Developing a Complex Educational–Behavioural Intervention: The TREAT Intervention for Patients with Atrial Fibrillation
Abstract
:1. Introduction
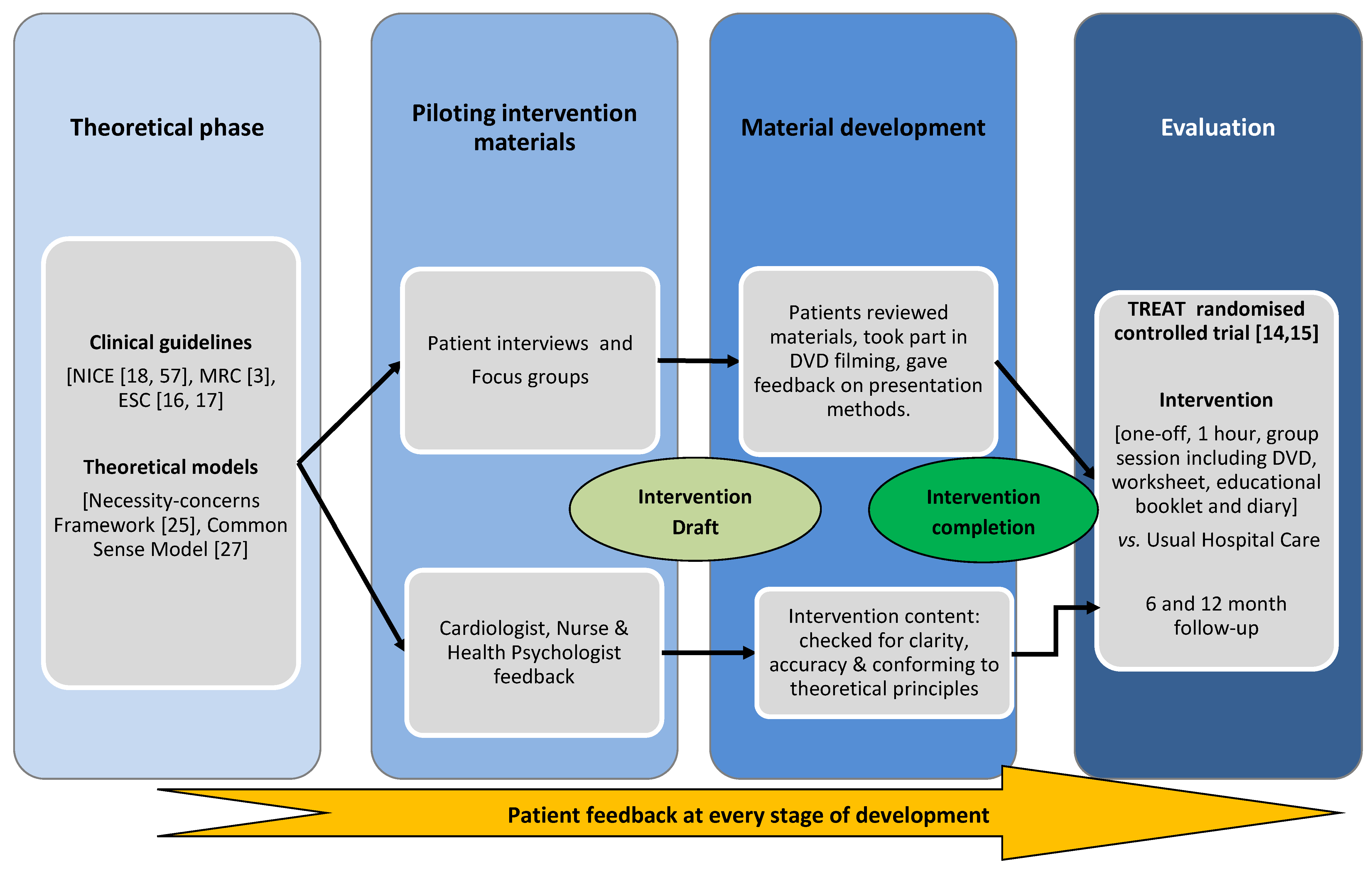
1.1. Intervention Development Process
1.2. Theoretical Approach
1.2.1. Application of the Necessity-Concerns Framework
| Key Recommendations |
|---|
|
1.2.2. Application of the Common Sense Model
| Create an illness identity | Help patients understand which symptoms are/are not associated with AF, common co-morbidities, the risks of stroke and the reasons for prescribing anticoagulant medication and the emotions individuals associate with the illness (e.g., “I am afraid of what will happen“). |
| Understand the consequences | Help patients understand the physical, social and economic implications of both AF and treatment with anticoagulation. Patients need to be provided with information about the risks associated with AF e.g., the main risk associated with AF is stroke. |
| Identifying their illness timeline | Patients can be made aware of the duration of their illness and treatment and given information about the different types of AF and how this relates to the risk of stroke. |
| Understanding the causes | Patients need to recognise their personal ideas about the causes of AF and how they relate to the scientific evidence. |
| Identifying a cure or control for their illness/symptoms | Patients can be presented with information pertaining to the control of their INR and pharmacological control of their AF symptoms, and explore the factors that may affect their symptoms including caffeine intake, exercise and alcohol. Of particular relevance are the key lifestyle factors that affect INR control including diet, alcohol intake and other medications and supplements, as for many patients there is no “cure” for AF. |
2. Experimental Section
2.1. Development of the Intervention Materials
2.2. Intervention Outline
2.3. Patient Involvement
2.3.1. Description of Symptoms
| Behavioural Change Technique | Method of Delivery | Intervention Components | Theoretical Model Targets |
|---|---|---|---|
| Provide general information on behaviour-health link |
|
|
|
| Provide information on consequences |
|
|
|
| Prompt barrier identification |
|
|
|
| Provide instruction |
|
|
|
| Prompt self-monitoring of behaviour |
|
|
|
| Teach to use prompts/cues |
|
|
|
| Provide opportunities for social comparison |
|
|
|
2.3.2. Types of AF
- Paroxysmal: multiple episodes that typically last less than 48 h and stop by themselves.
- Persistent: episodes that last longer than 7 days, or stop when treated.
- Permanent: continuous atrial fibrillation for more than 1 year.
- [M2]:
- “out of that... number 3 will be the nearest to me... permanent continuous atrial fibrillation... for more than 1 year and I would say 50 years... that's how long I can go back... I can only say its number 3 for me because of the length of time”.
- I –
- “how about everybody else?”
- [F1]:
- “I have spent 3 years since I was diagnosed with it. With atrial fibrillation”
- I–
- “and which category would you fall into?”
- [F1:]
- “mmm I would say probably the first one... comes and goes...”
2.3.3. Risk Presentation
2.3.4. Diagram of the Heart and Formation of Clots
- [F1]:
- “well its explaining what can happen in the sections of the heart and how erm, as it there, clots can form and erm”
- [M1]:
- “go to the brain”
- [F1]:
- “yeah… can go to the brain and that can cause erm strokes or whatever and at the same time, it is also showing how the different movements of the heart, the pumping of the heart”
- [M1]:
- “oh yes”
- [F1]:
- “can effect erm… this distribution shall we say unless it is controlled with a thinning… drug or whatever. That’s how I look at it”
3. Results
3.1. Evaluation of the Intervention
| Trial No | ISRCTN93952605 |
|---|---|
| Target group | N Randomised: 46 intervention vs. 51 usual care Diagnosis: Warfarin-naive AF patients Demographics of cohort: Mean (SD) age 72.9 (8.2) years; 64.9% male; 99% White British, Irish or European. No significant differences in demographic variable between groups. Inclusion/exclusion criteria: AF patients newly referred for warfarin therapy, with ECG-documented AF, will be eligible for inclusion. Patients were excluded if they were aged <18 years old, had any contraindication to warfarin, had previously received warfarin, had valvular heart disease, cognitively impaired, unable to speak or read English or had any disease likely to cause their death within 12 months. |
| Intervention | Type: One-off group [1–6 patients] theory-driven educational intervention Content: DVD, educational booklet, worksheet, group discussion. Duration: 1 h session Facilitator: Health-Psychologist (could be delivered by trained lay educator) Setting: Hospital outpatients clinic |
| Outcomes | Primary outcome: Time within therapeutic range (TTR) calculated using the Rosendaal method Secondary outcomes: Patient knowledge, Beliefs about Medication, Quality of Life, Anxiety and Depression, Hospital Admissions and Adverse events. |
| Comparison group | Usual Hospital Care |
| Random sequence generation | A computer generated list stratified by (a) age (<70 and ≥70 years)/sex and (b) specialist AF clinic versus ‘general’ cardiology clinic, in blocks of four, randomised patients on an individual basis to receive either ‘usual care’ or the intensive educational intervention, in addition to ‘usual care’. The randomisation schedule was designed by an independent trials unit. |
| Blinding | A researcher not involved in the data analysis or intervention delivery matched patient ID numbers with randomisation codes and checked follow-up questionnaires for completeness. The researcher analysing the data was blinded to which arm of the intervention patients were randomised to. |
3.2. Beliefs about Medication
3.3. Illness Perceptions
4. Discussion
| N (%) | Baseline | χ2 | 1 Month | χ2 | 2 Months | χ2 | 6 Months | χ2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (n = 25) | Usual care (n = 34) | Intervention (n = 24) | Usual care (n = 24) | Intervention (n = 19) | Usual care (n = 19) | Intervention (n = 23) | Usual care (n = 21) | |||||
| Psychological | 10 (40.0) | 14 (41.2) | 1.38 | 8 (33.3) | 8 (33.3) | 0.15 | 3 (15.8) | 9 (47.4) | 4.47 | 3 (13.0) | 9 (42.9) | 6.31 * |
| External | 6 (24.0) | 12 (35.3) | 11 (45.8) | 12 (50.0) | 12 (63.2) | 8 (42.1) | 17 (73.9) | 8 (38.1) | ||||
| Lifestyle | 9 (36.0) | 8 (23.5) | 5 (20.8) | 4 (16.7) | 4 (21.1) | 2 (10.5) | 3 (!3.0) | 4 (19.0) |
5. Conclusions
Practice Implications
- Provide educational materials and discuss the health-behaviour link, enabling patients to understand why and how they make lifestyle changes.
- Provide educational materials and risk information on the consequences of AF and treatment with/or without warfarin
- Provide opportunities for social comparison with other patients
- Encourage patients to self-monitor, create action plans, and use their own memory aids/cues for remembering to take tablets
- Discuss patients concerns and barriers to changing their lifestyle and adopting a new treatment regime; correct any misconceptions with accurate information.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. Int. J. Nurs. Stud. 2013, 50, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Treweek, S.; Dreischulte, T.; Foy, R.; Guthrie, B. Process evaluations for cluster-randomised trials of complex interventions: A proposed framework for design and reporting. Trials 2013. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council. A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health; Medical Research Council: London, UK, 2000; p. 18. [Google Scholar]
- Clarkesmith, D.E.; Pattison, H.M.; Lane, D.A. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Cannegieter, S.C.; van der Meer, F.J.; Briët, E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993, 69, 236–239. [Google Scholar] [PubMed]
- Lane, D.A.; Ponsford, J.; Shelley, A.; Sirpal, A.; Lip, G.Y. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: Effects of an educational intervention programme. The West Birmingham Atrial Fibrillation Project. Int. J. Cardiol. 2006, 110, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Barker, R.V.; Lip, G.Y.H. Best practice for atrial fibrillation patient education. Curr. Pharm. Des. 2015, 21, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Aguinaga, L.; Blomström-Lundqvist, C.; Boriani, G.; Dan, G.A.; Hills, M.T.; Hylek, E.M.; LaHaye, S.A.; Lip, G.Y.; Lobban, T.; et al. Cardiac tachyarrhythmias and patient values and preferences for their management: The European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2015. [Google Scholar] [CrossRef]
- Connolly, S.J.; Pogue, J.; Eikelboom, J.; Flaker, G.; Commerford, P.; Franzosi, M.G.; Healey, J.S.; Yusuf, S. ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008, 118, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.L.; McEwan, P.; Tukiendorf, A.; Robinson, P.A.; Clemens, A.; Plumb, J.M. Warfarin treatment in patients with atrial fibrillation: Observing outcomes associated with varying levels of INR control. Thromb Res. 2009, 124, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Thrall, G.; Lip, G.Y.; Carroll, D.; Lane, D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest 2007, 132, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Langman, C.M.; Lip, G.Y.; Nouwen, A. Illness perceptions, affective response, and health-related quality of life in patients with atrial fibrillation. J. Psychosom. Res. 2009, 66, 203–210. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.J.; Barnason, S.A.; Houfek, J. Illness beliefs in patients with recurrent symptomatic atrial fibrillation. Pacing Clin. Electrophysiol. 2011, 34, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Xuereb, C.B.; Pattison, H.M.; Lip, G.Y.; Lane, D.A. TRial of an Educational intervention on patients’ knowledge of Atrial fibrillation and anticoagulant therapy, INR control, and outcome of Treatment with warfarin (TREAT). BMC Cardiovasc. Disord. 2010. [Google Scholar] [CrossRef] [PubMed]
- Clarkesmith, D.E.; Pattison, H.M.; Lip, G.Y.; Lane, D.A. Educational intervention improves anticoagulation control in atrial fibrillation patients: The TREAT randomised trial. PLoS ONE 2013, 8, e74037. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation. Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [PubMed]
- Camm, A.J.; Lip, G.Y.; de Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar] [PubMed]
- National Institute for Health and Care Excellence (NICE). Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care; National Clinical Guideline Centre (UK): London, UK, 2006. [Google Scholar]
- Horne, R.; Weinmann, J.; Hankins, M. The Beliefs about Medicines Questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health 1999, 14, 1–24. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Neame, R.; Hammond, A. Beliefs about medications: A questionnaire survey of people with rheumatoid arthritis. Rheumatology 2005, 44, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Jessop, D.C.; Rutter, D.R. Adherence to asthma medications: The role of illness representations. Psychol. Health 2003, 18, 595–612. [Google Scholar] [CrossRef]
- Farmer, A.; Kinmonth, A.L.; Sutton, S. Measuring beliefs about taking hypoglycaemic medication among people with Type 2 diabetes. Diabet. Med. 2006, 23, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Aikens, J.E.; Nease, D.E., Jr.; Nau, D.P.; Klinkman, M.S.; Schwenk, T.L. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann. Fam. Med. 2005, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Horne, R. Treatment perceptions and self regulation. In The Self-Regulation of Health and Illness Behaviour; Cameron, L.D., Leventhal, H., Eds.; Routledge Taylor Francis Group: London, UK, 2003; pp. 138–153. [Google Scholar]
- Clifford, S.; Barber, N.; Horne, R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: Application of the Necessity-Concerns Framework. J. Psychosom. Res. 2008, 64, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Leventhal, H.; Gutmann, M. Common-sense models of illness: The example of hypertension. Health Psychol. 1985, 4, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.D.; Leventhal, H. The Self-Regulation of Health and Illness Behaviour; Routledge: New York, NY, USA, 2003. [Google Scholar]
- Skelton, J.A.; Croyle, R.T. Mental Representation Health and Illness; Springer: New York, NY, USA, 1991. [Google Scholar]
- Horowitz, C.R.; Rein, S.B.; Leventhal, H. A story of maladies, misconceptions and mishaps: Effective management of heart failure. Soc. Sci. Med. 2004, 58, 631–643. [Google Scholar] [CrossRef]
- Cameron, L.; Leventhal, E.A.; Leventhal, H. Symptom representations and affect as determinants of care seeking in a community-dwelling, adult sample population. Health Psychol. 1993, 12, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Skinner, T.C.; Hampson, S.E. Personal models of diabetes in relation to self-care, well-being, and glycemic control. A prospective study in adolescence. Diabetes Care 2001, 24, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Hagger, M.S.; Orbell, S. A meta-analytic review of the common-sense model of illness representations. Psychol. Health 2003, 18, 141–184. [Google Scholar] [CrossRef]
- Leventhal, H.; Weinman, J.; Leventhal, E.A.; Phillips, L.A. Health psychology: The search for pathways between behavior and health. Annu. Rev. Psychol. 2008, 59, 477–505. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Heller, S.; Skinner, T.C.; Campbell, M.J.; Carey, M.E.; Cradock, S.; Dallosso, H.M.; Daly, H.; Doherty, Y.; Eaton, S.; et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: Cluster randomised controlled trial. BMJ 2008, 336, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.J.; Cameron, L.D.; Ellis, C.J.; Buick, D.; Weinman, J. Changing illness perceptions after myocardial infarction: An early intervention randomized controlled trial. Psychosom. Med. 2002, 64, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.R.; Bernard, T.M.; Hartman, K.A. Further explorations of common-sense representations of common illnesses. Health Psychol. 1989, 8, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, H.; Nerenz, D.R.; Steele, D.J. Illness representations and coping with health threats. In Handbook of Psychology and Health, Volume IV: Social and Psycholgical Aspects of Health; Baum, A., Taylor, S.E., Singer, J.E., Eds.; Erlbaum: Hillsdale, MI, USA, 1984; Volume 5, pp. 219–252. [Google Scholar]
- McAndrew, L.M.; Musumeci-Szabó, T.J.; Mora, P.A.; Vileikyte, L.; Burns, E.; Halm, E.A.; Leventhal, E.A.; Leventhal, H. Using the common sense model to design interventions for the prevention and management of chronic illness threats: From description to process. Br. J. Health Psychol. 2008, 13, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27, 379–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; le Heuzey, J.Y.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. JACC 2006, 114, e257–e354. [Google Scholar]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Man-Son-Hing, M.; Laupacis, A.; O’Connor, A.M.; Biggs, J.; Drake, E.; Yetisir, E.; Hart, R.G. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: A randomized controlled trial. JAMA 1999, 282, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Lip, G.Y. Quality of life in older people with atrial fibrillation. J. Interv. Card Electrophysiol. 2009, 25, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.; Dudley, N.; Blacktop, J. Avoidance hierarchies and preferences for anticoagulation-semi-qualitative analysis of older patients’ views about stroke prevention and the use of warfarin. Age Ageing 2004, 33, 608–611. [Google Scholar] [CrossRef] [PubMed]
- ISRCTNregistry. Educational Intervention for Atrial Fibrillation. Available online: http://www.controlled-trials.com/ISRCTN93952605 (accessed on 17 September 2013).
- Devereaux, P.J.; Anderson, D.R.; Gardner, M.J.; Putnam, W.; Flowerdew, G.J.; Brownell, B.F.; Nagpal, S.; Cox, J.L. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: Observational study. BMJ 2001, 323, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, S.; Regpala, S.; Lacombe, S.; Sharma, M.; Gibbens, S.; Ball, D.; Francis, K. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost 2014, 111, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Arnar, D.O.; Thorvaldsson, S.; Manolio, T.A.; Thorgeirsson, G.; Kristjansson, K.; Hakonarson, H.; Stefansson, K. Familial aggregation of atrial fibrillation in Iceland. Eur. Heart J. 2006, 27, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Parise, H.; D’Agostino, R.B.; Lloyd-Jones, D.M.; Vasan, R.S.; Wang, T.J.; Levy, D.; Wolf, P.A.; Benjamin, E.J. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004, 291, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
- Van Oort, L.; Schröder, C.; French, D.P. What do people think about when they answer the Brief Illness Perception Questionnaire? A “think-aloud” study. Br. J. Health Psychol. 2011, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, N.C.; de Ridder, D.T.; Bensing, J.M.; Rutten, G.E. Manipulation of patient-provider interaction: Discussing illness representations or action plans concerning adherence. Patient Educ. Couns. 2003, 51, 247–258. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J.; Barber, N.; Elliot, R.; Morgan, M. Concordance, Adherence and Compliance in Medicine Taking. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation (NCCSDO). 2005. Available online: http://www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1412-076_V01.pdf (accessed on 12 November 2014).
- National Institute for Health and Clinical Excellence (NICE). Behaviour Change at Population, Community and Individual Levels; National Clinical Guideline Centre (UK): London, UK, 2007. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarkesmith, D.E.; Pattison, H.M.; Borg Xuereb, C.; Lane, D.A. Developing a Complex Educational–Behavioural Intervention: The TREAT Intervention for Patients with Atrial Fibrillation. Healthcare 2016, 4, 10. https://doi.org/10.3390/healthcare4010010
Clarkesmith DE, Pattison HM, Borg Xuereb C, Lane DA. Developing a Complex Educational–Behavioural Intervention: The TREAT Intervention for Patients with Atrial Fibrillation. Healthcare. 2016; 4(1):10. https://doi.org/10.3390/healthcare4010010
Chicago/Turabian StyleClarkesmith, Danielle E., Helen M. Pattison, Christian Borg Xuereb, and Deirdre A. Lane. 2016. "Developing a Complex Educational–Behavioural Intervention: The TREAT Intervention for Patients with Atrial Fibrillation" Healthcare 4, no. 1: 10. https://doi.org/10.3390/healthcare4010010





