Revisiting the Corticomotor Plasticity in Low Back Pain: Challenges and Perspectives
Abstract
:1. Lower Back Pain: A Growing Burden for Society
Single Pulse TMS
Paired-Pulse TMS
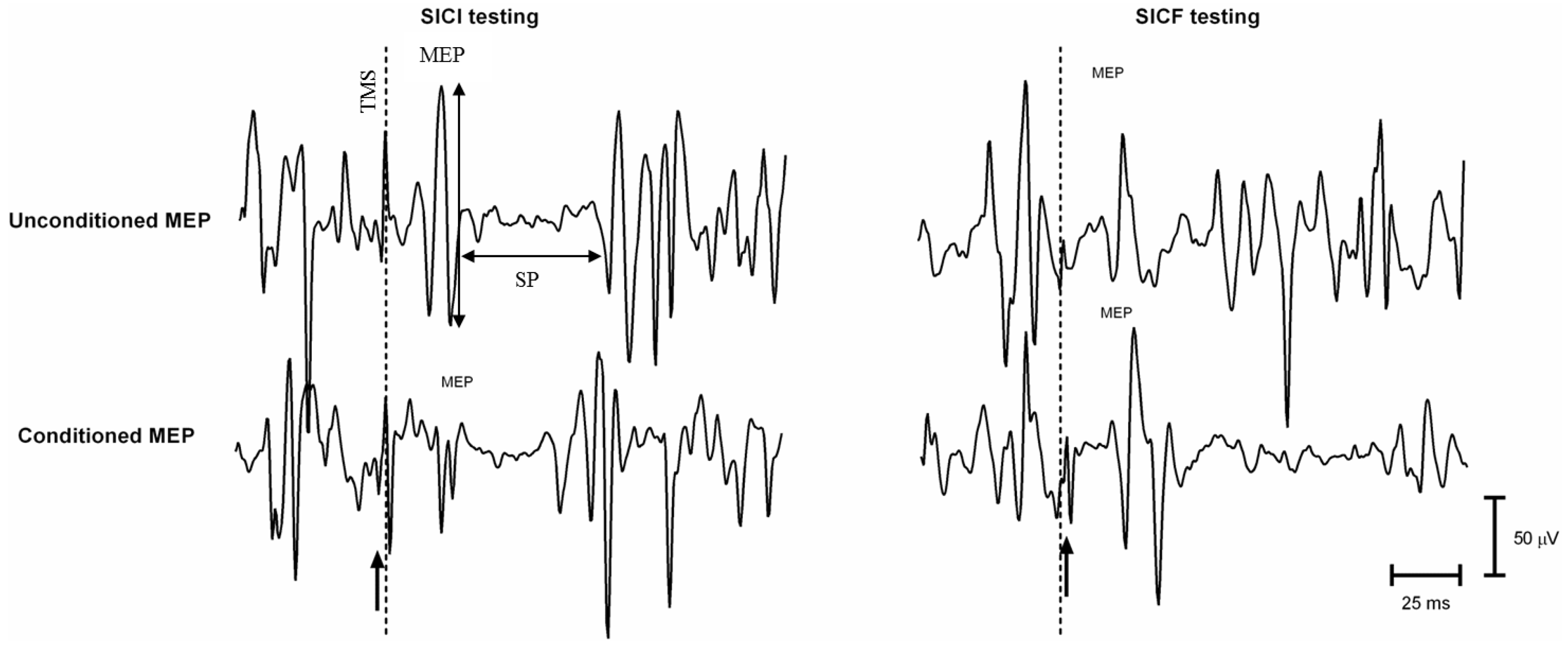
Double-Coil TMS
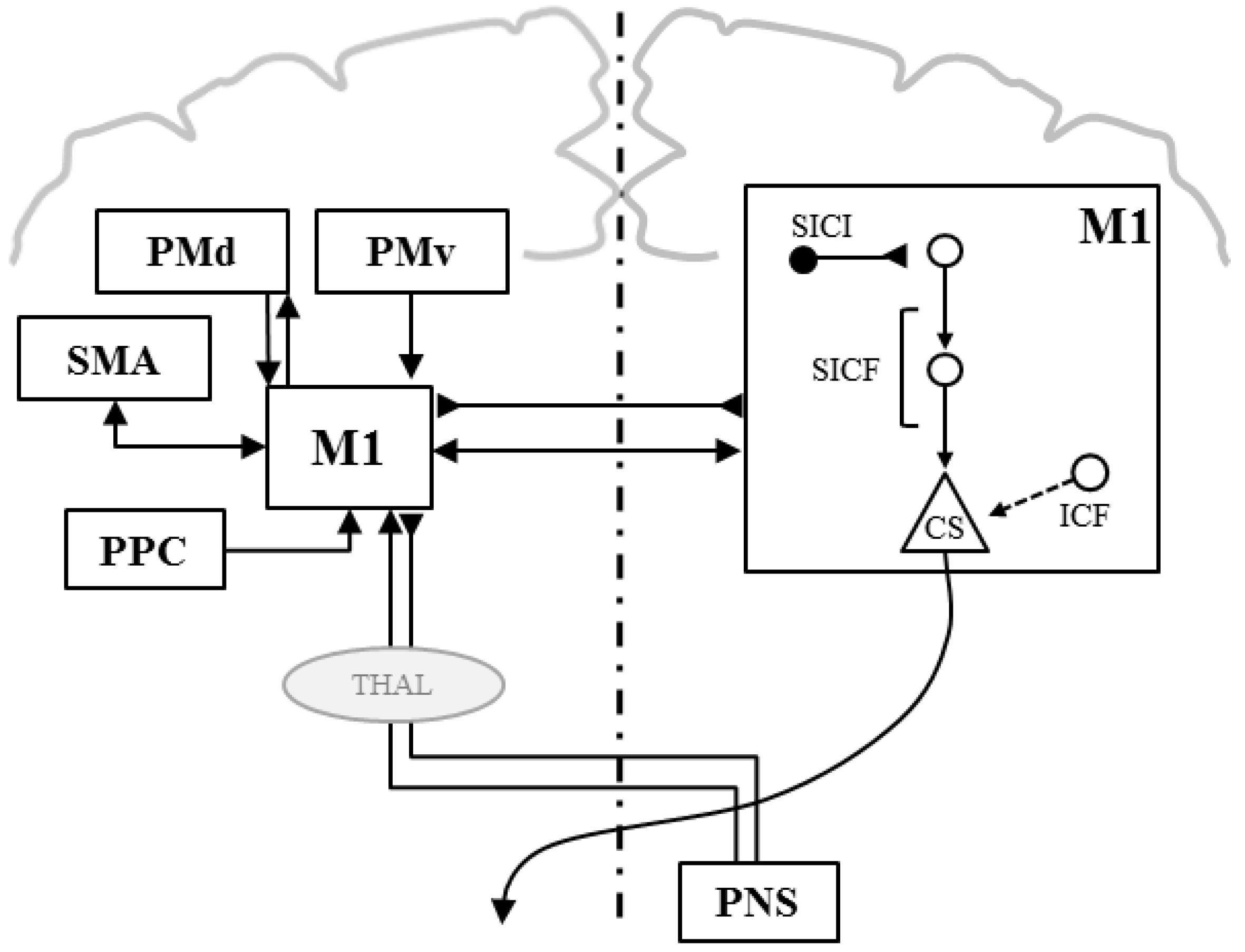
2. Plasticity in M1 and Motor-Related Cerebral Areas
2.1. Can M1 Plasticity Explain Motor Impairment in People with CLBP?
2.2. Discrepancies between TMS Studies in CLBP: How to Reconcile the Controversial Results?
3. Subgrouping of CLBP in Neuroplasticity Studies
3.1. Subgroups Based on the Nature of CLBP
3.2. Subgroups Based on the Nociceptive Somatosensory Processing: Mechanical vs. Non-Mechanical CLBP
3.3. Subgroups Bbased on Movement Disorders
4. Interventions Targeting M1 Plasticity
4.1. Learning-Dependent Plasticity in CLBP: How Motor Training Impacts M1?
4.2. Noninvasive and Painless Neuromodulation in CLBP
4.3. Central Stimulation
4.4. Peripheral Stimulation
4.5. Combination of Interventions
5. How Can Neuroplasticity Studies Better Reduce Pain and Disability in CLBP?
5.1. Identifying Brain Biomarkers in CLBP
5.1.1. “Central Sensitization” or Non-Mechanical CLBP
5.1.2. Nociceptive or Mechanical CLBP
6. Conclusions: Avoiding One Size Fits All Treatments to M1 Plasticity in CLBP
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Freburger, J.K.; Holmes, G.M.; Agans, R.P.; Jackman, A.M.; Darter, J.D.; Wallace, A.S.; Castel, L.D.; Kalsbeek, W.D.; Carey, T.S. The rising prevalence of chronic low back pain. Arch. Intern. Med. 2009, 169, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Blyth, F.M.; March, L.M.; Brooks, P.; Woolf, A.D.; Hoy, D.G. Placing the global burden of low back pain in context. Best Pract. Res. Clin. Rheumatol. 2013, 27, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the united states and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.M.; Hestbaek, L.; Cassidy, J.D. Low back pain across the life course. Best Pract. Res. Clin. Rheumatol. 2013, 27, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Lemeunier, N.; Leboeuf-Yde, C.; Gagey, O. The natural course of low back pain: A systematic critical literature review. Chiropr. Man. therap. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, L.G.; Maher, C.G.; Latimer, J.; McAuley, J.H.; Hodges, P.W.; Rogers, W.T. Nature and determinants of the course of chronic low back pain over a 12-month period: A cluster analysis. Phys. Ther. 2014, 94, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Vasseljen, O.; Woodhouse, A.; Bjorngaard, J.H.; Leivseth, L. Natural course of acute neck and low back pain in the general population: The hunt study. Pain 2013, 154, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Krismer, M.; van Tulder, M. Strategies for prevention and management of musculoskeletal conditions. Low back pain (non-specific). Best Pract. Res. Clin. Rheumatol. 2007, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.; van Tulder, M.W.; Malmivaara, A.; Koes, B.W. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Rubinstein, S.M.; van Middelkoop, M.; Assendelft, W.J.; de Boer, M.R.; van Tulder, M.W. Spinal manipulative therapy for chronic low-back pain. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef] [PubMed]
- Kregel, J.; Meeus, M.; Malfliet, A.; Dolphens, M.; Danneels, L.; Nijs, J.; Cagnie, B. Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin. Arthritis. Rheum. 2015, 45, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wilcke, T.; Leinisch, E.; Ganssbauer, S.; Draganski, B.; Bogdahn, U.; Altmeppen, J.; May, A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 2006, 125, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.; Shir, Y.; Ouellet, J.A.; Ware, M.A.; Stone, L.S.; Seminowicz, D.A. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum. Brain Mapp. 2015, 36, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Ferreira, H.F.; Ferreira, M.L. Lumbar spine: Treatment of instability and disorders of movement control. In Pathology and Intervention in Musculoskeletal Rehabilitation; Magee, D.J., Zachazewski, J.E., Quillen, W.S., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2009; pp. 389–425. [Google Scholar]
- Lee, D.G. The Pelvic Girdle: An Integration of Clinical Expertise and Research; Elsevier Health Sciences: New York, NY, USA, 2011. [Google Scholar]
- McGill, S.M. Low Back Disorders: Evidence-Based Prevention and Rehabilitation, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2007; p. 328. [Google Scholar]
- Richardson, C.A.; Paul, H.; Hides, J.A. Therapeutic Exercise for Lumbopelvic Stabilization: A Motor Control Approach for the Treatment and Prevention of Low Back Pain, 2nd ed.; Churchill Livingston: London, UK, 2004; p. 271. [Google Scholar]
- Sahrmann, S. Diagnosis and Treatment of Movement Impairment Syndromes, 1st ed.; Mosby: St. Louis, MO, USA, 2002; p. 380. [Google Scholar]
- Hodges, P.W.; Richardson, C.A. Inefficient muscular stabilization of the lumbar spine associated with low back pain: A motor control evaluation of transversus abdominis. Spine 1996, 21, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Kulig, K. Altered multifidus recruitment during walking in young asymptomatic individuals with a history of low back pain. J. Orthop. Sports Phys. Ther. 2016, 46, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Radebold, A.; Cholewicki, J.; Polzhofer, G.K.; Greene, H.S. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic low back pain. Spine 2001, 26, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Stanton, W.; Mendis, M.D.; Sexton, M. The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man. Ther. 2011, 16, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Massion, J. Movement, posture and equilibrium: Interaction and coordination. Prog. Neurobiol. 1992, 38, 35–56. [Google Scholar] [CrossRef]
- Ferbert, A.; Caramia, D.; Priori, A.; Bertolasi, L.; Rothwell, J.C. Cortical projection to erector spinae muscles in man as assessed by focal transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 382–387. [Google Scholar] [CrossRef]
- Strutton, P.H.; Beith, I.D.; Theodorou, S.; Catley, M.; McGregor, A.H.; Davey, N.J. Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. Exp. Brain Res. 2004, 158, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Danneels, L.; Hodges, P.W. Individual fascicles of the paraspinal muscles are activated by discrete cortical networks in humans. Clin. Neurophysiol. 2011, 122, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Galea, M.P.; Hodges, P.W. Concurrent excitation of the opposite motor cortex during transcranial magnetic stimulation to activate the abdominal muscles. J. Neurosci. Methods 2008, 171, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp. Brain Res. 2001, 141, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Galea, M.P.; Hodges, P.W. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 2008, 131, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Strutton, P.H.; Theodorou, S.; Catley, M.; McGregor, A.H.; Davey, N.J. Corticospinal excitability in patients with chronic low back pain. J. Spinal Disord. Tech. 2005, 18, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Danneels, L.A.; Hodges, P.W. Issls prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine 2011, 36, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Elgueta-Cancino, E.L.; Hodges, P.W. Smudging of the motor cortex is related to the severity of low back pain. Spine 2015. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. Corticomotor control of lumbar multifidus muscles is impaired in chronic low back pain: Concurrent evidence from ultrasound imaging and double-pulse transcranial magnetic stimulation. Exp. Brain Res. 2016, 234, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Flamand, V.H.; Moffet, H.; Schneider, C. Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments. Exp. Brain Res. 2012, 218, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Ivry, R.B. Role of corticospinal suppression during motor preparation. Cereb. Cortex. 2009, 19, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Byblow, W.D. Role of intracortical inhibition in selective hand muscle activation. J. Neurophysiol. 2003, 89, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.; Jalinous, R.; Freeston, I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Muller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2014, 126, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an i.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inghilleri, M.; Berardelli, A.; Cruccu, G.; Manfredi, M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J. Physiol. 1993, 466, 521–534. [Google Scholar] [PubMed]
- Wassermann, E.M.; McShane, L.M.; Hallett, M.; Cohen, L.G. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 1–8. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Tokimura, H.; Ridding, M.C.; Tokimura, Y.; Amassian, V.E.; Rothwell, J.C. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 263–272. [Google Scholar] [CrossRef]
- Ziemann, U.; Tergau, F.; Wassermann, E.M.; Wischer, S.; Hildebrandt, J.; Paulus, W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J. Physiol. 1998, 511, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Descending pathways in motor control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Petre, B.; Torbey, S.; Herrmann, K.M.; Huang, L.; Schnitzer, T.J.; Fields, H.L.; Apkarian, A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012, 15, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M.; Brumagne, S.; Caeyenberghs, K.; Janssens, L.; Goossens, N.; Marinazzo, D.; Swinnen, S.P.; Claeys, K.; Siugzdaite, R. Resting-state functional connectivity of the sensorimotor network in individuals with nonspecific low back pain and the association with the sit-to-stand-to-sit task. Brain Connect. 2015, 5, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Wideman, T.H.; Naso, L.; Hatami-Khoroushahi, Z.; Fallatah, S.; Ware, M.A.; Jarzem, P.; Bushnell, M.C.; Shir, Y.; Ouellet, J.A.; et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011, 31, 7540–7550. [Google Scholar] [CrossRef] [PubMed]
- Bingel, U.; Lorenz, J.; Glauche, V.; Knab, R.; Gläscher, J.; Weiller, C.; Büchel, C. Somatotopic organization of human somatosensory cortices for pain: A single trial fmri study. Neuroimage 2004, 23, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Isnard, J.; Mauguiere, F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with si and insular responses. Cerebral. Cortex. 2006, 16, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Karhu, J.; Tesche, C.D. Simultaneous early processing of sensory input in human primary (SI) and secondary (SII) somatosensory cortices. J. Neurophysiol. 1999, 81, 2017–2025. [Google Scholar] [PubMed]
- Schabrun, S.M.; Jones, E.; Kloster, J.; Hodges, P.W. Temporal association between changes in primary sensory cortex and corticomotor output during muscle pain. Neuroscience 2013, 235, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Ridding, M.C.; Galea, M.P.; Hodges, P.W.; Chipchase, L.S. Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS ONE 2012, 7, e51298. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Spaeth, R.B.; Wey, H.Y.; Cheetham, A.; Cook, A.H.; Jensen, K.; Tan, Y.; Liu, H.; Wang, D.; Loggia, M.L.; et al. S1 is associated with chronic low back pain: A functional and structural mri study. Mol. Pain 2013. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Braun, C.; Elbert, T.; Birbaumer, N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997, 224, 5–8. [Google Scholar] [CrossRef]
- Luomajoki, H.; Moseley, G.L. Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br. J. Sports Med. 2011, 45, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L. I can’t find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain 2008, 140, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Henry, S.M.; Nagle, K.J. People with chronic low back pain exhibit decreased variability in the timing of their anticipatory postural adjustments. Behav. Neurosci. 2009, 123, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Pijnenburg, M.; Caeyenberghs, K.; Janssens, L.; Goossens, N.; Swinnen, S.P.; Sunaert, S.; Brumagne, S. Microstructural integrity of the superior cerebellar peduncle is associated with an impaired proprioceptive weighting capacity in individuals with non-specific low back pain. PLoS ONE 2014, 9, e100666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliki, M.N.; Schnitzer, T.J.; Bauer, W.R.; Apkarian, A.V. Brain morphological signatures for chronic pain. PLoS ONE 2011, 6, e26010. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.P.; Yang, H.J. Smaller amygdala volumes in patients with chronic low back pain compared with healthy control individuals. J. Pain 2015, 16, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.L.; Stampfli, P.; Vrana, A.; Humphreys, B.K.; Seifritz, E.; Hotz-Boendermaker, S. Neural correlates of fear of movement in patients with chronic low back pain vs. Pain-free individuals. Front. Hum. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Alschuler, K.N.; Neblett, R.; Wiggert, E.; Haig, A.J.; Geisser, M.E. Flexion-relaxation and clinical features associated with chronic low back pain: A comparison of different methods of quantifying flexion-relaxation. Clin. J. Pain 2009, 25, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Geisser, M.E.; Haig, A.J.; Wallbom, A.S.; Wiggert, E.A. Pain-related fear, lumbar flexion, and dynamic emg among persons with chronic musculoskeletal low back pain. Clin. J. Pain 2004, 20, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.J.; Booker, C.K.; Main, C.J. Evidence for the role of psychological factors in abnormal paraspinal activity in patients with chronic low back pain. J. Musculoskelatal Pain 1997, 5, 41–56. [Google Scholar] [CrossRef]
- Karayannis, N.V.; Smeets, R.J.; van den Hoorn, W.; Hodges, P.W. Fear of movement is related to trunk stiffness in low back pain. PLoS ONE 2013, 8, e67779. [Google Scholar] [CrossRef] [PubMed]
- Marras, W.S.; Davis, K.G.; Heaney, C.A.; Maronitis, A.B.; Allread, W.G. The influence of psychosocial stress, gender, and personality on mechanical loading of the lumbar spine. Spine 2000, 25, 3045–3054. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.R.; Baliki, M.N.; Huang, L.; Torbey, S.; Herrmann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Brain white matter structural properties predict transition to chronic pain. Pain 2013, 154, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.Y.; Shih, Y.F.; Chou, L.W.; McGregor, A.H.; Strutton, P.H. Impaired neural drive in patients with low back pain. Eur. J. Pain 2014, 18, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. Impairment of Corticomotor Control of Lumbar Multifidus in Chronic Low Back Pain. In Proceedings of the 35th Annual Scientific Meeting of the Canadian Pain Society, Quebec City, QC, Canada, 20–23 May 2014.
- Chiou, S.Y.; Jeevathol, A.; Odedra, A.; Strutton, P.H. Voluntary activation of trunk extensors appears normal in young adults who have recovered from low back pain. Eur. J. Pain 2015, 19, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. The side of chronic low back pain matters: Evidence from the primary motor cortex excitability and the postural adjustments of multifidi muscles, experimental brain research. Exp. Brain Res. Unpublished work. 2016. [Google Scholar]
- Karayannis, N.V.; Jull, G.A.; Hodges, P.W. Physiotherapy movement based classification approaches to low back pain: Comparison of subgroups through review and developer/expert survey. BMC Musculoskelet. Disord. 2012. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.E.; Hill, J.C.; O’Sullivan, P.; Hancock, M. Stratified models of care. Best Pract. Res. Clin. Rheumatol. 2013, 27, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Apeldoorn, A.; Hallegraeff, H.; Clark, J.; Smeets, R.; Malfliet, A.; Girbes, E.L.; De Kooning, M.; Ickmans, K. Low back pain: Guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015, 18, E333–E346. [Google Scholar] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical indicators of “nociceptive”, “peripheral neuropathic” and “central” mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man. Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. The discriminative validity of “nociceptive”, “peripheral neuropathic”, and “central sensitization” as mechanisms-based classifications of musculoskeletal pain. Clin. J. Pain 2011, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Strutton, P.H.; Catley, M.; McGregor, A.H.; Davey, N.J. Corticospinal excitability in patients with unilateral sciatica. Neurosci. Lett. 2003, 353, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Chistyakov, A.V.; Yudashkin, M.; Kaplan, B.; Hafner, H.; Feinsod, M. Evidence for cortical hyperexcitability of the affected limb representation area in crps: A psychophysical and transcranial magnetic stimulation study. Pain 2005, 113, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Schwenkreis, P.; Janssen, F.; Rommel, O.; Pleger, B.; Volker, B.; Hosbach, I.; Dertwinkel, R.; Maier, C.; Tegenthoff, M. Bilateral motor cortex disinhibition in complex regional pain syndrome (crps) type I of the hand. Neurology 2003, 61, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Mhalla, A.; de Andrade, D.C.; Baudic, S.; Perrot, S.; Bouhassira, D. Alteration of cortical excitability in patients with fibromyalgia. Pain 2010, 149, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Schwenkreis, P.; Scherens, A.; Ronnau, A.K.; Hoffken, O.; Tegenthoff, M.; Maier, C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 2010. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, V.; Luomajoki, H.; Leinonen, V.; Gibbons, S.; Airaksinen, O. Sub-classification based specific movement control exercises are superior to general exercise in sub-acute low back pain when both are combined with manual therapy: A randomized controlled trial. BMC Musculoskelet. Disord. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bareš, M.; Kaňovský, P.; Klajblová, H.; Rektor, I. Intracortical inhibition and facilitation are impaired in patients with early parkinson's disease: A paired tms study. Eur. J. Neurol. 2003, 10, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Pilato, F.; Saturno, E.; Dileone, M.; Marra, C.; Daniele, A.; Ghirlanda, S.; Gainotti, G.; Tonali, P. Motor cortex hyperexcitability to transcranial magnetic stimulation in alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983, 306, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Rabey, M.; Slater, H.; O'Sullivan, P.; Beales, D.; Smith, A. Somatosensory nociceptive characteristics differentiate subgroups in people with chronic low back pain: A cluster analysis. Pain 2015, 156, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Meeus, M.; Cagnie, B.; Roussel, N.A.; Dolphens, M.; Van Oosterwijck, J.; Danneels, L. A modern neuroscience approach to chronic spinal pain: Combining pain neuroscience education with cognition-targeted motor control training. Phys. Ther. 2014, 94, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with “nociceptive”, “peripheral neuropathic” and “central sensitisation” pain. The discriminant validity of mechanisms-based classifications of low back (+/−leg) pain. Man. Ther. 2012, 17, 119–125. [Google Scholar] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Thacker, M.; Doody, C. Mechanisms-based classifications of musculoskeletal pain: Part 1 of 3: Symptoms and signs of central sensitisation in patients with low back (+/–leg) pain. Man. Ther. 2012, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Thacker, M.; Doody, C. Mechanisms-based classifications of musculoskeletal pain: Part 2 of 3: Symptoms and signs of peripheral neuropathic pain in patients with low back (+/−leg) pain. Man. Ther. 2012, 17, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Thacker, M.; Doody, C. Mechanisms-based classifications of musculoskeletal pain: Part 3 of 3: Symptoms and signs of nociceptive pain in patients with low back (+/−leg) pain. Man. Ther. 2012, 17, 352–357. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P.; Waller, R.; Wright, A.; Gardner, J.; Johnston, R.; Payne, C.; Shannon, A.; Ware, B.; Smith, A. Sensory characteristics of chronic non-specific low back pain: A subgroup investigation. Man. Ther. 2014, 19, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rabey, M.; Beales, D.; Slater, H.; O’Sullivan, P. Multidimensional pain profiles in four cases of chronic non-specific axial low back pain: An examination of the limitations of contemporary classification systems. Man. Ther. 2015, 20, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Erpelding, N.; Moayedi, M.; Davis, K.D. Cortical thickness correlates of pain and temperature sensitivity. Pain 2012, 153, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Dankaerts, W.; O’Sullivan, P.; Burnett, A.; Straker, L. Altered patterns of superficial trunk muscle activation during sitting in nonspecific chronic low back pain patients: Importance of subclassification. Spine 2006, 31, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Dankaerts, W.; O’Sullivan, P.; Burnett, A.; Straker, L.; Davey, P.; Gupta, R. Discriminating healthy controls and two clinical subgroups of nonspecific chronic low back pain patients using trunk muscle activation and lumbosacral kinematics of postures and movements: A statistical classification model. Spine 2009, 34, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, M.; Mannig, A.; Waldvogel, A.; Wand, B.M.; Luomajoki, H. The effect of motor control and tactile acuity training on patients with non-specific low back pain and movement control impairment. J. Bodyw. Mov. Ther. 2015, 19, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Iosa, M.; Paolucci, T.; Fusco, A.; Alcuri, R.; Spadini, E.; Saraceni, V.M.; Paolucci, S. Efficacy of perceptive rehabilitation in the treatment of chronic nonspecific low back pain through a new tool: A randomized clinical study. Clin. Rehabil. 2012, 26, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Galea, M.P.; Hodges, P.W. Driving plasticity in the motor cortex in recurrent low back pain. Eur. J. Pain. 2010, 14, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. Influence of paravertebral muscles training on brain plasticity and postural control in chronic low back pain. Scand. J. Pain. 2016, 12, 74–83. [Google Scholar] [CrossRef]
- Adkins, D.L.; Boychuk, J.; Remple, M.S.; Kleim, J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006, 101, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Classen, J.; Cohen, L.G.; Hallett, M. Task-dependent changes of intracortical inhibition. Exp. Brain Res. 1998, 118, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Grafman, J.; Hallett, M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science 1994, 263, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.; Brasil-Neto, J.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [PubMed]
- Ziemann, U.; Ilic, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, M.G.; Rasmussen-Barr, E.; Grooten, W.J. Motor control exercises reduces pain and disability in chronic and recurrent low back pain: A meta-analysis. Spine 2013, 38, E350–E358. [Google Scholar] [CrossRef] [PubMed]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.; Menezes Costa, L.C.; Ostelo, R.W.; Macedo, L.G. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Peyron, R.; Mertens, P.; Gregoire, M.C.; Lavenne, F.; Le Bars, D.; Convers, P.; Mauguiere, F.; Sindou, M.; Laurent, B. Electrical stimulation of motor cortex for pain control: A combined pet-scan and electrophysiological study. Pain 1999, 83, 259–273. [Google Scholar] [CrossRef]
- Galhardoni, R.; Correia, G.S.; Araujo, H.; Yeng, L.T.; Fernandes, D.T.; Kaziyama, H.H.; Marcolin, M.A.; Bouhassira, D.; Teixeira, M.J.; de Andrade, D.C. Repetitive transcranial magnetic stimulation in chronic pain: A review of the literature. Arch. Phys. Med. Rehabil. 2015, 96, S156–S172. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Andre-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rtms). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Summers, J.; Pridmore, S. Changes to somatosensory detection and pain thresholds following high frequency repetitive tms of the motor cortex in individuals suffering from chronic pain. Pain 2006, 123, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Rushton, A.; Wright, C.; Jurgens, T.; Polzer, A.; Mueller, G.; May, A. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: Sham controlled double blinded randomised controlled trial. BMJ 2015. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, N.E.; Cossar, J.; Marston, L.; Wand, B.M.; Bunce, D.; De Souza, L.H.; Maskill, D.W.; Sharp, A.; Moseley, G.L. Transcranial direct current stimulation of the motor cortex in the treatment of chronic nonspecific low back pain: A randomized, double-blind exploratory study. Clin. J. Pain 2013, 29, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Schabrun, S.M.; Jones, E.; Elgueta Cancino, E.L.; Hodges, P.W. Targeting chronic recurrent low back pain from the top-down and the bottom-up: A combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 2014, 7, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Angerer, B.; Havel, P. Modulation of sensorimotor performances and cognition abilities induced by rpms: Clinical and experimental investigations. Suppl. Clin. Neurophysiol. 2003, 56, 358–367. [Google Scholar] [PubMed]
- Struppler, A.; Havel, P.; Müller-Barna, P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS)—A new approach in central paresis. NeuroRehabilitation 2003, 18, 69–82. [Google Scholar] [PubMed]
- Krause, P.; Straube, A. Peripheral repetitive magnetic stimulation induces intracortical inhibition in healthy subjects. Neurol. Res. 2008, 30, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007, 36, T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Fook-Chong, S.; Huerto, A.P.; George, J.M. A randomized, placebo-controlled trial of repetitive spinal magnetic stimulation in lumbosacral spondylotic pain. Pain Med. 2011, 12, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Flamand, V.H.; Moffet, H.; Schneider, C. Peripheral neurostimulation and specific motor training of deep abdominal muscles improve posturomotor control in chronic low back pain. Clin. J. Pain 2013, 29, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Maeda, F.; Keenan, J.; Tormos, J.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Siebner, H.R. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008, 1, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Schneider, C. Cerebral reorganization in chronic low back pain and neurostimulation to improve motor control. Neurophysiol. Clin. 2011, 41, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. Peripheral magnetic neurostimulation of multifidus muscles combined with motor training influenced spine motor control and chronic low back pain. Clin. Neurophysiol. Unpublished work. 2016. [Google Scholar]
- Schabrun, S.M.; Chipchase, L.S. Priming the brain to learn: The future of therapy? Man. Ther. 2012, 17, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Vlaeyen, J.W. Beyond nociception: The imprecision hypothesis of chronic pain. Pain 2015, 156, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.S.; Moseley, G.L. Explain Pain, 2nd ed.; NOI group: Adelaide, Austrlia, 2013. [Google Scholar]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Addressing neuroplastic changes in distributed areas of the nervous system associated with chronic musculoskeletal disorders. Phys. Ther. 2015, 95, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Baliki, M.N.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape shifting pain: Chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013, 136, 2751–2768. [Google Scholar] [CrossRef] [PubMed]
- Krummenacher, P.; Candia, V.; Folkers, G.; Schedlowski, M.; Schonbachler, G. Prefrontal cortex modulates placebo analgesia. Pain 2010, 148, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Mantyh, P.W. The cerebral signature for pain perception and its modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Vibe Fersum, K.; O’Sullivan, P.; Skouen, J.S.; Smith, A.; Kvale, A. Efficacy of classification-based cognitive functional therapy in patients with non-specific chronic low back pain: A randomized controlled trial. Eur. J. Pain 2013, 17, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Chan, S.; Pringle, E.; Schallert, K.; Procaccio, V.; Jimenez, R.; Cramer, S.C. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006, 9, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Flor, H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabil. Neural. Repair. 2012, 26, 646–652. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massé-Alarie, H.; Schneider, C. Revisiting the Corticomotor Plasticity in Low Back Pain: Challenges and Perspectives. Healthcare 2016, 4, 67. https://doi.org/10.3390/healthcare4030067
Massé-Alarie H, Schneider C. Revisiting the Corticomotor Plasticity in Low Back Pain: Challenges and Perspectives. Healthcare. 2016; 4(3):67. https://doi.org/10.3390/healthcare4030067
Chicago/Turabian StyleMassé-Alarie, Hugo, and Cyril Schneider. 2016. "Revisiting the Corticomotor Plasticity in Low Back Pain: Challenges and Perspectives" Healthcare 4, no. 3: 67. https://doi.org/10.3390/healthcare4030067




