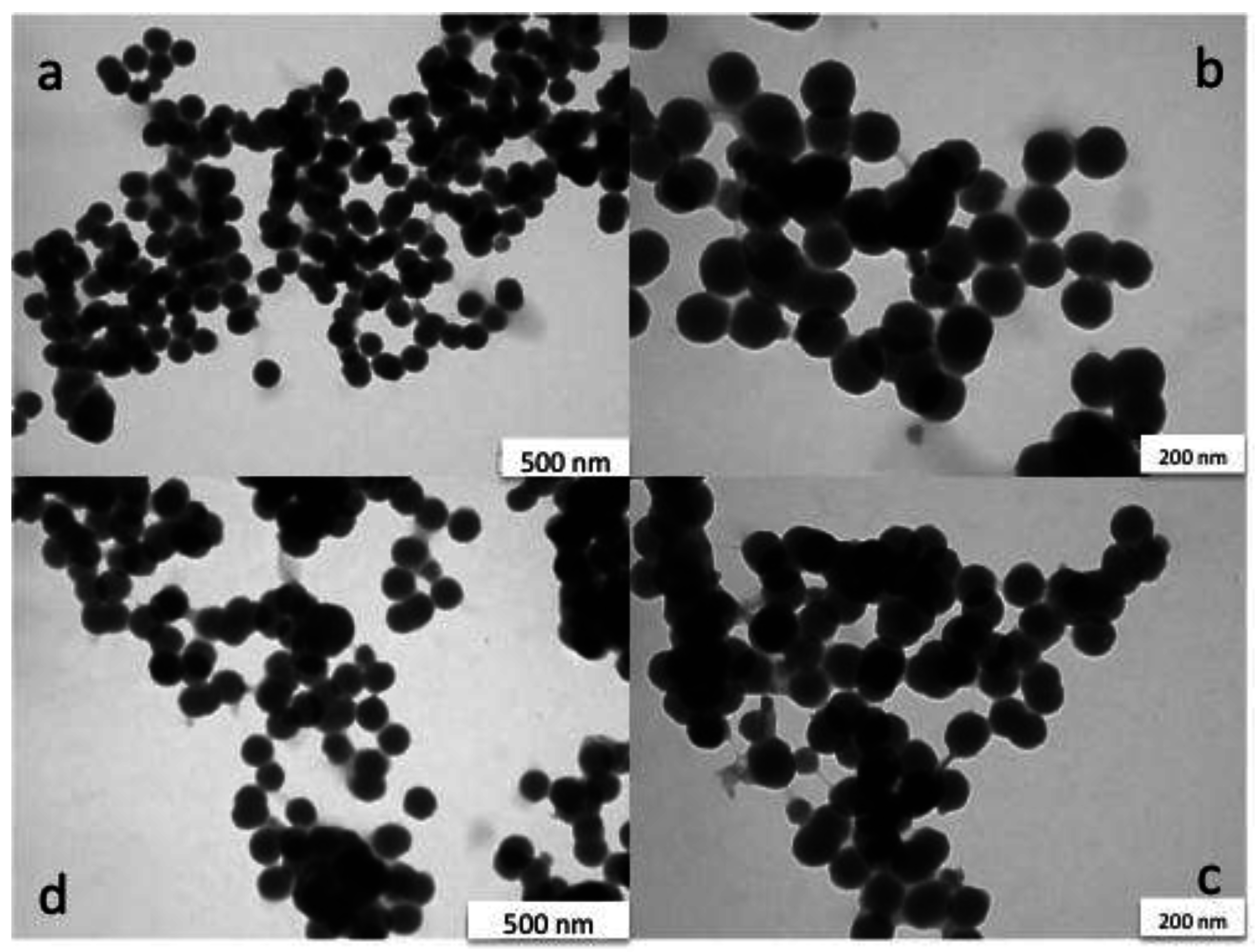
3.2. Characterisation of Silica Nanoparticles
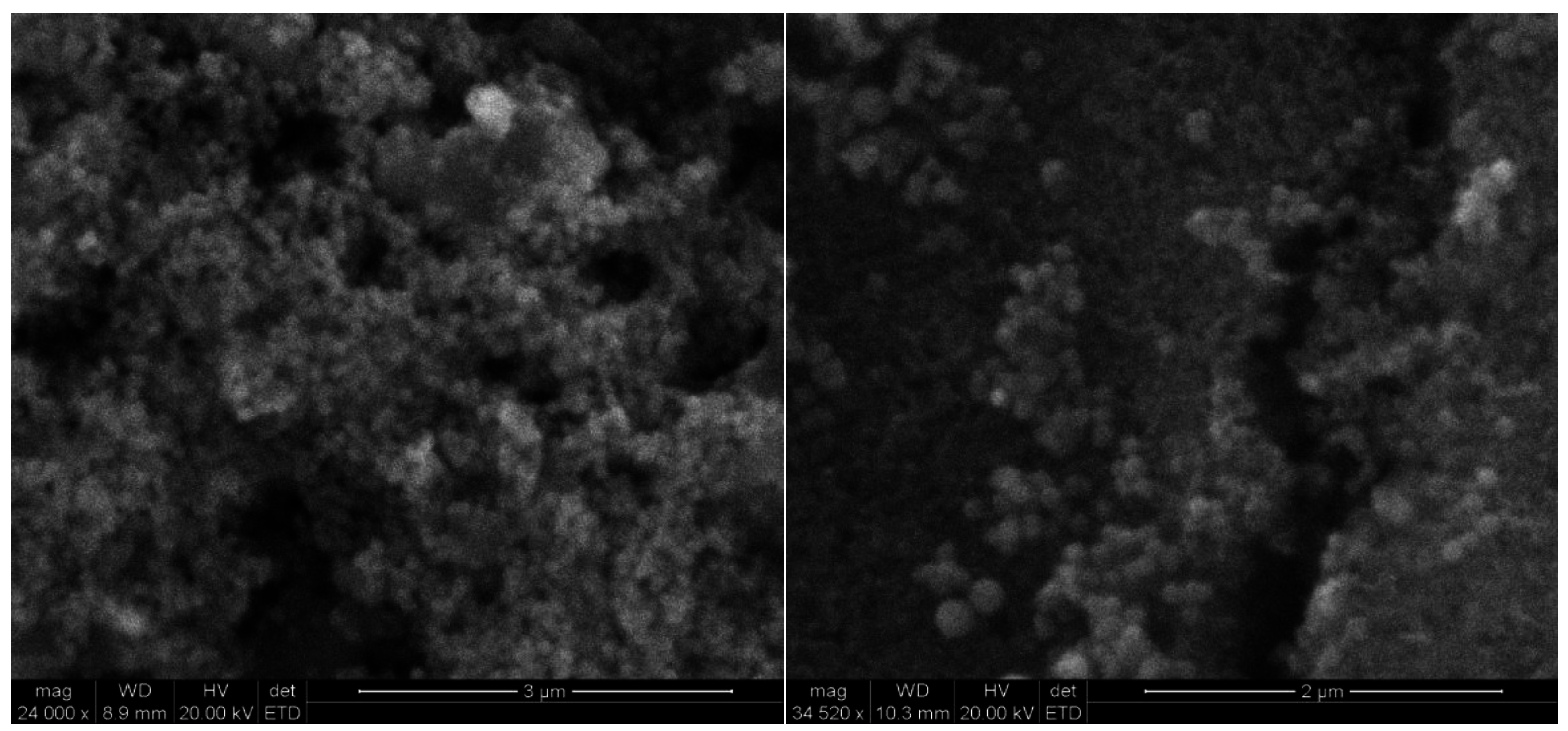
3.3. Characterisation of the Modified Carbon Surface
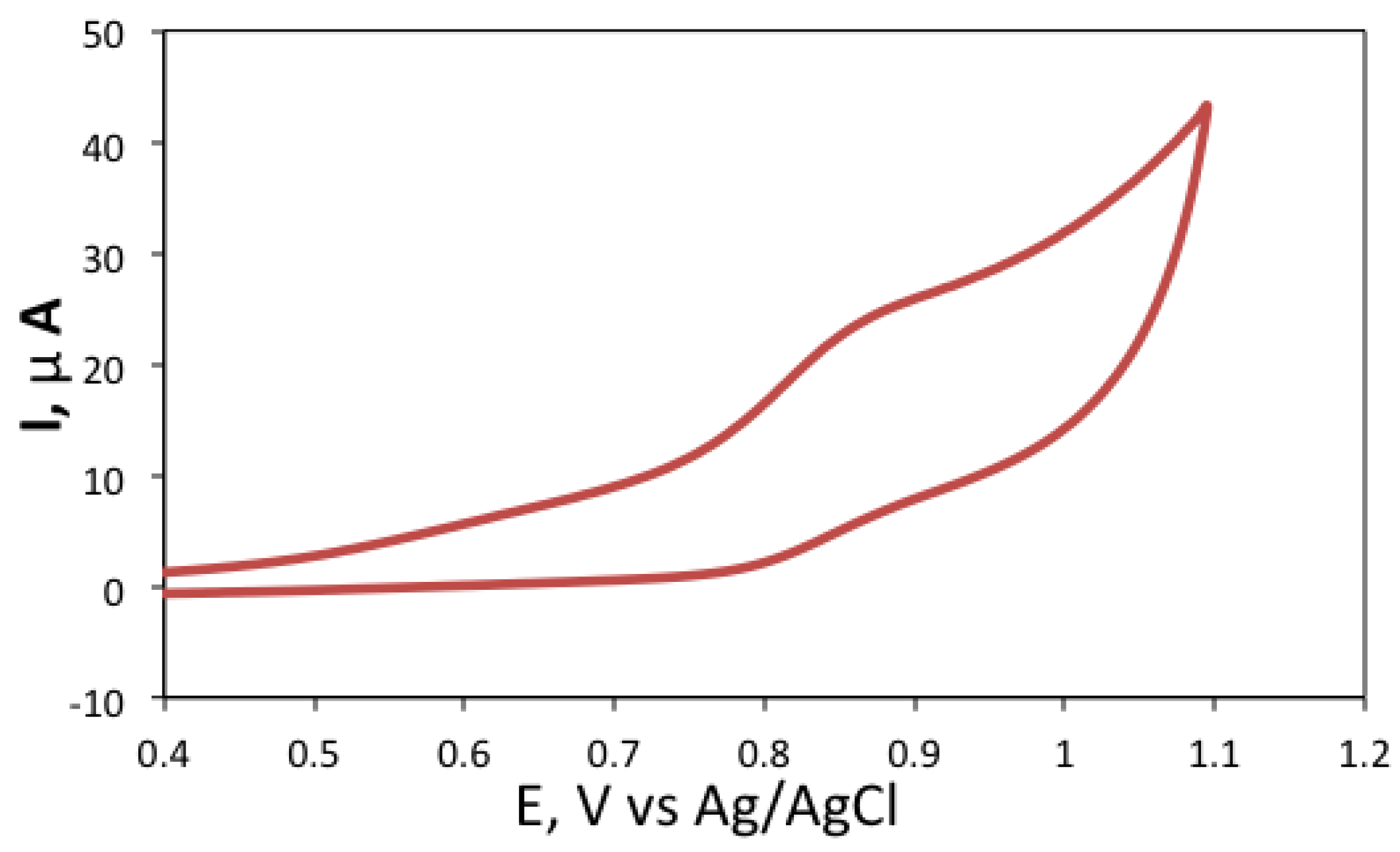
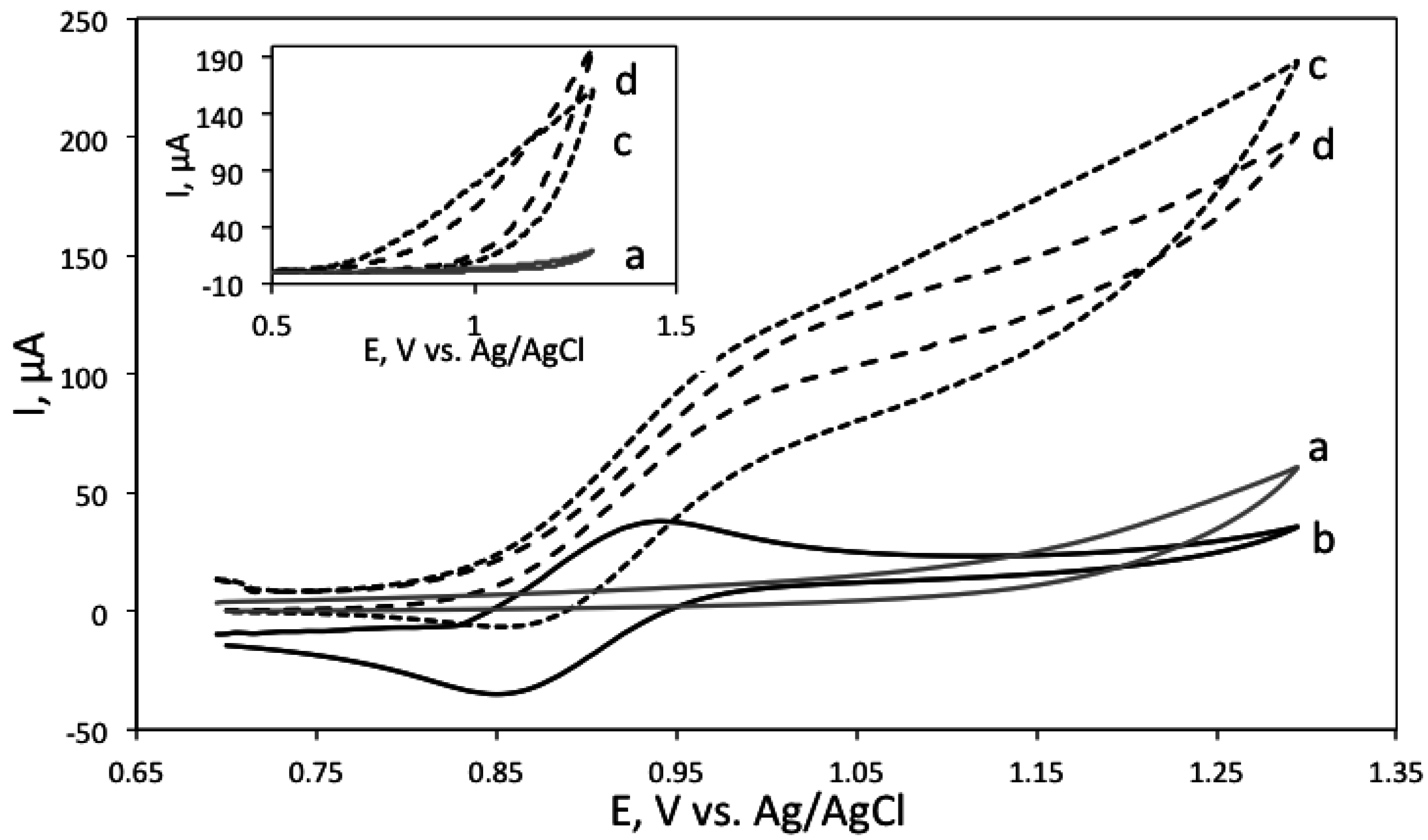
3.4. ECL Detection of the Biogenic Amines
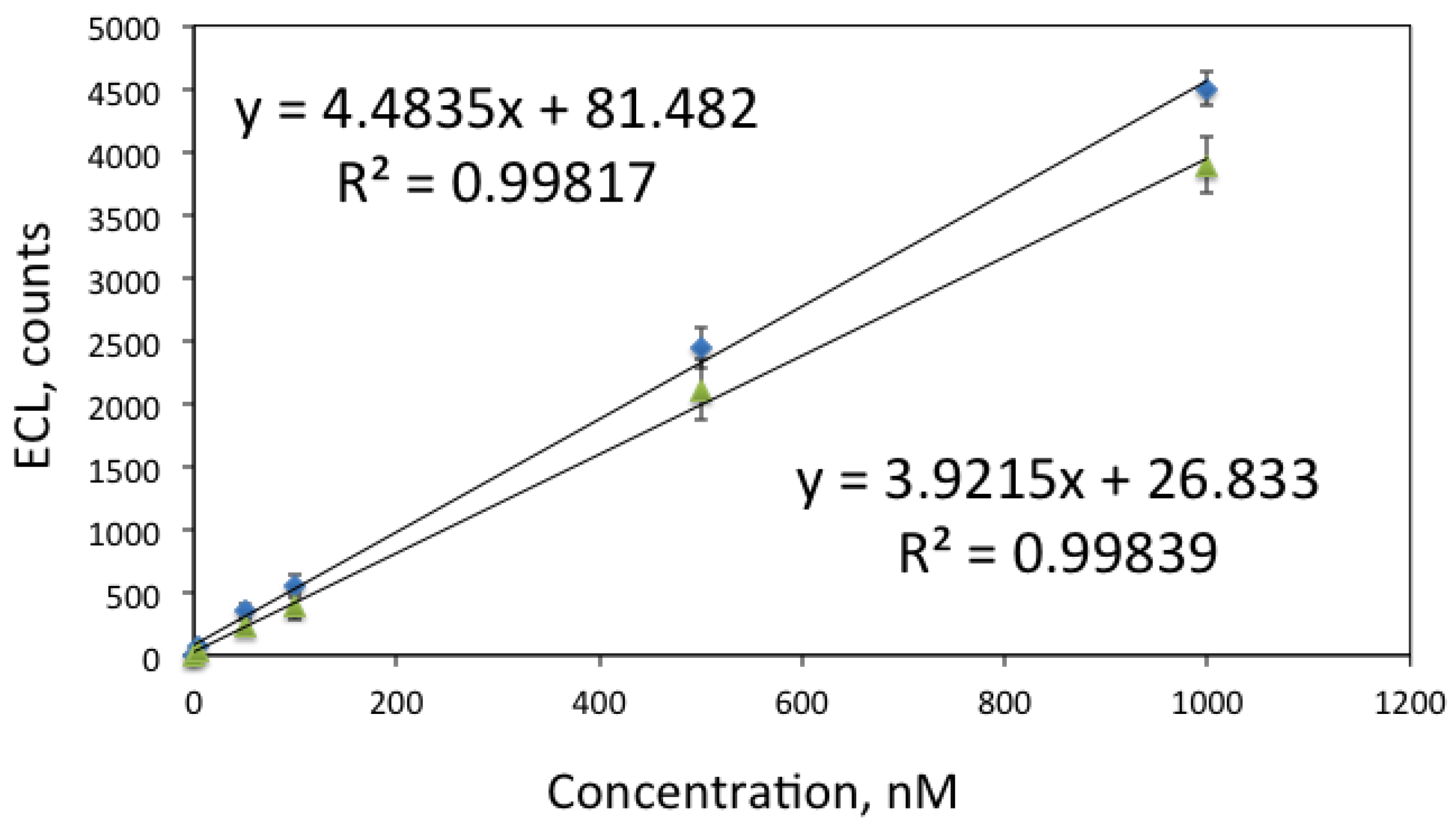

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Su, Y.; Chen, H.; Wang, Z.; Lv, Y. Recent advances in Chemiluminescence. Appl. Spectros. Rev. 2007, 42, 139–176. [Google Scholar] [CrossRef]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef] [PubMed]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; Forster, R.J.; Keyes, T.E. Nanostructured materials for electrochemiluminescence (ECL)-based detection methods: Recent advances and future perspectives. Biosens. Bioelectron. 2009, 24, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Electrochemiluminescence of tris(2,2'-bipyridyl)ruthenium and its applications in bioanalysis: A review. Luminescence 2011, 26, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, K. Current trends in the development of the electrochemicaluminescent immunosensors. Biosens. Bioelectron. 2014, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E.K. Solid-state electrochemiluminescence of tris(2,2'-bipyridyl) ruthenium. TrAC Trends Anal. Chem. 2008, 27, 447–459. [Google Scholar] [CrossRef]
- Blackburn, G.F.; Shah, H.P.; Kenten, J.H.; Leland, J.; Kamin, R.A.; Link, J.; Peterman, J.; Powell, M.J.; Shah, A.; Talley, D.B. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin. Chem. 1991, 37, 1534–1539. [Google Scholar] [PubMed]
- Spehar-Délèze, A.; Schmidt, L.; Neier, R.; Kulmala, S.; de Rooij, N.; Koudelka-Hep, M. Electrochemiluminescent hybridization chip with electric field aided discrimination. Biosens. Bioelectron. 2006, 22, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Leland, J.K.; Powell, M.J. Electrogenerated chemiluminescence: an oxidative-reduction type ECL reaction sequence using tripropyl amine. J. Electrochem. Soc. 1990, 137, 3127–3131. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.-P.; Bard, A.J. Electrogenerated chemiluminescence 69: The tris(2,2'-bipyridine)ruthenium(II)/tri-n-propylamine (TPrA) system revisited—A new route involving TPrA cation radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jia, J.; Yang, X.; Dong, S.; Wang, E. Capillary electrophoresis with solid-state electrochemiluminescence detector. Electrophoresis 2002, 23, 3692–3698. [Google Scholar] [CrossRef] [PubMed]
- Dennany, L.; Foster, R.J.; Rusling, J.F. Simultaneous Direct Electrochemiluminescence and Catalytic Voltammetry Detection of DNA in Ultrathin Films. J. Am. Chem. Soc. 2003, 125, 5213–5218. [Google Scholar] [CrossRef] [PubMed]
- Greenway, G.M.; Nelstrop, L.J.; Port, S.N. Tris(2,2-bipyridyl)ruthenium(II) chemiluminescence in a microflow injection system for codeine determination. Anal. Chim. Acta 2000, 405, 43–50. [Google Scholar] [CrossRef]
- Eunsook, S.J.; Norris, B.J.; Puntano, P. An electrogenerated chemiluminescence imaging fiber electrode chemical sensor for NADH. Electroanalysis 2001, 13, 1287–1290. [Google Scholar] [CrossRef]
- Choi, J.-P.; Bard, A.J. Electrogenerated chemiluminescence (ECL) 79. Reductive-oxidation ECL of tris(2,2'-bipyridine)ruthenium(II) using hydrogen peroxide as a coreactant in pH 7.5 phosphate buffer solution. Anal. Chim. Acta 2005, 541, 143–150. [Google Scholar]
- Rubinstein, I.; Bard, A.J. Polymer Films on Electrodes. 5. Electrochemistry and Chemiluminescence at Nafion-Coated Electrodes. J. Am. Chem. Soc. 1981, 103, 5007–5013. [Google Scholar] [CrossRef]
- Su, M.; Liu, S. Solid state electrochemiluminescence analysis with coreactant of the immobilized tris(2,2'-bipyridyl) ruthenium. Anal. Biochem. 2010, 402, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, A.; Dennany, L.; Dickinson, C.; Keyes, T.E.; Forster, R.J. Enhanced Electrochemiluminescence and Charge Transport through Films of Metallopolymer-Gold Nanoparticle Composites. Electrochem. Comm. 2012, 19, 43–45. [Google Scholar] [CrossRef]
- Venkatanarayanan, A.; Spehar-Délèze, A.; Dennany, L.; Pellegrin, Y.; Keyes, T.E.; Forster, R.J. Ruthenium Amino-phenanthroline Films Deposited from an Ionic Liquid: Deposition, Electrochemical and Photonic Properties. Langmuir 2008, 24, 11233–11238. [Google Scholar] [CrossRef] [PubMed]
- Piper, D.J.E.; Barbante, G.J.; Brack, N.; Pigram, P.J.; Hogan, C.F. Highly Stable ECL Active Films Formed by the Electrografting of a Diazotized Ruthenium Complex Generated in situ from the Amine. Langmuir 2011, 27, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.P.; Du, Y.; Dong, S.J.; Wang, E.K. Method for effective immobilization of Ru(bpy)32+ on an electrode surface for solid-state electrochemiluminescence detection. Anal. Chem. 2005, 77, 8166–8169. [Google Scholar] [CrossRef] [PubMed]
- Bertoncello, P.; Dennany, L.; Forster, R.J.; Unwin, P.R. Nafion-Tris(2–2'-bipyridyl)ruthenium(II) Ultrathin Langmuir-Schaefer Films: Redox Catalysis and Electrochemiluminescent Properties. Anal. Chem. 2007, 79, 7549–7553. [Google Scholar] [CrossRef] [PubMed]
- Moretto, L.M.; Kohls, T.; Badocco, D.; Pastore, P.; Sojic, N.; Ugo, P. Electrochemiluminescence of Ru(bpy)32+ loaded in Nafion Langmuir-Blodget films: Role of the interfacial ultrathin film. J. Electroanal. Chem. 2010, 1–2, 35–41. [Google Scholar]
- Qian, L.; Yang, X.R. One-step synthesis of Ru(2,2'-bipyridine)3Cl2-immobilized silica nanoparticles for use in electrogenerated chemiluminescence detection. Adv. Funct. Mater. 2007, 17, 1353–1358. [Google Scholar] [CrossRef]
- Zhang, L.H.; Dong, S.J. Electrogenerated chemiluminescence sensors using Ru(bpy)32+ doped in silica nanoparticles. Anal. Chem. 2006, 78, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, N.P.; Barron, J.; Rusling, J.F. Carbon nanotube microwell array for sensitive electrochemiluminescent detection of cancer biomarker proteins. Anal. Chem. 2011, 83, 6698–6703. [Google Scholar] [CrossRef] [PubMed]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in food. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Tombelli, S.; Mascini, M. Electrochemical biosensors for biogenic amines: A comparison between different approaches. Anal. Chim. Acta 1998, 358, 277–284. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry based saliva metabolomics identifies oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Igarachi, K.; Ueda, S.; Yoshida, K.; Kashiwagi, K. Polyamines in renal failure. Amino Acids 2006, 31, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; Wang, E. Direct tris(2,2'-bipyridyl)ruthenium(II) electrochemiluminescence detection of polyamines separated by capillary electrophoresis. Electrophoresis 2003, 24, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.W.; Greenway, G.M. Relationship between structural attributes and observed electrogenerated chemiluminescence (ECL) activity of tertiary amines as potential analytes for the tris(2,2-bipyridine)ruthenium(II) ECL reaction. A review. Analyst 1996, 121, 101R–106R. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. 501–503. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spehar-Délèze, A.-M.; Almadaghi, S.; O'Sullivan, C.K. Development of Solid-State Electrochemiluminescence (ECL) Sensor Based on Ru(bpy)32+-Encapsulated Silica Nanoparticles for the Detection of Biogenic Polyamines. Chemosensors 2015, 3, 178-189. https://doi.org/10.3390/chemosensors3020178
Spehar-Délèze A-M, Almadaghi S, O'Sullivan CK. Development of Solid-State Electrochemiluminescence (ECL) Sensor Based on Ru(bpy)32+-Encapsulated Silica Nanoparticles for the Detection of Biogenic Polyamines. Chemosensors. 2015; 3(2):178-189. https://doi.org/10.3390/chemosensors3020178
Chicago/Turabian StyleSpehar-Délèze, Anna-Maria, Sallam Almadaghi, and Ciara K. O'Sullivan. 2015. "Development of Solid-State Electrochemiluminescence (ECL) Sensor Based on Ru(bpy)32+-Encapsulated Silica Nanoparticles for the Detection of Biogenic Polyamines" Chemosensors 3, no. 2: 178-189. https://doi.org/10.3390/chemosensors3020178