Building a Biopsychosocial Conceptual Framework to Explore Pressure Ulcer Pain for Hospitalized Patients
Abstract
:1. Introduction
2. Method
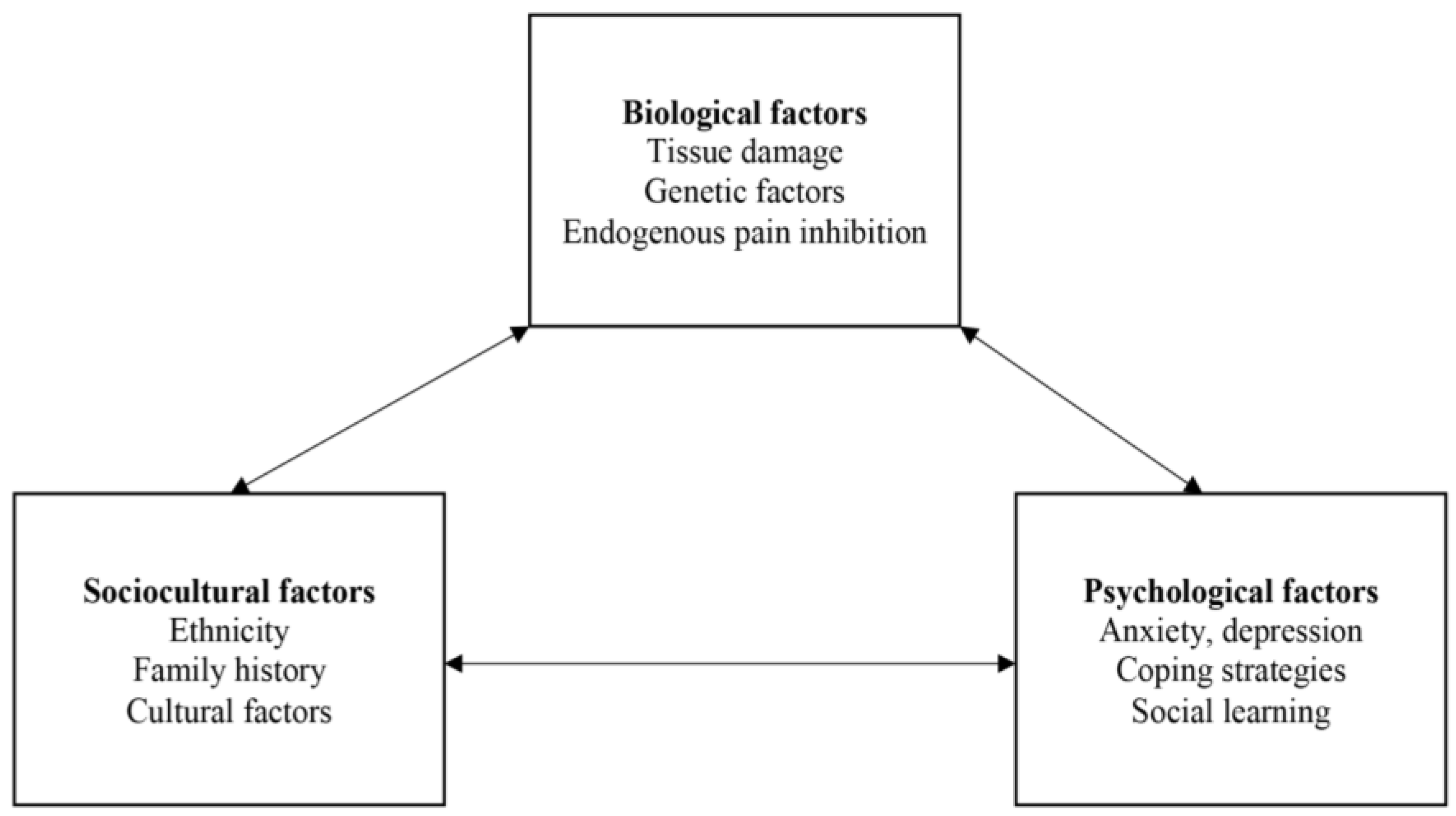
3. Results
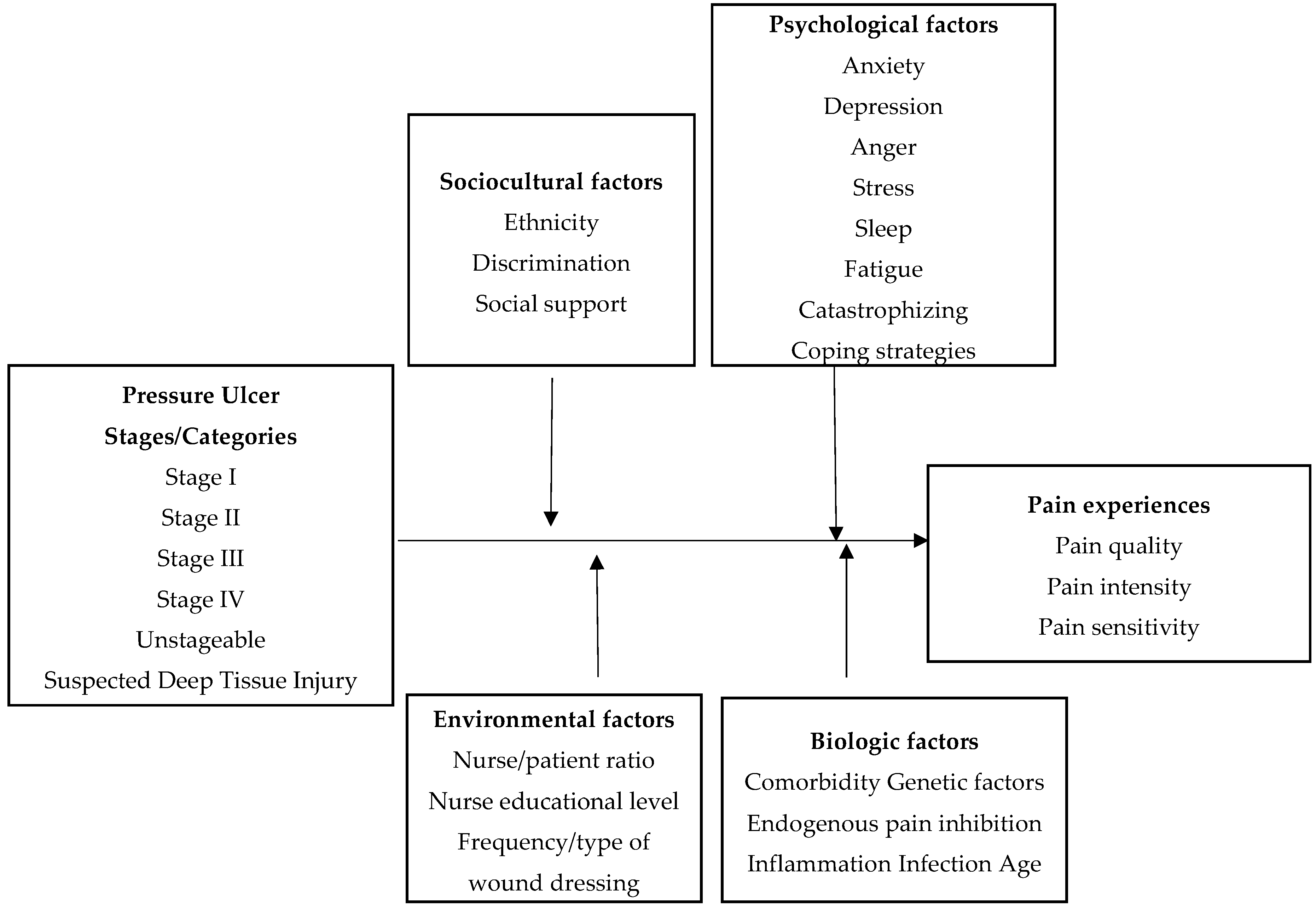
| Factors and Components | Definitions | Possible Empirical Indicators |
|---|---|---|
| Pressure Ulcer | ||
| Pressure ulcer stages/categories | Stage of localized injury to the skin and/or underlying tissue, usually over bony prominence, as a result of pressure, or pressure in combination with shear | National Pressure Ulcer Advisory Panel (NPUAP) Pressure Ulcer Stages/categories |
| Biological Factors | ||
| Comorbidity | Two or more coexisting medical conditions or unrelated disease processes | Charlson Comorbidity Index (CCI) |
| Genetic factors | The factors pertaining to or produced by a gene | catechol-O-methyltransferase gene (COMT), and mu-opioid receptor gene (OPRM1) |
| Endogenous pain inhibition | The pain inhibition originating from within the body | Quantitative Sensory Testing |
| Inflammation | The protective response of body tissue to irritation or injury | C-reactive protein, tumor necrosis factor-alpha, and interleukins |
| Infection | The invasion of the body by pathogenic microorganisms that reproduce and multiply, causing disease by local cellular injury, secretion of a toxin, or antigen-antibody reaction in the host | Plantonic bacteria and biofilm |
| Age | The number of years of life | Years of life |
| Sociocultural Factors | ||
| Ethnicity | Discrete groups of people that are similar according to behaviors, culture, and biophysical characteristics | Multigroup Ethnic Identity Measure (MEIM) |
| Discrimination | Unfair or different treatment based on membership in a group | Experience of Discrimination Scale (EOD) |
| Social support | The availability of resources from others who are connected by social networks to an individual | Multidimensional Scale of Perceived Social Support |
| Psychological Factors | ||
| Anxiety | Anticipation of impending danger and dread accompanied by restlessness, tension, tachycardia, and breathing difficulty not associated with an apparent stimulus | The State and Trait Anxiety Inventory (STAI) |
| Depression | A condition of loss of interest or pleasure in life activities that cause significant impairment in social, work, or other important areas of functioning | Patient Health Questionnaire (PHQ9) and Center for Epidemiological Studies-Depression Scale (CES-D) |
| Anger | An emotional reaction characterized by extreme displeasure, rage, indignation or hostility | The Anger Regulation and Expression Scale (ARES), and State Trait Anger Expression Inventory (STAXI) |
| Stress | Stressors or anxiety that acts as a stimulus or events that evoke physiologic responses | Perceived Stress Scale (PSS) |
| Sleep | A state marked by reduced consciousness, diminished activity of skeletal muscles and depressed metabolism | Pittsburg Sleep Quality Index (PSQI), Sleep Hygiene Practice Scale (SHPS), and Insomnia Severity Index (ISI) |
| Fatigue | A nonspecific, common subjective feeling of low vitality that disrupts daily functioning | Fatigue Severity Scale (FSS) |
| Catastrophizing | A negative cognitive and affective process involving magnification of pain-related symptoms, helplessness, pessimism, and rumination about pain | Pain Catastrophizing Scale (PCS) |
| Coping strategy | The cognitive and behavioral adjustments that an individual uses to confront and manage health and daily-life stressors | Coping Strategies Questionnaire-Revised (CSQ-R) |
| Environmental Factors | ||
| Nurse/patient ratio | The number of patients assigned to RN | Number of patients per RN |
| Nurse educational level | The level of education of the nurse | Educational Preparation |
| Frequency/type of wound dressing | The number of dressing changes/type during 24 h | Frequency/type of dressing per a day |
| Pain Experiences | ||
| Pain intensity | The level of intensity of pain | Numerical Rating Scale (NRS), Visual Analog Scale (VAS), and Wong-Baker FACES Pain Rating Scale |
| Pain quality | Any of the features that make pain | McGill Pain Questionnaire |
| Pain sensitivity | A condition of being sensitive to pain | Heat pain threshold/tolerance, cold pain threshold/tolerance, and pressure pain threshold |
3.1. Pain Quality, Intensity, and Sensitivity
3.2. Sociocultural Factors
3.3. Biological Factors
3.4. Psychosocial Factors
3.5. Environmental Factors
3.6. Major Propositions of the Proposed Conceptual Framework
- If other predictors are controlled, pressure ulcer stages/categories will be significantly associated with pain experiences.
- Biological factors, including comorbidities, single nucleotide polymorphisms of specific candidate genes (e.g., OPRM1, COMT), endogenous pain inhibition, specific inflammation markers (e.g., cortisol, beta-endorphin, and cytokines), and age will moderate pain experiences associated with pressure ulcers.
- Sociocultural factors, including ethnicity, discrimination, and social support, will moderate pain experiences associated with pressure ulcers.
- Psychological factors, including anxiety, depression, anger, stress, sleep, fatigue, catastrophizing, and coping strategies, will moderate pain experiences associated with pressure ulcers.
- Environmental factors, including nurse/patients ratio, nurse educational level and frequency/type of dressing changes, will moderate pain experiences associated with pressure ulcers in hospitalized patients.
- These biological, sociocultural, psychological, and environmental factors are assumed to combine in a sequence to influence pain experiences with pressure ulcers.
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Russo, A.; Steiner, C.; Spector, W. Hospitalizations Related to Pressure Ulcers among Adults 18 Years and Older, 2006. Available online: http://www.hcup-us.ahrq.gov/reports/statbriefs/statbriefs.jsp (accessed on 3 November 2015).
- Moore, Z. US medicare data show incidence of hospital-acquired pressure ulcers is 4.5%, and they are associated with longer hospital stay and higher risk of death. Evid. Based Nurs. 2013, 16, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, E.T.; Buerhaus, P.I. New medicare payment rules: Danger or opportunity for nursing? Am. J. Nurs. 2008, 108, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mattie, A.S.; Webster, B.L. Centers for medicare and medicaid services’ “never events”: An analysis and recommendations to hospitals. Health Care Manag. 2008, 27, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Agency for Healthcare Research and Quality. Saving Lives and Saving Money: Hospital-Acquired Conditions Update. Available online: http://www.ahrq.gov/professionals/quality-patient-safety/pfp/interimhacrate2014.html (accessed on 2 December 2015).
- Park-Lee, E.; Caffrey, C. Pressure ulcers among nursing home residents: United States, 2004. NCHS Data Brief 2009, 14, 1–8. [Google Scholar] [PubMed]
- Gorecki, C.; Brown, J.M.; Nelson, E.A.; Briggs, M.; Schoonhoven, L.; Dealey, C.; Defloor, T.; Nixon, J. Impact of pressure ulcers on quality of life in older patients: A systematic review. J. Am. Geriatr. Soc. 2009, 57, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.; Dealey, C.; Bale, S.; Defloor, T.; Worboys, F. Patient stories of living with a pressure ulcer. J. Adv. Nurs. 2006, 56, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Langemo, D.K.; Melland, H.; Hanson, D.; Olson, B.; Hunter, S. The lived experience of having a pressure ulcer: A qualitative analysis. Adv. Skin Wound Care 2000, 13, 225–235. [Google Scholar] [PubMed]
- Gunes, U.Y. A descriptive study of pressure ulcer pain. Ostomy Wound Manag. 2008, 54, 56–61. [Google Scholar]
- Pieper, B.; Langemo, D.; Cuddigan, J. Pressure ulcer pain: A systematic literature review and national pressure ulcer advisory panel white paper. Ostomy Wound Manag. 2009, 55, 16–31. [Google Scholar]
- Rastinehad, D. Pressure ulcer pain. J. Wound Ostomy Cont. Nurs. 2006, 33, 252–257. [Google Scholar] [CrossRef]
- Spilsbury, K.; Nelson, A.; Cullum, N.; Iglesias, C.; Nixon, J.; Mason, S. Pressure ulcers and their treatment and effects on quality of life: Hospital inpatient perspectives. J. Adv. Nurs. 2007, 57, 494–504. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Ulcer Advisory Panel. Npuap Pressure Ulcer Stages/Categories. Available online: http://www.npuap.org/resources/educational-and-clinical-resources/npuap-pressure-ulcer-stagescategories/ (accessed on 8 August 2015).
- Ratliff, C.R.; Rodeheaver, G.T. Pressure ulcer assessment and management. Lippincott’s Prim. Care Pract. 1999, 3, 242–258. [Google Scholar]
- Coleman, S.; Nixon, J.; Keen, J.; Wilson, L.; McGinnis, E.; Dealey, C.; Stubbs, N.; Farrin, A.; Dowding, D.; Schols, J.M.; et al. A new pressure ulcer conceptual framework. J. Adv. Nurs. 2014, 70, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, C.; Closs, S.J.; Nixon, J.; Briggs, M. Patient-reported pressure ulcer pain: A mixed-methods systematic review. J. Pain Symptom Manag. 2011, 42, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Girouard, K.; Harrison, M.B.; van den Kerkof, E. The symptom of pain with pressure ulcers: A review of the literature. Ostomy Wound Manag. 2008, 54, 30–40. [Google Scholar]
- Ahn, H.; Stechmiller, J.; Fillingim, R.; Lyon, D.; Garvan, C. Bodily pain intensity in nursing home residents with pressure ulcers: Analysis of national minimum data set 3.0. Res. Nurs. Health 2015, 38, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Stechmiller, J.; Horgas, A. Pressure ulcer-related pain in nursing home residents with cognitive impairment. Adv. Skin Wound Care 2013, 26, 375–380. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, E.; Briggs, M.; Collinson, M.; Wilson, L.; Dealey, C.; Brown, J.; Coleman, S.; Stubbs, N.; Stevenson, R.; Nelson, E.A.; et al. Pressure ulcer related pain in community populations: A prevalence survey. BMC Nurs. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- National Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Available online: http://www.npuap.org/wp-content/uploads/2014/08/Updated-10-16-14-Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-16Oct2014.pdf (accessed on 1 December 2015).
- European Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Available online: http://www.epuap.org/guidelines/guidelines-old/ (accessed on 1 December 2015).
- Walker, L.O.; Avant, K.C. Strategies for Theory Construction in Nursing, 5th ed.; Appleton and Lange: Norwalk, CT, USA, 2010. [Google Scholar]
- Melzack, R.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Summers, S. Evidence-based practice part 1: Pain definitions, pathophysiologic mechanisms, and theories. J. Perianesthesia Nurs. 2000, 15, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.L.; O’Malley, K.J.; Cody, M.; Kunik, M.E.; Ashton, C.M.; Beck, C.; Bruera, E.; Novy, D. A conceptual model of pain assessment for noncommunicative persons with dementia. Gerontologist 2004, 44, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Wong, T.K.; Yang, J.C. The lens model: Assessment of cancer pain in a chinese context. Cancer Nurs. 2000, 23, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B. Individual differences in pain responses. Curr. Rheumatol. Rep. 2005, 7, 342–347. [Google Scholar] [CrossRef] [PubMed]
- International Association for the Study of Pain. Iasp Taxonomy. Available online: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576#Pain (accessed on 19 May 2015).
- Bates, M.S. Ethnicity and pain: A biocultural model. Soc. Sci. Med. 1987, 24, 47–50. [Google Scholar] [CrossRef]
- Edwards, C.L.; Fillingim, R.B.; Keefe, F. Race, ethnicity and pain. Pain 2001, 94, 133–137. [Google Scholar] [CrossRef]
- Lasch, K.E. Culture, pain, and culturally sensitive pain care. Pain Manag. Nurs. 2000, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Rahim-Williams, F.B.; Riley, J.L., 3rd; Herrera, D.; Campbell, C.M.; Hastie, B.A.; Fillingim, R.B. Ethnic identity predicts experimental pain sensitivity in african americans and hispanics. Pain 2007, 129, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; France, C.R.; Robinson, M.E.; Logan, H.L.; Geffken, G.R.; Fillingim, R.B. Ethnic differences in diffuse noxious inhibitory controls. J. Pain 2008, 9, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Sibille, K.T.; Goodin, B.R.; Petrov, M.E.; Bartley, E.J.; Riley, J.L., 3rd; King, C.D.; Glover, T.L.; Sotolongo, A.; Herbert, M.S.; et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthr. Rheumatol. 2014, 66, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Lavizzo-Mourey, R.; Warren, R.C. The concept of race and health status in America. Public Health Rep. 1994, 109, 26–41. [Google Scholar] [PubMed]
- Garbez, R.; Puntillo, K. Acute musculoskeletal pain in the emergency department: A review of the literature and implications for the advanced practice nurse. AACN Clin. Issues 2005, 16, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.G. Pain documentation and predictors of analgesic prescribing for elderly patients during emergency department visits. J. Pain Symptom Manag. 2011, 41, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, M.J.; Kertesz, S.G.; Kohn, M.A.; Gonzales, R. Trends in opioid prescribing by race/ethnicity for patients seeking care in us emergency departments. JAMA 2008, 299, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bisconti, T.L.; Bergeman, C.S. Perceived social control as a mediator of the relationships among social support, psychological well-being, and perceived health. Gerontologist 1999, 39, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kahana, B.; Kahana, E. Social support and cognitive functioning as resources for elderly persons with chronic arthritis pain. Aging Ment. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Yamamoto-Mitani, N.; Abe, Y.; Suzuki, M. Literature review of pain management for people with chronic pain. Jpn. J. Nurs. Sci. 2015, 12, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.M.; Sherman, A.M. The relationship of optimism, pain and social support to well-being in older adults with osteoarthritis. Aging Ment. Health 2007, 11, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Keith, J.; Novak, P.D.; Elliot, M.A. Mosby’s Dictionary of Medicine, Nursing and Health Professions, 9th ed.; Elsevier/Mosby: Philadelphia, PA, USA, 2012. [Google Scholar]
- Onubogu, U.D. Pain and depression in older adults with arthritis. Orthop. Nurs. 2014, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Caporali, R.; Cimmino, M.A.; Sarzi-Puttini, P.; Scarpa, R.; Parazzini, F.; Zaninelli, A.; Ciocci, A.; Montecucco, C. Comorbid conditions in the amica study patients: Effects on the quality of life and drug prescriptions by general practitioners and specialists. Sem. Arthr. Rheum. 2005, 35, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Horjales-Araujo, E.; Dahl, J.B. Is the experience of thermal pain genetics dependent? BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, J.K.; Heitzeg, M.M.; Smith, Y.R.; Bueller, J.A.; Xu, K.; Xu, Y.; Koeppe, R.A.; Stohler, C.S.; Goldman, D. Comt val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 2003, 299, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Hastie, B.A.; Riley, J.L., 3rd; Kaplan, L.; Herrera, D.G.; Campbell, C.M.; Virtusio, K.; Mogil, J.S.; Wallace, M.R.; Fillingim, R.B. Ethnicity interacts with the oprm1 gene in experimental pain sensitivity. Pain 2012, 153, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; Kaplan, L.; Staud, R.; Ness, T.J.; Glover, T.L.; Campbell, C.M.; Mogil, J.S.; Wallace, M.R. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J. Pain 2005, 6, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Burns, J.W.; Gupta, R.; Buvanendran, A.; Chont, M.; Schuster, E.; France, C.R. Endogenous opioid inhibition of chronic low-back pain influences degree of back pain relief after morphine administration. Reg. Anesthesia Pain Med. 2014, 39, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, A.R.; Lyon, D.E.; Schubert, C.M. Pain and inflammation in women with early-stage breast cancer prior to induction of chemotherapy. Biol. Res. Nurs. 2013, 15, 234–241. [Google Scholar] [CrossRef] [PubMed]
- DeVon, H.A.; Piano, M.R.; Rosenfeld, A.G.; Hoppensteadt, D.A. The association of pain with protein inflammatory biomarkers: A review of the literature. Nurs. Res. 2014, 63, 51–62. [Google Scholar] [CrossRef] [PubMed]
- White, R.J. Wound infection-associated pain. J. Wound Care 2009, 18, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Juozapaviciene, L.; Rimdlka, R.; Karbonskiene, A. Problem with the post burn wound pain: Chronic profiles. EWMA J. 2012, 12, 33–38. [Google Scholar]
- Tengvall, O.M.; Bjornhagen, V.C.; Lindholm, C.; Jonsson, C.E.; Wengstrom, Y. Differences in pain patterns for infected and noninfected patients with burn injuries. Pain Manag. Nurs. 2006, 7, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Vangilder, C.; Macfarlane, G.D.; Meyer, S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manag. 2008, 54, 40–54. [Google Scholar]
- National Pressure Ulcer Advisory Panel. Pressure Ulcers: Prevalence, Incidence and Implications for Future, 2nd ed.; NPUAP: Washington, DC, USA, 2012. [Google Scholar]
- Niska, R.; Bhuiya, F.; Xu, J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl. Health Stat. Rep. 2010, 28, 1–31. [Google Scholar]
- Baumgarten, M.; Margolis, D.J.; Localio, A.R.; Kagan, S.H.; Lowe, R.A.; Kinosian, B.; Holmes, J.H.; Abbuhl, S.B.; Kavesh, W.; Ruffin, A. Pressure ulcers among elderly patients early in the hospital stay. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 749–754. [Google Scholar] [CrossRef]
- Jaul, E.; Calderon-Margalit, R. Systemic factors and mortality in elderly patients with pressure ulcers. Int. Wound J. 2015, 12, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Schlüer, A.B.; Halfens, R.J.; Schols, J.G.A. Pediatric pressure ulcer prevalence: A multicenter, cross-sectional, point prevalence study in switzerland. Ostomy Wound Manag. 2012, 58, 18–31. [Google Scholar]
- Kottner, J.; Wilborn, D.; Dassen, T. Frequency of pressure ulcers in the paediatric population: A literature review and new empirical data. Int. J. Nurs. Stud. 2010, 47, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Wandner, L.D.; Scipio, C.D.; Hirsh, A.T.; Torres, C.A.; Robinson, M.E. The perception of pain in others: How gender, race, and age influence pain expectations. J. Pain 2012, 13, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.J.; Farrell, M.J.; Gibson, S.J.; Egan, G.F. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiol. Aging 2010, 31, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Lautenbacher, S.; Kunz, M.; Strate, P.; Nielsen, J.; Arendt-Nielsen, L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain 2005, 115, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Lillie, A.K.; Read, S.; Mallen, C.; Croft, P.; McBeth, J. Musculoskeletal pain in older adults at the end-of-life: A systematic search and critical review of the literature with priorities for future research. BMC Palliat. Care 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Baharestani, M.M.; Ratliff, C.R. Pressure ulcers in neonates and children: An npuap white paper. Adv. Skin Wound Care 2007, 20, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Smeijers, L.; van de Pas, H.; Nyklicek, I.; Notten, P.J.; Pedersen, S.S.; Kop, W.J. The independent association of anxiety with non-cardiac chest pain. Psychol. Health 2014, 29, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, M.; McPhee, S.J.; Rabow, M.W. Current Medical Diagnosis and Treatment, 54th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Zakoscielna, K.M.; Parmelee, P.A. Pain variability and its predictors in older adults: Depression, cognition, functional status, health, and pain. J. Aging Health 2013, 25, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, L.; Visscher, C.M.; van Houtem, C.M.; Geels, L.M.; Vink, J.M.; de Jongh, A.; Boomsma, D.I. Comorbidity among multiple pain symptoms and anxious depression in a dutch population sample. J. Pain 2014, 15, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Chung, O.Y.; Burns, J.W. Anger expression and pain: An overview of findings and possible mechanisms. J. Behav. Med. 2006, 29, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Van Middendorp, H.; Lumley, M.A.; Moerbeek, M.; Jacobs, J.W.; Bijlsma, J.W.; Geenen, R. Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: A diary study. Eur. J. Pain 2010, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.A.; Nguyen, M.L. Psychosocial stress and abdominal pain in adolescents. Ment. Health Fam. Med. 2010, 7, 65–69. [Google Scholar] [PubMed]
- White, K.S.; Farrell, A.D. Anxiety and psychosocial stress as predictors of headache and abdominal pain in urban early adolescents. J. Pediatr. Psychol. 2006, 31, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Osteras, B.; Sigmundsson, H.; Haga, M. Perceived stress and musculoskeletal pain are prevalent and significantly associated in adolescents: An epidemiological cross-sectional study. BMC Public Health 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, B.; Lallukka, T.; Petrie, K.J.; Steingrimsdottir, O.A.; Stubhaug, A.; Nielsen, C.S. Sleep and pain sensitivity in adults. Pain 2015, 156, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Edwards, R.R.; McCann, U.D.; Haythornthwaite, J.A. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 2007, 30, 494–505. [Google Scholar] [PubMed]
- Schuh-Hofer, S.; Wodarski, R.; Pfau, D.B.; Caspani, O.; Magerl, W.; Kennedy, J.D.; Treede, R.D. One night of total sleep deprivation promotes a state of generalized hyperalgesia: A surrogate pain model to study the relationship of insomnia and pain. Pain 2013, 154, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Schrimpf, M.; Liegl, G.; Boeckle, M.; Leitner, A.; Geisler, P.; Pieh, C. The effect of sleep deprivation on pain perception in healthy subjects: A meta-analysis. Sleep Med. 2015, 16, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Addington, A.M.; Gallo, J.J.; Ford, D.E.; Eaton, W.W. Epidemiology of unexplained fatigue and major depression in the community: The baltimore eca follow-up, 1981–1994. Psychol. Med. 2001, 31, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Erturk, M.; Yildirim, Y.; Kilic, S.P.; Ozer, S.; Aykar, F.S. Pain and fatigue in elderly cancer patients: Turkey example. Holist. Nurs. Pract. 2015, 29, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.A.; Darnall, B.D.; Kao, M.C.; Mackey, S.C. Physical and psychological correlates of fatigue and physical function: A collaborative health outcomes information registry (choir) study. J. Pain 2015, 16, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Van Dartel, S.A.; Repping-Wuts, J.W.; van Hoogmoed, D.; Bleijenberg, G.; van Riel, P.L.; Fransen, J. Association between fatigue and pain in rheumatoid arthritis: Does pain precede fatigue or does fatigue precede pain? Arthr. Care Res. 2013, 65, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Creavin, S.T.; Dunn, K.M.; Mallen, C.D.; Nijrolder, I.; van der Windt, D.A. Co-occurrence and associations of pain and fatigue in a community sample of dutch adults. Eur. J. Pain 2010, 14, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Buenaver, L.F.; Edwards, R.R.; Smith, M.T.; Gramling, S.E.; Haythornthwaite, J.A. Catastrophizing and pain-coping in young adults: Associations with depressive symptoms and headache pain. J. Pain 2008, 9, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing: A critical review. Expert Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef] [PubMed]
- George, S.Z.; Hirsh, A.T. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J. Pain 2009, 10, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Pelaez-Ballestas, I.; Boonen, A.; Vazquez-Mellado, J.; Reyes-Lagunes, I.; Hernandez-Garduno, A.; Goycochea, M.V.; Bernard-Medina, A.G.; Rodriguez-Amado, J.; Casasola-Vargas, J.; Garza-Elizondo, M.A.; et al. Coping strategies for health and daily-life stressors in patients with rheumatoid arthritis, ankylosing spondylitis, and gout: Strobe-compliant article. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.; Stewart, I.; Barnes-Holmes, D.; Barnes-Holmes, Y.; McGuire, B.E. Comparison of acceptance and distraction strategies in coping with experimentally induced pain. J. Pain Res. 2015, 8, 139–151. [Google Scholar] [PubMed]
- Rejeh, N.; Heravi-Karimooi, M.; Vaismoradi, M.; Jasper, M. Effect of systematic relaxation techniques on anxiety and pain in older patients undergoing abdominal surgery. Int. J. Nurs. Pract. 2013, 19, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Boyle, D.K.; Bergquist-Beringer, S.; Staggs, V.S.; Dunton, N.E. Concurrent and lagged effects of registered nurse turnover and staffing on unit-acquired pressure ulcers. Health Serv. Res. 2014, 49, 1205–1225. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Staggs, V.S. Comparability of nurse staffing measures in examining the relationship between rn staffing and unit-acquired pressure ulcers: A unit-level descriptive, correlational study. Int. J. Nurs. Stud. 2014, 51, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.G.; Anderson, R.A. Caregiver staffing in nursing homes and their influence on quality of care: Using dynamic panel estimation methods. Med. Care 2011, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.; Gorecki, C.; Nelson, E.A.; Closs, S.J.; Defloor, T.; Halfens, R.; Farrin, A.; Brown, J.; Schoonhoven, L.; Nixon, J. Patient risk factors for pressure ulcer development: Systematic review. Int. J. Nurs. Stud. 2013, 50, 974–1003. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.K.; Karadag, A. Assessment of nurses’ knowledge and practice in prevention and management of deep tissue injury and stage i pressure ulcer. J. Wound Ostomy Cont. Nurs. 2010, 37, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Pancorbo-Hidalgo, P.L.; Garcia-Fernandez, F.P.; Lopez-Medina, I.M.; Lopez-Ortega, J. Pressure ulcer care in spain: Nurses’ knowledge and clinical practice. J. Adv. Nurs. 2007, 58, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Chiummariello, S.; Guarro, G.; Pica, A.; Alfano, C. Evaluation of negative pressure vacuum-assisted system in acute and chronic wounds closure: Our experience. G. Chir. 2012, 33, 358–362. [Google Scholar] [PubMed]
- Woo, K.Y.; Sibbald, R.G. Chronic wound pain: A conceptual model. Adv. Skin Wound Care 2008, 21, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.; DeSanto-Madeya, S. Contemporary Nursing Knowledge: Analysis and Evaluation of Nursing Models and Theories, 3rd ed.; F.A. Davis Company: Philadelphia, PA, USA, 2012. [Google Scholar]
- Meleis, A.I. Theoretical Nursing: Development and Progress, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Burns, N.B.; Grove, S.K. The Practice of Nursing Research: Conduct, Critique, and Utilization, 5th ed.; Elsevier Saunders: St. Louis, MO, USA, 2005. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ahn, H.; Lyon, D.E.; Stechmiller, J. Building a Biopsychosocial Conceptual Framework to Explore Pressure Ulcer Pain for Hospitalized Patients. Healthcare 2016, 4, 7. https://doi.org/10.3390/healthcare4010007
Kim J, Ahn H, Lyon DE, Stechmiller J. Building a Biopsychosocial Conceptual Framework to Explore Pressure Ulcer Pain for Hospitalized Patients. Healthcare. 2016; 4(1):7. https://doi.org/10.3390/healthcare4010007
Chicago/Turabian StyleKim, Junglyun, Hyochol Ahn, Debra E. Lyon, and Joyce Stechmiller. 2016. "Building a Biopsychosocial Conceptual Framework to Explore Pressure Ulcer Pain for Hospitalized Patients" Healthcare 4, no. 1: 7. https://doi.org/10.3390/healthcare4010007






